Abstract
Knowledge and understanding of the appearance of normal bone marrow (BM) and therefore normal haematopoiesis is essential for both general pathologists and specialist haematopathologists. It is only once normal cytology and histology is understood that abnormalities can be identified and defined, leading to the accurate diagnosis of pathologies seen in the BM.
Introduction
Haematopoiesis: the production of blood cells and platelets, which occurs in the bone marrow [1].
Knowledge and understanding of the appearance of normal bone marrow (BM) and therefore normal haematopoiesis is essential for both general pathologists and specialist haematopathologists. It is only once normal cytology and histology is understood that abnormalities can be identified and defined, leading to the accurate diagnosis of pathologies seen in the BM.
The BM is located within the medulla of the bone, supported by a meshwork of cancellous bone, forming bony trabeculae. The BM is found within the intertrabecular spaces and comprises haematopoietic tissue, a relatively small number of other cells (including lymphocytes, plasma cells and mast cells), along with stromal tissues.
At first glance the BM, whether assessed in an aspirate or trephine sample, may appear busy and disorderly. This largely reflects the dynamic nature of this tissue, with a large number of different cell types present, all at varying stages of maturation. However, by considering each of these lineages individually, and in the context of one another and the supporting stroma, a clear, organised and repeating picture can be easily appreciated.
Table 2.1 summarizes the normal components of the BM.
Table 2.1 Normal components of the bone marrow.
| Pluripotent stem cells |
|---|
| Cell lineages derived from the common myeloid progenitor: |
| Megakaryocytes |
| Erythrocytes |
| Neutrophils |
| Basophils |
| Eosinophils |
| Mast cells |
| Monocyte/macrophages (and other dendritic cells) |
| Cell lineages derived from the common lymphoid progenitor: |
| Lymphocytes |
| Plasma cells |
| Bone (including osteocytes, osteoblasts, osteoclastsa) |
| Stromal tissues: |
| Fibroblasts |
| Blood vessels |
| Adipose tissue |
| Reticulin network |
a Osteoclasts are derived from myeloid progenitors and form part of the monocytic lineage.
The aim of this chapter is to provide an overview of these components, the structure of the normal BM and their relationship to one another.
Tinctorial Stains
Prior to reviewing the components of the BM, one must consider the stains used to achieve optimal assessment. Immunohistochemical stains have greatly facilitated analysis of the BM and will be considered in more detail separately (see Chapter 19). However, a range of tinctorial stains can provide additional information over and above a routine haematoxylin and eosin (H&E) and/or Giemsa-stained section of a BM biopsy (BMB) or Giemsa-stained aspirate. Perls’ Prussian blue stain for iron and reticulin staining are frequently performed routinely as additional stains.
Table 2.2 summarizes the tinctorial stains used in assessment of the bone marrow.
| Stain | Use |
|---|---|
| Haematoxylin and eosin (H&E) | A routine stain for assessment of bone marrow trephine biopsies, enabling assessment of architecture and cytomorphology. |
| Giemsa | The routine stain used for bone marrow aspirates and an alternative stain to H&E in biopsies. In a bone marrow trephine, this is particularly helpful for visualizing mast cells, erythroblasts and plasma cells. |
| Periodic acid–Schiff (PAS) | Stains polysaccharides, such as glycogen, and mucosubstances, such as glycoproteins, glycolipids and mucins. Can aid in distinguishing erythroblasts from the granulocytic lineage and highlights megakaryocytes. Largely superseded by immunohistochemistry in this context. Should always be used in immunosuppressed patients to visualize organisms. |
| Reticulin (by silver impregnation, various methods) | Demonstration of normal stromal structural fibres and the assessment of the degree of reticulin fibrosis in various disorders. |
| Perls’ Prussian blue | Assessment of the presence of haemosiderin within macrophages and the interstitium; and in erythroid precursors in aspirate samples. Staining may be reduced by decalcification. |
| Masson trichrome | Demonstration of collagen in the normal bone marrow and in various disorders, where these stains should be performed to assess the presence and grade of collagen fibrosis (see Chapter 10). |
| Martius scarlet blue (MSB) | |
| Toluidine blue | A metachromatic stain. Infrequently used. Stains mast cell granules purple, connective tissues, especially acid mucins, purple to red and amyloid blue with bright red birefringence under polarized light. |
In a BMB, the process of decalcification removes a proportion of the iron present. Therefore iron detected by Perls’ Prussian blue staining in a trephine biopsy may be considered as ‘stainable iron’, rather than a reflection of the total iron present in vivo. Perls’ staining rarely detects iron within erythroid precursors in BMB. Accurate quantification and characterization of the iron present is best performed on an aspirate, especially for low quantities of iron. A grading system for iron present in the BM (as assessed on BM aspirate samples) was proposed by Gale et al. [2], and is summarized in Table 2.3.
Table 2.3 Grading of iron in bone marrow aspirate samples, modified from Gale et al. [2].
| Grade | Description |
|---|---|
| 0 | No visible iron under oil immersion |
| 1+ | Small iron particles just visible in macrophages under oil immersion |
| 2+ | Small, sparsely distributed iron particles usually visible under low-power magnification |
| 3+ | Numerous small particles present in macrophages throughout the marrow particles |
| 4+ | Larger particles throughout the marrow with tendency to aggregate into clumps |
| 5+ | Dense, large clumps of iron throughout the marrow |
| 6+ | Very large deposits of iron, both intra- and extracellular, which obscure cellular detail in the marrow particles |
The assessment and grading of reticulin and collagen fibrosis is covered in detail in Chapter 10.
Stem Cells
A very small proportion of nucleated BM cells are haematopoietic stem cells (<0.1%) and these are virtually impossible to identify by morphological means alone.
These BM haematopoietic stem cells give rise to not only the myeloid and lymphoid progenitors, but also to osteoclasts [3].
Once the stem cells have differentiated into lineage-specific progenitors, such as erythroblasts or myeloblasts, they rapidly develop morphological features that enable their identification. The term blast cell is usually used to encompass not only the pluripotent stem cells but also those that have differentiated into lineage-specific progenitors, and taken together these account for 2–3% of nucleated marrow cells.
These haematopoietic stem cells express CD34 and CD117. However, these proteins continue to be expressed in the early blast stages once lineage-specific differentiation has occurred, so on a BMB will highlight around 2–3% of nucleated cells in total.
Bone
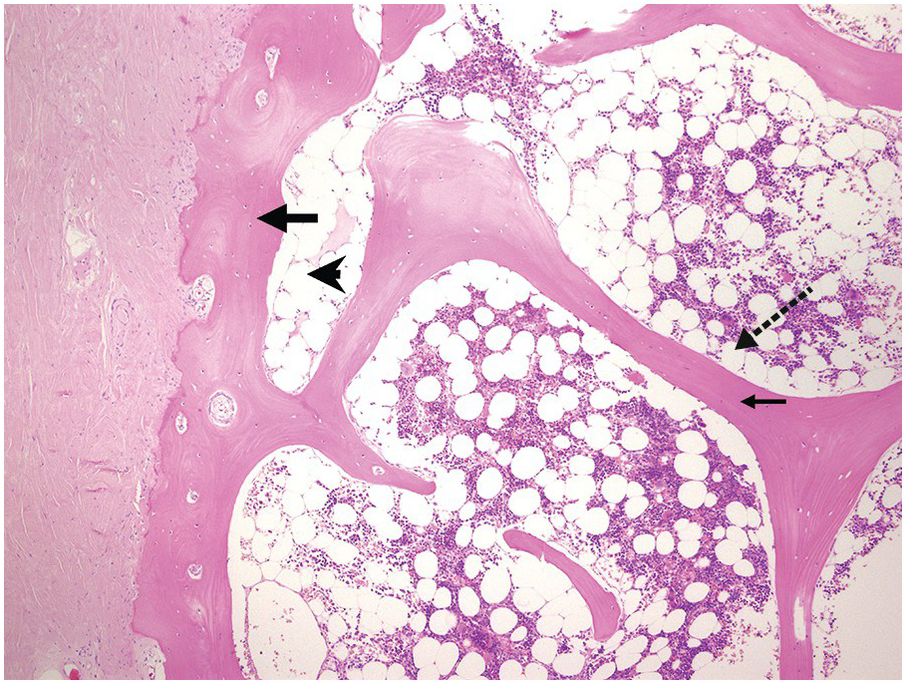
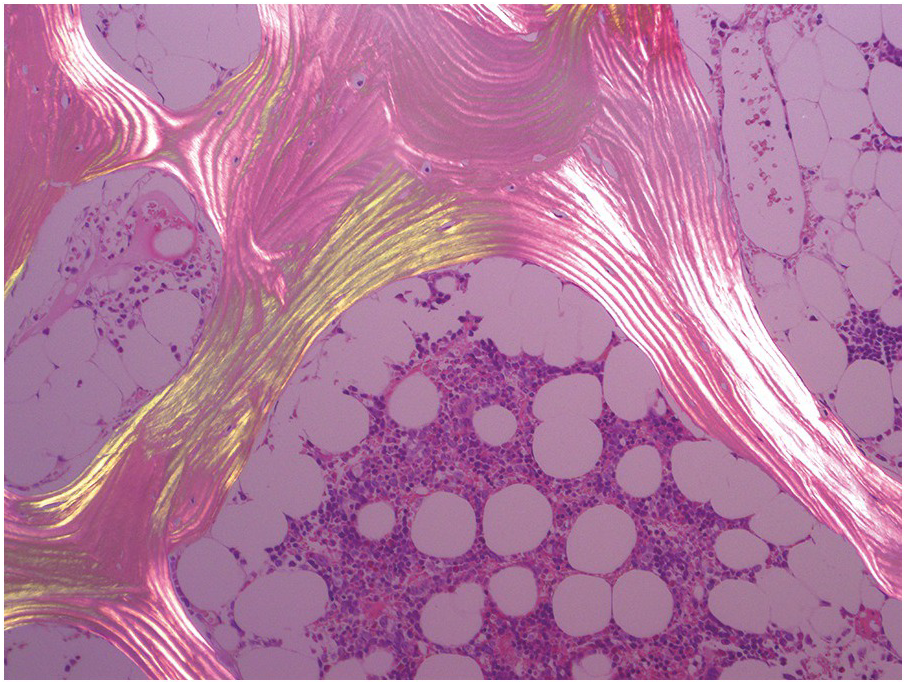
The bony trabeculae are formed of lamellar bone (Figure 2.1). The lamellar nature of the bone can often be appreciated using polarized light (Figure 2.2). The bony trabeculae are covered by a thin layer of endosteum.
Figure 2.2 Lamellar bone under polarized light.
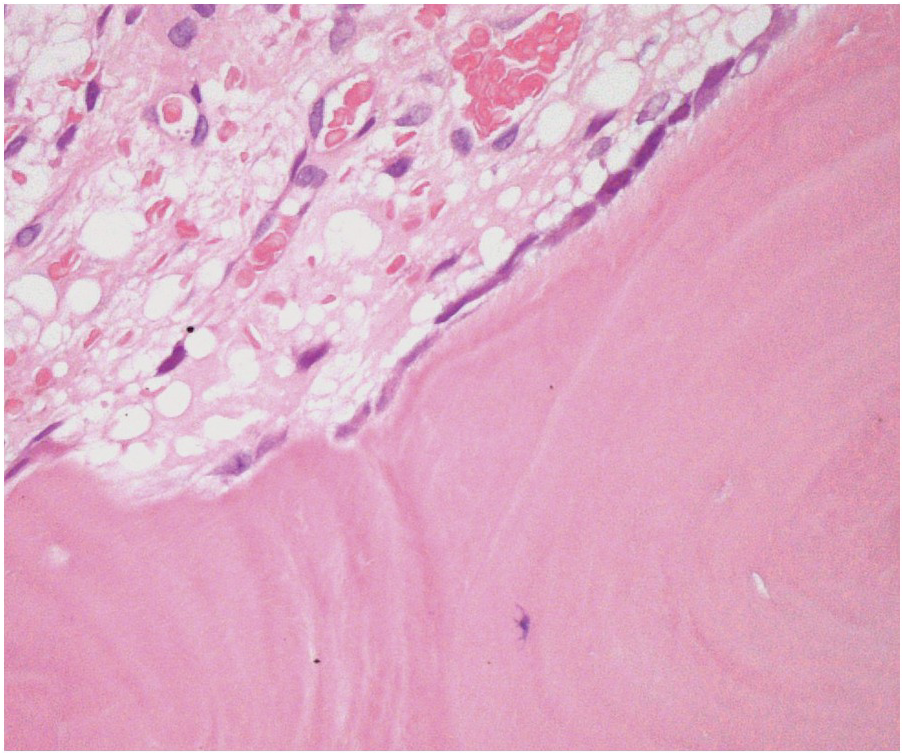
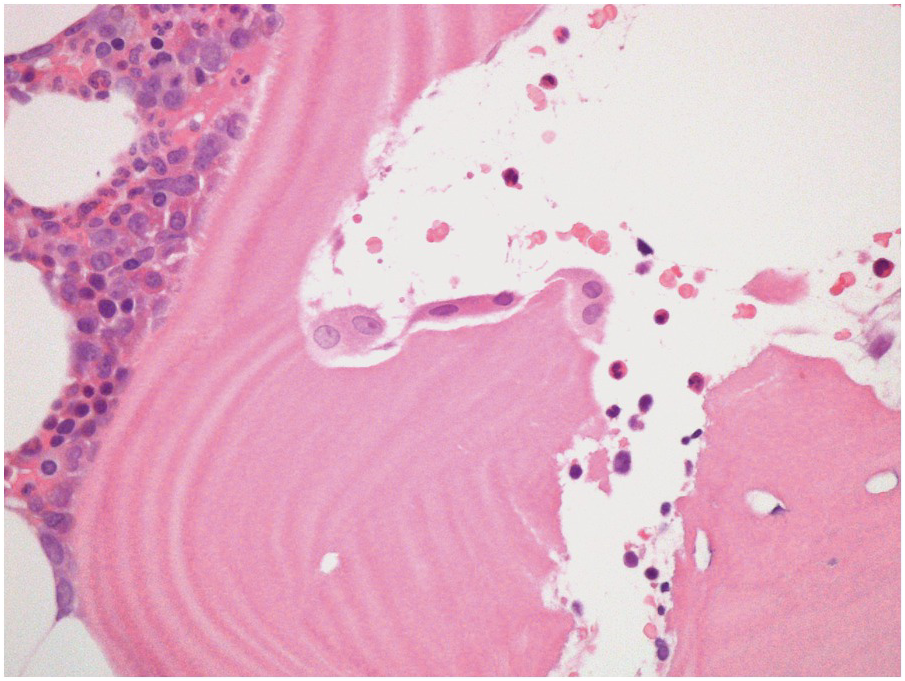
Three main cell types can be identified in viable bone: osteocytes, osteoblasts (Figure 2.3) and osteoclasts (Figure 2.4). Osteocytes and osteoblasts are derived from the same lineage, with osteoblasts becoming osteocytes once they are surrounded by newly formed bone. Osteoblasts line the endosteal surface of the bone. Osteoclasts, which are large, multinucleate cells, form part of the monocytic lineage.
Normal osteoblasts express CD56 and act as a helpful positive control in the normal BM. They may be confused with other CD56+ cells such as neoplastic plasma cells if this is not borne in mind.
Viable bone can be identified by the presence of nucleated osteocytes.
The appearance of the bone changes significantly with age. In young children there is very active bone remodelling with visible osteoblast activity. In infants the bone may not be fully ossified. In adults (with the exception of some young adults), physiological bone remodelling has ceased, and therefore osteoblasts and osteoclasts should be inconspicious, although occasional osteoclasts in normal adults bone may be identified in Howslip’s lacunae.
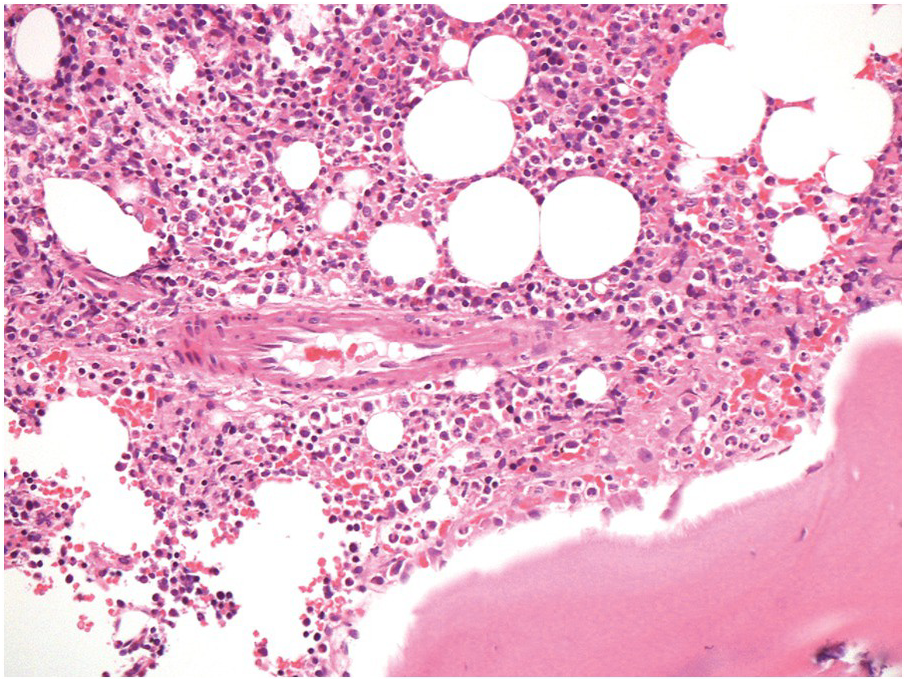
Sometimes the BMB may include compact cortical bone at one end, merging with periosteal soft tissue (Figure 2.1). The periosteal soft tissue may harbour amyloid deposits and must be carefully examined (see Chapter 3).
Stromal Tissues
The BM stroma is composed of a mixture of adipose tissue, fibroblasts, blood vessels, nerve fibres, reticulin fibres and macrophages. The stroma and stromal cells are recognized to play a crucial role in the regulation and support of normal haemopoiesis [4, 5] and are considered in Chapter 3.
Adipose Tissue
Within the intertrabecular spaces, the majority of non-haematopoietic tissue is adipose tissue.
Bone marrow cellularity is assessed as the relative percentage of haematopoietic tissue compared to adipose tissue.
When considering cellularity and the distribution of cellular haematopoietic tissue, two additional points must be considered.
1. The subcortical bone marrow is usually hypocellular. Therefore if only subcortical BM is represented in a biopsy, for example due to tangential sampling or an inadequate biopsy, the cellularity of the BM sample may not be representative (Figure 2.1).
2. Bony trabeculae are usually surrounded by a single layer of adipocytes, separating them from the haematopoietic tissue (Figure 2.1). Loss of this layer can be useful in identifying abnormalities in the marrow.
In aspirate samples, adipocytes are most often seen in particles. However, occasional single adipocytes may be identified, as large cells, with abundant clear-to-pale cytoplasm and an oval nucleus [6].
Macrophages
Macrophages are derived from the common myeloid progenitor and differentiate from monocytes within tissues.
Macrophages are distributed throughout the bone marrow interstitium, where they play an important role in phagocytosis and frequently contain apoptotic debris, which may be mistaken for micro-organisms.
Macrophages overall (assessed on aspirate samples) account for <2% of nucleated marrow cells [7].
Blood Vessels
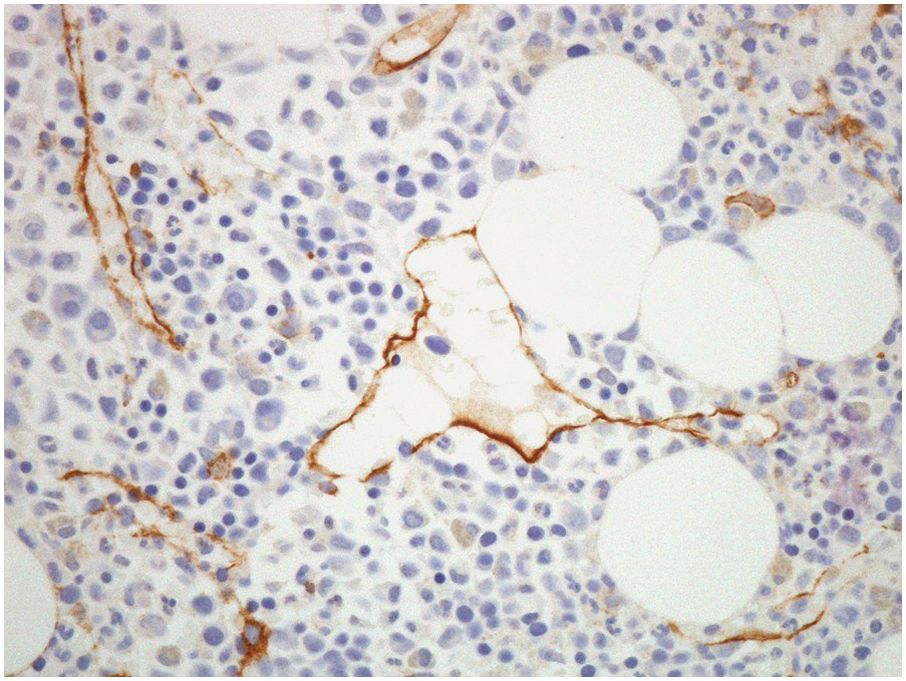
The arterial supply of the BM is derived from arteries that traverse the cortical bone (both from the main nutrient artery of the bone, as well as from surrounding soft tissues). Once within the BM, these arteries form a capillary network, which drains into thin-walled sinusoids within the intertrabecular spaces [8]. Bone marrow sinusoids are lined by endothelial cells, which sit on a discontinuous basement membrane. In normal BM, these sinusoids are usually collapsed and difficult to visualize. CD34 IHC stains endothelial cells lining the sinusoids and may be utilized to highlight their presence (Figure 2.5) as well as forming a useful internal control for CD34 staining.
Figure 2.5 CD34 immunostain highlighting sinusoids.
The sinusoids provide the route by which the mature haematopoietic cells enter the circulation, draining into a central sinus and, eventually, into venules and then the nutrient vein.
Small arterioles and venules through which the blood enters and leaves the BM may also be identified; these may only be appreciated in occasional intertrabecular spaces in a core biopsy (Figure 2.6). In amyloidosis, the small arterioles are frequently involved and should be carefully examined in relevant cases (see Chapter 3).