Abstract
Trepanning of the bone is one of the oldest known procedures carried out by man and the use of the modern trephine biopsy has a venerable history. Parapia has published an admirable summary of the history of the topic and this should be consulted for the excellent illustrations of historical instruments [1]. The history is briefly summarized here [1]. Trepanning of the skull is the oldest known surgical procedure in humans and evidence of this practice has been found in Europe, North Africa, South America, Asia and New Zealand. In Peru, where the procedure is likely to have been carried out to treat headache, mental illness and to relieve intracranial pressure, sharp knives of obsidian, stone and bronze were used for trephination. Celsus, the Roman physician, described a modiolus – an iron instrument with a serrated cylinder that was rotated over a central pin by means of a strap. The early interventions were therapeutic and the first diagnostic biopsy was undertaken in Pianese in Italy in 1903. In 1922, Morris and Falconer used a drill-like instrument to biopsy the tibia, producing similar specimens to modern biopsies and, in the same year, Seyfarth developed a puncture needle for open biopsy of the sternum, producing smears, touch preparations and blocks for sectioning. The modern era probably began in 1958 when McFarland and Dameshek described a technique for biopsy of the right posterior iliac crest using a Silverman needle, which had been described in 1938. Further improvements followed, with modified instruments described by Jamshidi in 1971 and an electric drill technique by Burkhardt in 1971. Recent developments are described later in the chapter.
Introduction
Trepanning of the bone is one of the oldest known procedures carried out by man and the use of the modern trephine biopsy has a venerable history. Parapia has published an admirable summary of the history of the topic and this should be consulted for the excellent illustrations of historical instruments [1]. The history is briefly summarized here [1]. Trepanning of the skull is the oldest known surgical procedure in humans and evidence of this practice has been found in Europe, North Africa, South America, Asia and New Zealand. In Peru, where the procedure is likely to have been carried out to treat headache, mental illness and to relieve intracranial pressure, sharp knives of obsidian, stone and bronze were used for trephination. Celsus, the Roman physician, described a modiolus – an iron instrument with a serrated cylinder that was rotated over a central pin by means of a strap. The early interventions were therapeutic and the first diagnostic biopsy was undertaken in Pianese in Italy in 1903. In 1922, Morris and Falconer used a drill-like instrument to biopsy the tibia, producing similar specimens to modern biopsies and, in the same year, Seyfarth developed a puncture needle for open biopsy of the sternum, producing smears, touch preparations and blocks for sectioning. The modern era probably began in 1958 when McFarland and Dameshek described a technique for biopsy of the right posterior iliac crest using a Silverman needle, which had been described in 1938. Further improvements followed, with modified instruments described by Jamshidi in 1971 and an electric drill technique by Burkhardt in 1971. Recent developments are described later in the chapter.
In modern practice, bone marrow (BM) examination, including both aspiration and biopsy sampling, can be performed on virtually any patient. However, patients with coagulation deficiencies or profound thrombocytopaenia may experience prolonged bleeding, which cannot be controlled by pressure bandages. In these rare cases, specific treatment (e.g. platelet transfusion) may be indicated. In a study of 19 259 procedures, including 13 147 combined procedures and 6112 aspirates, a total of 16 adverse events were reported, representing 0.08% of all reported procedures. The adverse events were eleven haemorrhages, three very serious, with other complications being two infections, two cases of persistent pain and a serous leak persisting for six days [2].
Indications for performing BM examination are summarized in Table 1.1. It has long been accepted that in the vast majority of cases, both aspiration and a biopsy should be performed and are complementary [3, 4]. Bone marrow aspiration provides excellent cytological detail; however, marrow architecture cannot be assessed. Bone marrow core biopsy (BMB) allows for an accurate analysis of architecture but fine cytological details may be lost. Table 1.2 shows the accepted indications for performing a BMB. These include cases with inadequate or failed aspiration, a need for accurate assessment of BM cellularity, architecture, stroma, vascularity and bone structure, and cases in which the presence of focal lesions, such as granulomatous disease or metastatic carcinoma, is suspected. In general, patients with hypocellular marrows or BM fibrosis are likely to need a BMB for adequate assessment. In such patients, an aspirate would probably be inadequate or even impossible. Unexplained pancytopaenia and unexplained leukoerythroblastic blood pictures are further indications for a BMB, because they are likely to indicate the presence of BM metastatic disease or fibrosis. Focal skeletal lesions may also require a BMB when focal manifestation of generalized disease, e.g. plasma cell myeloma or metastatic malignancy, may be found. Targeted BMB may also be used.
Table 1.1 Indications for bone marrow aspiration with or without a trephine biopsy.
| Investigation and/or follow-up of: |
|---|
| Unexplained microcytosis |
| Unexplained macrocytosis |
| Unexplained anaemia |
| Unexplained thrombocytopaenia |
| Pancytopaenia (including suspected aplastic anaemia) |
| Leukoerythroblastic blood smear and suspected bone marrow infiltration |
| Suspected acute leukaemia |
| Suspected myelodysplasia or myelodysplastic/myeloproliferative disorder |
| Suspected myeloproliferative neoplasms |
| Suspected chronic lymphocytic leukaemia, hairy cell leukaemia and other leukaemic lymphoproliferative disorders |
| Suspected non-Hodgkin lymphoma |
| Staging of non-Hodgkin lymphoma |
| Suspected plasma cell myeloma or other plasma cell dyscrasias |
| Suspected storage disease |
| Fever of unknown origin (with microbial culture) |
| Investigation of anaemia in immunodeficiency |
| Confirmation of normal bone marrow if bone marrow is being aspirated for allogeneic transplantation |
| Follow-up and monitoring of therapy |
Table 1.2 Indications for performing a bone marrow biopsy.
| Investigation and/or follow-up of: |
|---|
| Diagnosis and/or staging of suspected Hodgkin lymphoma and non-Hodgkin lymphoma |
| Chronic lymphocytic leukaemia, hairy cell leukaemia and other leukaemic lymphoproliferative disorders |
| Diagnosis of suspected metastatic carcinoma |
| Diagnosis, staging and follow-up of small cell tumours of childhood |
| Myeloproliferative neoplasms, myeloproliferative/myelodysplastic neoplasms, systemic mastocytosis |
| Diagnosis of aplastic anaemia |
| Investigation of an unexplained leukoerythroblastic blood smear |
| Investigation of a fever of unknown origin and/or granulomatous infection |
| Investigation of suspected haemophagocytic syndrome |
| Evaluation of any patient in whom an adequate bone marrow aspirate cannot be obtained |
| Suspected plasma cell myeloma and other plasma cell dyscrasias |
| Suspected acute myeloid leukaemia |
| Suspected myelodysplastic syndrome |
| Investigation of suspected storage disease |
| Suspected primary amyloidosis |
| Investigation of bone diseases |
The BMB specimen differs from biopsy material from most other organs, because a proper interpretation of the BM in haematological disorders requires the incorporation of clinical data, BM aspirate studies, peripheral blood smears and complete blood count data, which should be evaluated in conjunction with flow cytometry and molecular investigations, to form a composite report in order to fulfil WHO disease definitions and comply with current guidelines [5–8]. Occasionally, BMB imprint smears may be used to assess cytology in cases with inaspirable marrows (dry taps). The benefits of synoptic reporting in a multiparameter diagnosis are well established and check lists and general recommendations have been published in the USA and UK [6, 8]. The following chapters will emphasize this multifactorial approach to BM evaluation, attempt to highlight the diagnostic questions that require the use of ancillary techniques for accurate diagnosis and provide guidance in their selection.
Obtaining the Bone Marrow Aspirate and Biopsy
The technique used to obtain BMB and aspirate smears has been described in detail in standard publications and the reader is referred to those for more detail [9, 10]. Separate guidelines for children have also been published [11]. If BM aspiration and biopsy are both being performed using the same needle, it is usually preferable to obtain the biopsy first, to avoid distortion of the biopsy specimen by aspiration artefacts. Another approach uses separate needles for each procedure. This requires that the needles be placed in different sites of the bone marrow; good-quality aspirate and biopsy can be obtained in either sequence. When two needles are used, it is often advantageous to aspirate the marrow through a smaller needle specifically designed for that purpose. This minimizes the contamination with peripheral blood, which is often observed when the aspirate is performed through a Jamshidi needle.
In recent years, new and potentially improved BMB devices have been developed, including powered devices. However, a recent study suggested that, despite the ability of powered devices to yield longer BMB lengths, the method is costlier than manual BMB and does not result in a discernible improvement in the quality of BM specimens [12]. A recent review of a number of different devices concluded that the newly launched Möller Medical bone marrow biopsy needle (Möller Medical, Fulda, Germany) had definite advantages over the others tested, i.e. the J-needle by Cardinal Health (Cardinal Health, Waukegan, Illinois, USA), the Jamshidi bone marrow biopsy needle with marrow acquisition cradle by CareFusion (CareFusion, Vernon Hills, Illinois, USA) and the OnControl powered drill system by Vidacare (Vidacare, Texas, USA) [13].
The initial aspirate sample should always be used for morphology. Subsequent aspirations may be obtained for flow cytometry, cytogenetics, microbiology culture or molecular studies. Non-anticoagulated specimens should be immediately handed to a technical assistant who will prepare various smears and BM particle crush preparations. The remaining BM aspirate material is allowed to clot and submitted for BM clot sections. Alternatively, aspirate smears can be made in the laboratory after the procedure. To this end, BM aspirate material should be immediately placed into tubes containing ethylenediaminetetraacetic acid (EDTA). This limits the clotting of the aspirate specimen and allows material to be submitted for ancillary studies as well as particle sections. Whether the smears are made at the bedside or from the EDTA tubes, the aspirate should be grossly evaluated for the presence of BM particles. The absence of particles on a smear limits its diagnostic usefulness in many cases. Such smears often show findings consistent with peripheral blood contamination. Many paediatric patients, however, will not demonstrate gross particles in the aspirate material despite numerous BM elements in the smear.
Representative aspirate smears and imprints are stained with a Romanovsky type of stain. The actual stain type varies among laboratories and includes Giemsa, Wright–Giemsa and May–Grünwald–Giemsa stains. Rapid review of these smears helps in determining the need for ancillary studies, such as cytochemistry, immunophenotyping, cytogenetic analysis and molecular genetic study.
Clot biopsy sections are often made from coagulated aspirate material. This material contains predominantly blood as well as small BM particles that can be embedded in paraffin, sectioned and stained with haematoxylin and eosin (H&E) or other stains. Alternatively, when EDTA-anticoagulated aspirate specimens are used, the BM particles that are left after smears are prepared can be filtered and embedded for histologic evaluation [14]. This method provides a more concentrated collection of BM particles but may not yield any material, particularly in paediatric patients [15].
Trephine core biopsy is not performed for all patients, but these specimens are essential in the evaluation of patients suspected of having disease that may only involve the BM focally, such as malignant lymphoma or metastatic carcinoma, and are preferred in all patients. Core biopsies allow for architectural assessment of the BM and offer a number of other benefits. The incidences of BM involvement by various types of malignancies are proportional to the amount of BM evaluated. The minimum adequate length is in the range 15 to 20 mm [8]. To increase the yield, bilateral BMBs have been recommended for patients undergoing BM staging by several authors [15, 16] but this is no longer regularly performed [8].
Imprint slides from the biopsy specimens may yield positive same-day results in some of the aspirate-negative or dry-tap cases [17]. The imprints can be made either at the bedside or in the laboratory. To make them in the laboratory, the BM core is submitted fresh, on saline-dampened gauze, with the imprints made immediately to allow adequate fixation of the biopsy specimen. Otherwise, the imprints are made at the bedside and the biopsy specimen is submitted in fixative.
Fixation, Decalcification and Processing of the Biopsy
Bone marrow biopsies differ from most others in that decalcification is mandatory and excellent morphology is obligatory. Furthermore, molecular diagnostic techniques, covered in detail in Chapter 20, can be applied to BM biopsies and preservation of DNA and RNA is thus required to maximize the diagnostic yield. Archival BM biopsies provide an opportunity to examine previous biopsies for cytogenetic abnormalities that may shed light on progression of the disease and to use new diagnostic techniques unavailable at the time of the original diagnosis. However, methods for the preparation, processing and reporting of BM aspirates and BMB specimens can vary considerably and these differences may thus result in inconsistencies in disease diagnosis or classification that may affect treatment and clinical outcomes [10]. An international Working Party for the Standardization of Bone Marrow Specimens and Reports was formed by the International Council for Standardization in Haematology (ICSH) to prepare a set of guidelines based on preferred best practices, which are summarized here, and the reader is referred to this comprehensive overview for further detail [10].
Decalcification using methods including EDTA, formic acid, acetic acid, nitric acid and various commercial agents varies in time required for complete decalcification and in the preservation of cytomorphology, tissue iron and the ability to perform reliable, quantifiable immunohistochemistry and molecular diagnostic analysis [10]. A recent study of the methods used by 28 different laboratories revealed that eight different fixatives and nine different decalcification methods were used [18]. While 93% of participants believed that they produced excellent results in BMB immunohistochemistry (IHC), only 21% of laboratories did not have any poor results. This study led to a call for a reduction in the number of processing methods and calibration of results in external quality assessment (EQA) programmes [18]. Elliot et al. have also addressed the variability of IHC staining in general and stressed the need for IHC laboratories to bring the same level of robust validation seen in the molecular pathology laboratories and called for the principles to be applied to all routine IHC tests participation, assessed in appropriate EQA schemes [19]. ICSH guidelines for the standardization of BM IHC have now been published based on currently available published evidence and modern understanding of quality assurance principles as applied to IHC in general [20]. The reader is referred to the article for detailed guidance but, in summary, the following core principles are defined.
Ischaemic Time
Depending on the availability of on-site technical support, preparation of BM biopsy imprints and aspirate smears at the bedside, to minimise cold ischaemic time and hence preserve protein epitopes relevant to IHC and RNA and DNA integrity, constitutes optimal practice. Transportation of fresh samples to the laboratory is suboptimal and increases epitope degradation and the risk of false negative results [20]. The BM biopsy should be rapidly placed into fixative for onward transmission to the laboratory after any additional techniques such as trephine biopsy roll preparations have been made.
Fixation
To avoid zoning, with areas of absent or suboptimal staining, fixation must be even and complete. 10% buffered formalin remains the common standard fixative but acetic acid–zinc–formalin (AZF) has been proposed as an alternative that improves laboratory throughput due to decreased decalcification time, while preserving IHC and molecular testing [21, 22]. Local experience has not supported AZF as a method due to poor molecular results and careful evaluation is recommended before adoption [unpublished data]. Bouin’s solution has fallen out of favour due to laboratory safety issues and poor IHC results, and Zenker’s solution and B5 are now unavailable in many countries due to their mercuric chloride content [20].
Decalcification
Current optimal practice requires that recommendations for the IHC processing methods also take into account DNA and RNA integrity, and this applies to decalcification as well as fixation, and there may be a conflict between the ability to perform both IHC and molecular tests and turnaround time (TAT). Decalcification is best achieved by 14% EDTA, which sequesters metallic ions, including calcium, in aqueous solutions as a chelating factor for 16–24 hours [20]. Strong mineral acids such as 10% hydrogen chloride (HCl) or weak organic acids, such as 5–10% formic acid (HCOOH) form soluble calcium salts in an ion exchange that moves calcium into the decalcification solution and are used for decalcification, particularly when rapid TAT is required. However, questions remain about suitability for IHC and molecular tests, and acid-based decalcification is not recommended unless steps are taken to ensure that processing is rigorously monitored and material is preserved for IHC and molecular tests, such as the aspirate or peripheral blood [20]. Improved TAT is achieved by shortening EDTA decalcification using a magnetic stirrer in a microwave or hot plate at 37–40°C [20]. Decalcification can be accelerated by using ultrasound energization at lower temperatures (e.g. 18°C), which reduces the decalcification time to approximately 6–12 hours and even as short as 2 hours [20, 23]. Novel decalcification solutions used at 37°C that resulted in superior nucleic acid preservation have been described and deserve further evaluation [24].
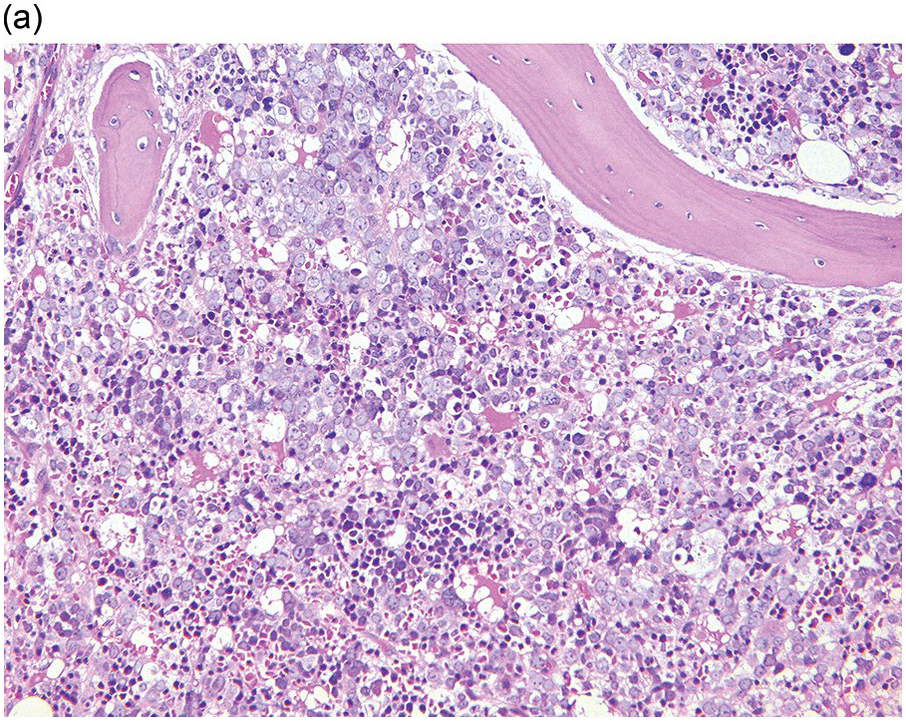
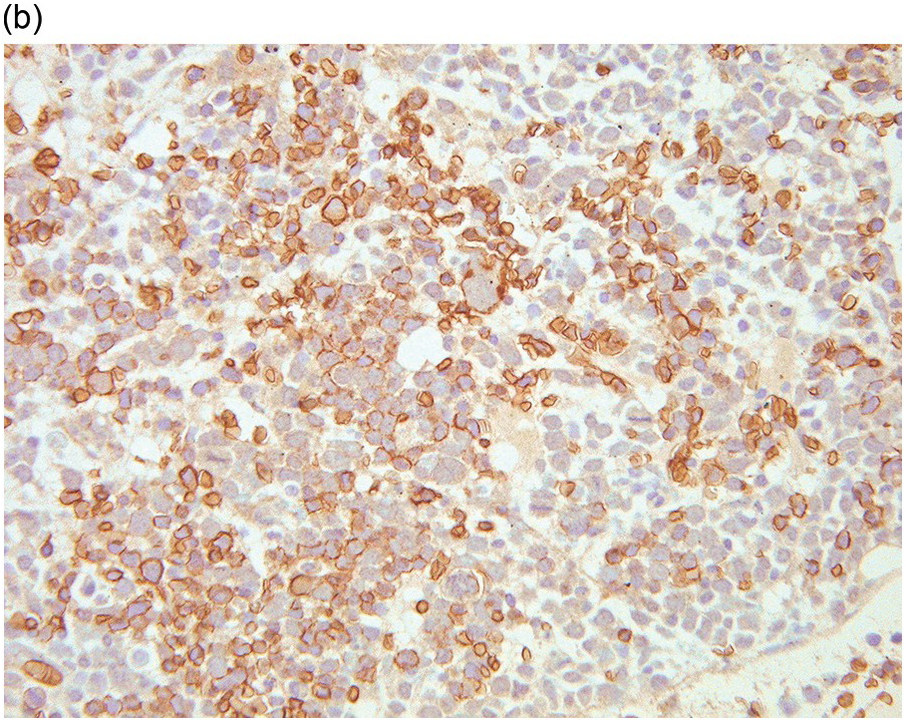
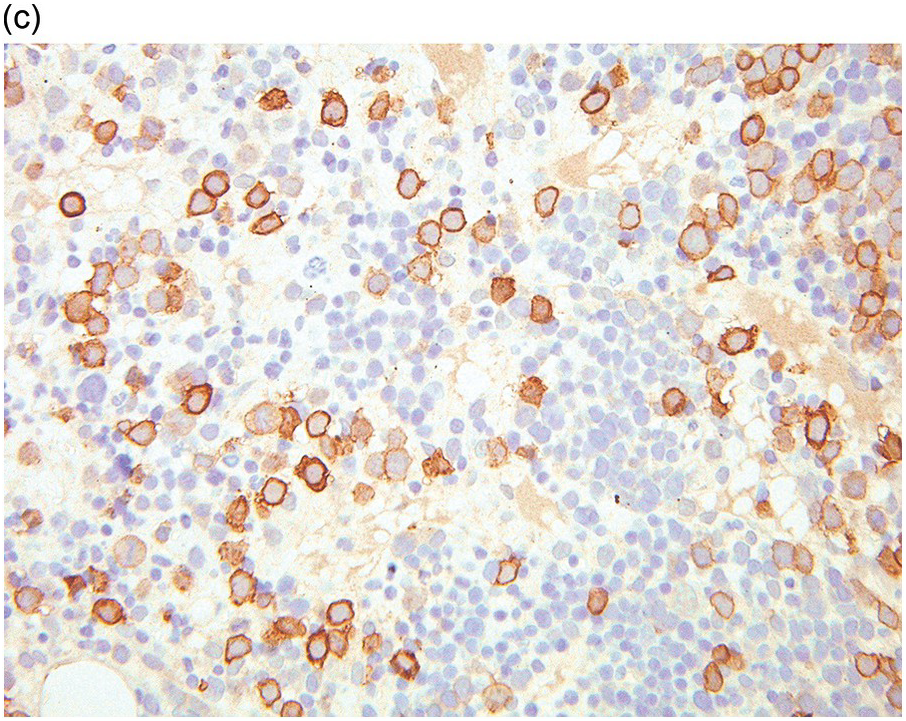
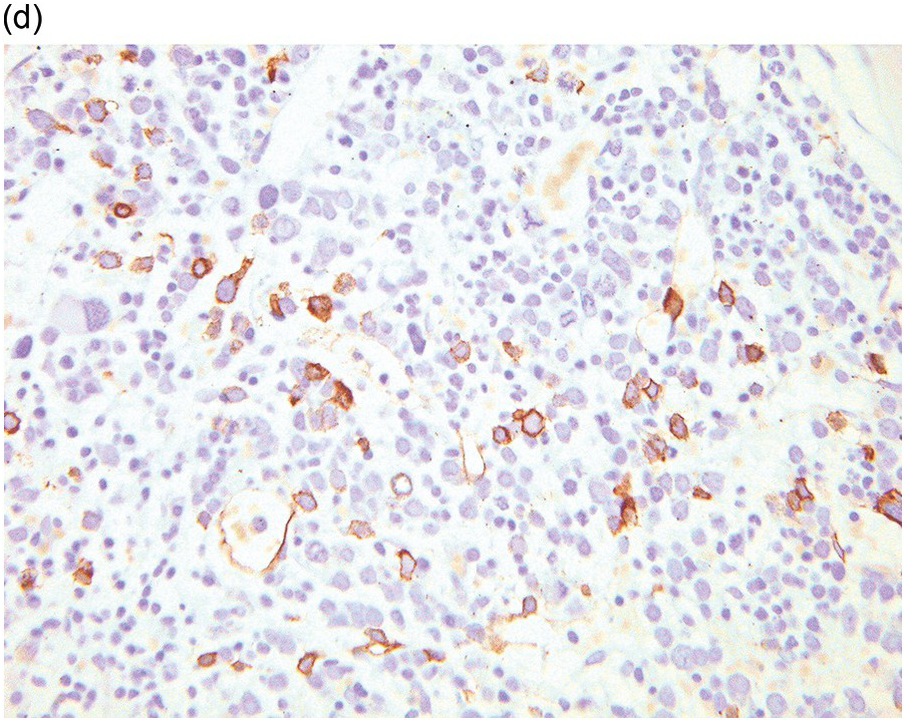
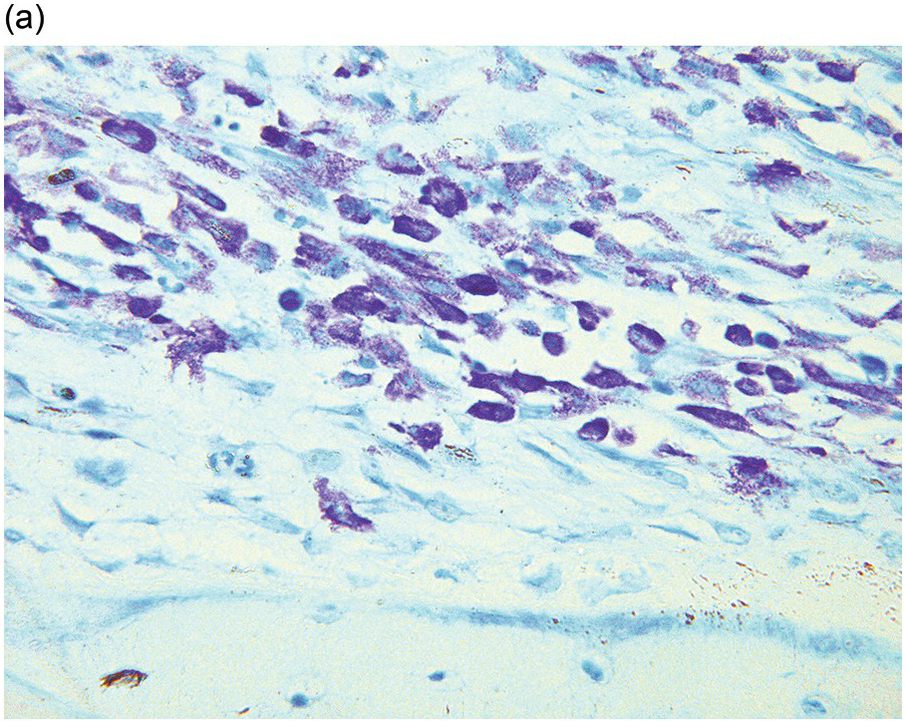
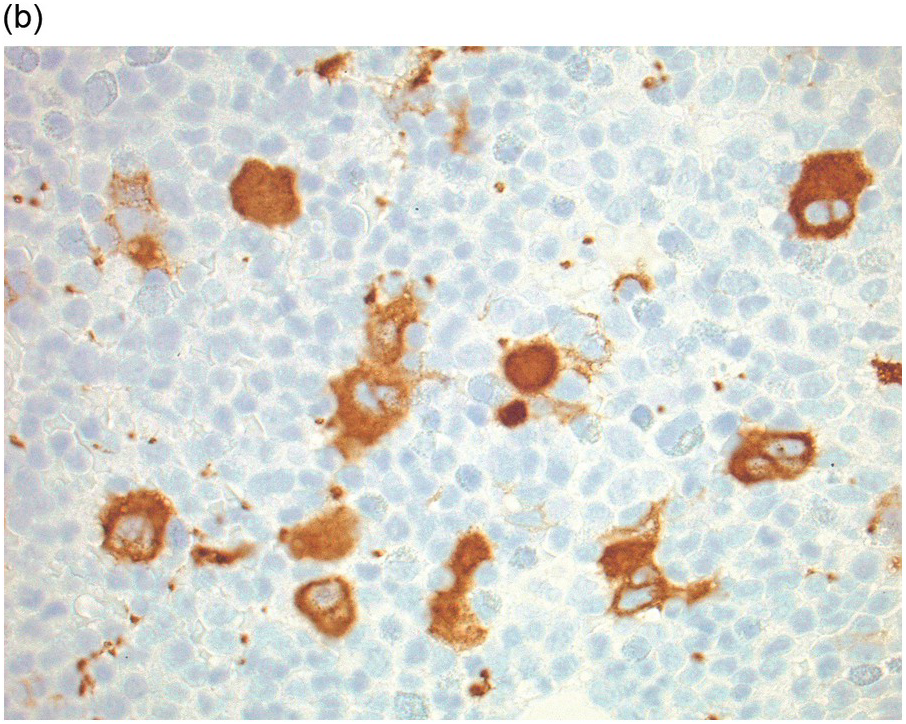
Whatever method is chosen, IHC results must be validated for both descriptive and quantitative tests according to international guidelines [20]. Methodology should take into account that decalcified BM biopsies may require a modified protocol in comparison with non-decalcified tissues. Bone marrow IHC should be validated by an experienced haematopathologist who is aware of the full range of IHC staining in haematological tissues, e.g. the difference in intensity of staining in CD117 staining for proerythroblasts versus mast cells, adequate staining of both being required for validation (Figure 1.1) [20]. External quality assurance is necessary for validation but many general schemes do not include decalcified BM biopsies and this is an issue that requires further action [20].
Similar considerations apply to the use of BM biopsies for fluorescence in situ hybridization (FISH) and polymerase chain reaction (PCR)-based molecular techniques for examination of DNA and RNA, and the technical aspects have been comprehensively reviewed [25, 26].
Embedding and Sectioning
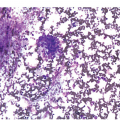
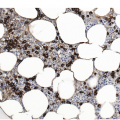
Historically, BM biopsies have been embedded in either paraffin or in plastic. Both methods have certain advantages and disadvantages and are suitable for IHC and molecular tests [20, 27]. However, excellent morphology may be obtained from carefully processed and sectioned paraffin-embedded sections and most laboratories no longer possess the expertise for plastic processing and sectioning (Figures 1.2–1.4). Modern fully automated systems that perform microwave-enhanced fixation, decalcification and paraffin impregnation in a closed system (LOGOS J, Milestone, Bergamo, Italy) are available and enable overnight protocols, improving TAT to sign-out within two days [28].
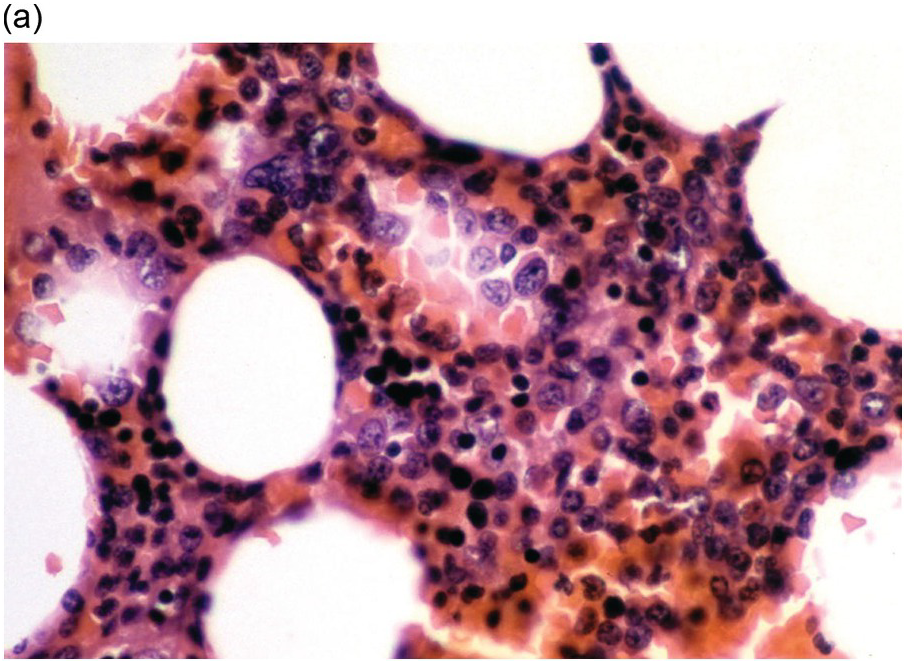
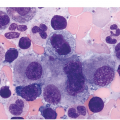
(a) An example of a poorly prepared bone marrow core biopsy. The specimen is too thick and not well stained, preventing adequate identification of different cell types and their stages of maturation.