Abstract
The myelodysplastic/myeloproliferative neoplasms (MDS/MPN) are clonal myeloid neoplasms characterized at the time of their initial presentation by the simultaneous presence of myelodysplastic and myeloproliferative features, which prevent them from being classified as either myelodysplastic syndrome (MDS) or myeloproliferative neoplasm (MPN). The incidence of MDS/MPN is estimated at 0.1 to 3/100,000 individuals. They are characterized by hypercellular bone marrow (BM) morphology due to proliferation in one or more of the myeloid lineages. Cytopaenias and dysplastic changes of any cell line may be seen in conjunction with elevated white blood cell (WBC) counts, thrombocytosis and organomegaly, features more commonly associated with MPN. Hepatosplenomegaly is frequently seen. The most common entities within the MDS/MPN group include chronic myelomonocytic leukaemia (CMML), atypical chronic myeloid leukaemia BCR-ABL1 negative (aCML) and juvenile myelomonocytic leukaemia (JMML), which is seen exclusively in paediatric patients. A less well-defined group of MDS/MPN-like diseases includes MDS/MPN unclassifiable (MDS/MPN-U) and a recently recognized entity of MDS/MPN with ring sideroblasts and thrombocytosis (MDS/MPN-RS-T), previously known as refractory anaemia with ring sideroblasts and thrombocytosis (RARS-T). It was considered a provisional entity within the group of MDS/MPN-U in the 2008 edition of the WHO, but has now been promoted to a true entry in the updated 2016 WHO edition. Since the publication of the last WHO Classification in 2008, multiple studies have examined the molecular pathogenetic features of the MDS/MPN entities (see Table 11.1). Many of these results have been incorporated into the updated 2016 WHO classification.
Introduction
The myelodysplastic/myeloproliferative neoplasms (MDS/MPN) are clonal myeloid neoplasms characterized at the time of their initial presentation by the simultaneous presence of myelodysplastic and myeloproliferative features, which prevent them from being classified as either myelodysplastic syndrome (MDS) or myeloproliferative neoplasm (MPN). The incidence of MDS/MPN is estimated at 0.1 to 3/100,000 individuals. They are characterized by hypercellular bone marrow (BM) morphology due to proliferation in one or more of the myeloid lineages. Cytopaenias and dysplastic changes of any cell line may be seen in conjunction with elevated white blood cell (WBC) counts, thrombocytosis and organomegaly, features more commonly associated with MPN. Hepatosplenomegaly is frequently seen. The most common entities within the MDS/MPN group include chronic myelomonocytic leukaemia (CMML), atypical chronic myeloid leukaemia BCR-ABL1 negative (aCML) and juvenile myelomonocytic leukaemia (JMML), which is seen exclusively in paediatric patients. A less well-defined group of MDS/MPN-like diseases includes MDS/MPN unclassifiable (MDS/MPN-U) and a recently recognized entity of MDS/MPN with ring sideroblasts and thrombocytosis (MDS/MPN-RS-T), previously known as refractory anaemia with ring sideroblasts and thrombocytosis (RARS-T). It was considered a provisional entity within the group of MDS/MPN-U in the 2008 edition of the WHO, but has now been promoted to a true entry in the updated 2016 WHO edition. Since the publication of the last WHO Classification in 2008, multiple studies have examined the molecular pathogenetic features of the MDS/MPN entities (see Table 11.1). Many of these results have been incorporated into the updated 2016 WHO classification.
Table 11.1 Molecular characteristics of the main MDS/MPN subtypes.
| Subtype | JAK2 | SETBP1 | SF3B1 | SRSF2 | TET2 | ASXL1 |
|---|---|---|---|---|---|---|
| CMML | 1–7% | 4–15% | 6% | 40–50% | 50–60% | 35–44% |
| aCML | 0–8% | 25% | n/a | n/a | 30% | 20–30% |
| MDS/MPN-RS-T | 50–60% | 10% | 80–90% | 1% | 25% | 10–20% |
| JMML | <1% | 8%a,b | <1% | <1% | <1% | 4%a |
Abbreviations: CMML, chronic myelomonocytic leukaemia; aCML, atypical chronic myeloid leukaemia; MDS/MPN-RS-T, myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis.
a SETBP1 and ASXL1 mutations in patients with JMML were often secondary and subclonal. These are likely involved in disease progression and are not driver mutations for the initiation of leukaemia [90–92].
b Subclonal SETBP1 mutations were identified in up to 30% of JMML patients using sensitive droplet digital polymerase chain reaction [93].
Chronic Myelomonocytic Leukaemia
Epidemiology
Chronic myelomonocytic leukaemia (CMML) occurs in older adults (median age at diagnosis, 70 years). There is a moderate male predominance. The precise incidence is not known. Estimates from cancer databases indicate approximately 1,000 cases diagnosed annually in the United States, with a crude annual incidence rate of 0.3 per 100,000 [1]. However, these figures likely underestimate the true incidence of CMML, since it has been historically grouped with myelodysplastic syndrome and also due to its non-specific clinical presentation [2].
Clinical Features
According to the revised WHO classification criteria, the diagnosis of CMML is made when there is a persistent, unexplained absolute monocytosis in the blood ≥1 × 109/L with monocytes accounting for ≥10% of the WBC (Table 11.2) [3]. Many patients may be asymptomatic and have abnormalities found on routine blood counts. Other patients present with complications resulting from cytopaenias, skin lesions or symptoms related to splenomegaly. Chronic myelomonocytic leukaemia is a heterogeneous disorder with a proportion of the cases that resembles an MPN (proliferative variant with high CBC values ≥13 × 109/L) and another subset that is more reminiscent of MDS (dysplastic CMML with low to normal WBC <13 × 109/L, and more prominent dysplasia). In patients with the proliferative form of CMML the WBC count is increased due to monocytosis and neutrophilia. In most cases the lymphocyte count as well as lactate dehydrogenase (LDH) and beta-2 microglobulin are generally higher than in patients with the dysplastic form of the disease. At times severe leukocytosis may be observed, generally associated with myeloid left-shift, reminiscent of chronic myeloid leukaemia (CML). Anaemia and thrombocytopaenia are common but thrombocytosis may also occasionally be observed. Splenomegaly is present in up to 50% of patients with CMML and is often accompanied by hepatomegaly, lymphadenopathy or nodular cutaneous leukaemic infiltrates [4].
| • Persistent monocytosis ≥1 × 109/L and ≥10% of the leukocytes | |
| • Not meeting WHO criteria for BCR/ABL positive CML, PMF, PV, or ET | |
| • No PDGFRA, PDGFRB, FGFR1 or PCM1-JAK2 gene rearrangements; <20% blasts in blood or bone marrow | |
| • Dysplasia in one or more of the lineages. If myelodysplasia is absent or minimal the diagnosis of CMML can still be made if the other requirements are met and: | |
| – an acquired clonal cytogenetic or molecular abnormality is present, or | |
| – the monocytosis has been present for at least 3 months and all causes of reactive monocytosis have been excluded. | |
| CMML-0: blastsa <2% in blood, <5% in bone marrow | |
| CMML-1: blastsa 2–4% in blood, 5–9% in bone marrow | |
| CMML-2: blastsa 5–19% in blood, 10–19% or Auer rods in bone marrow, and/or when any Auer rods are present | |
a Blasts = myeloblasts + promonocytes.
In a subset of patients CMML may be associated with immune-mediated processes and/or autoimmune disorders, such as systemic vasculitis, immune thrombocytopaenia, psoriasis and gout [5, 6]. Patients with ‘proliferative’ type CMML tend to have more constitutional symptoms (fevers, unexplained weight loss and night sweats) and organomegaly, as well as pleural and pericardial effusions and ascites [7].
Oligomonocytic CMML
The term ‘oligomonocytic CMML’ refers to cases of myeloid neoplasms showing increased peripheral blood monocytes (≥10% peripheral blood monocytes with absolute monocyte count of 0.5 to 1 × 109/L) that is below the level of absolute monocytosis required by the WHO classification [8, 8b]. Currently these cases do not fit any well-defined WHO category and are usually classified as MDS or as MDS/MPN, U. Based on results of one study, oligomonocytic CMML patients have low to normal WBC values and typically present with anaemia, macrocytosis, thrombocytopaenia and/or neutropaenia [8]. Most of the cases of oligomonocytic CMML show a mutation profile similar to that seen in cases of overt CMML. Approximately one third of the patients progress to overt CMML. Clearly this subset represents an early phase of the disease. A significant subset (25%) of patients in the study went on to develop AML.
Morphology
Peripheral Blood
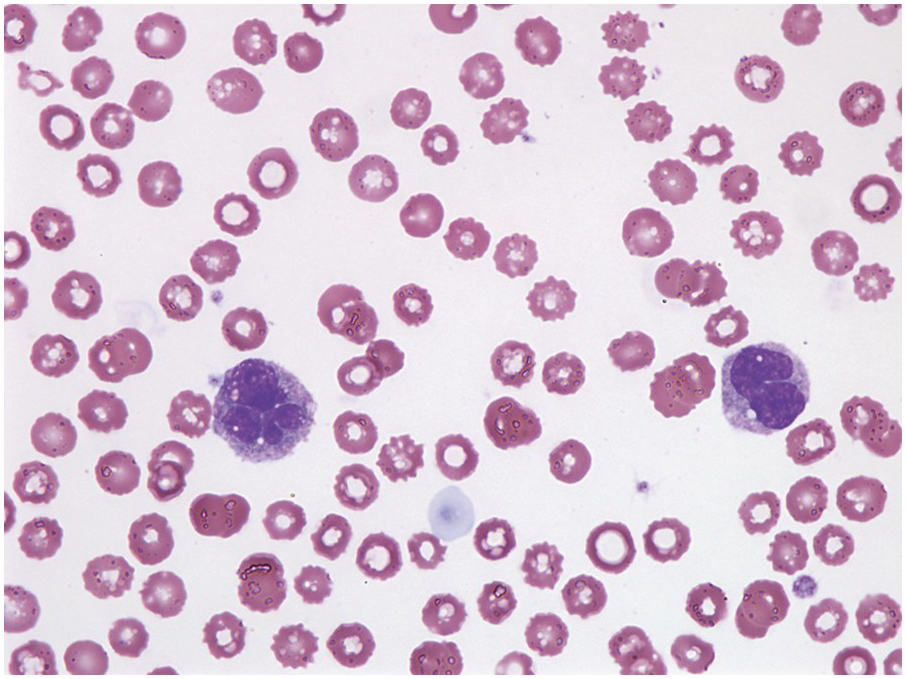
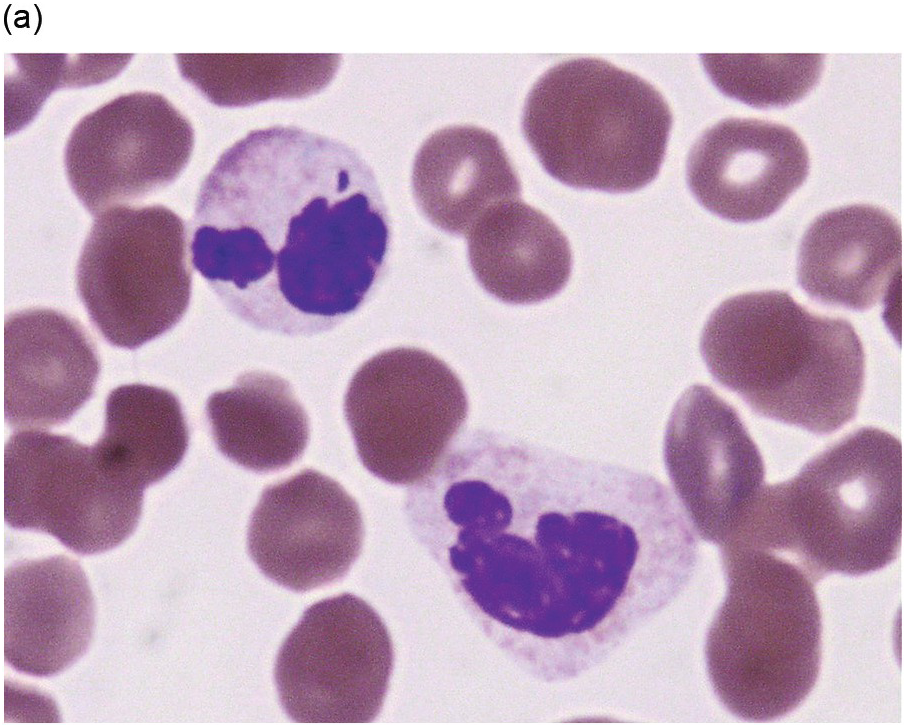
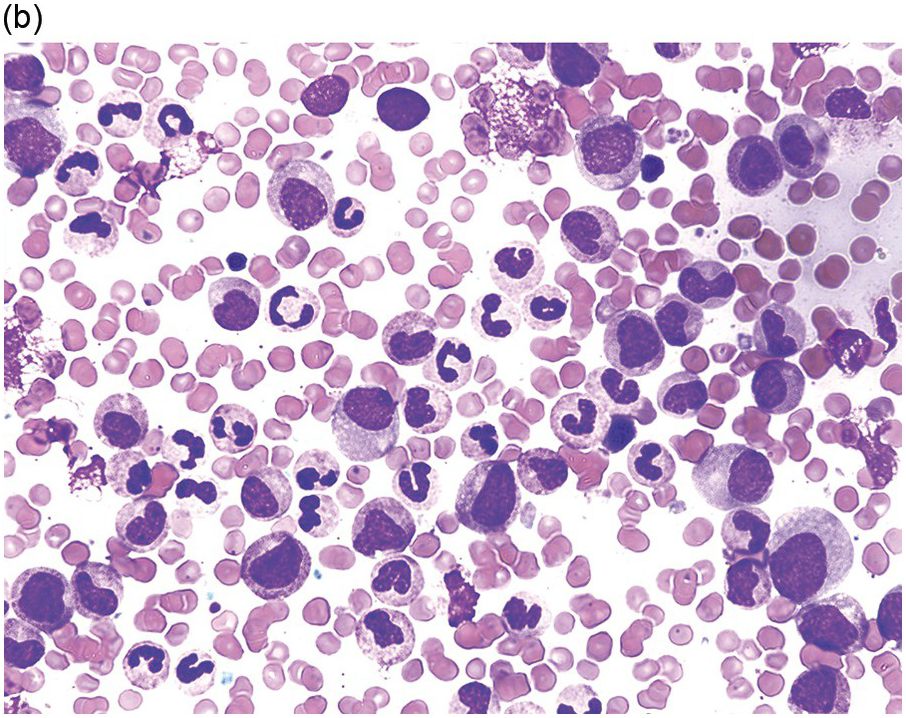
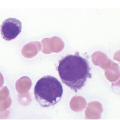
Peripheral blood monocytes usually range from 1 to 5 × 109/L and represent ≥10% of the leukocytes. Some cases have mature-appearing monocytes, while others are characterized by abnormal monocytes with unusual nuclear lobulation, delicate nuclear chromatin and abnormal cytoplasmic granules [9] (Figure 11.1). Blasts and promonocytes may be seen but represent <20% of the differential cellularity. There is often evidence of dysgranulopoiesis, especially in patients with lower WBC counts [9].
Figure 11.1 Chronic myelomonocytic leukaemia. Peripheral blood shows evidence of immature monocytosis.
Bone Marrow
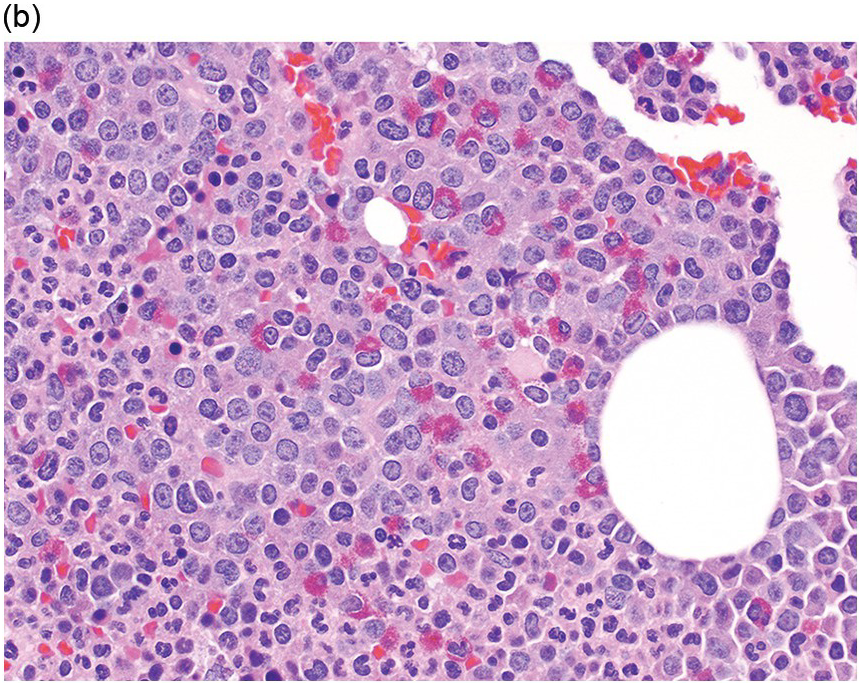
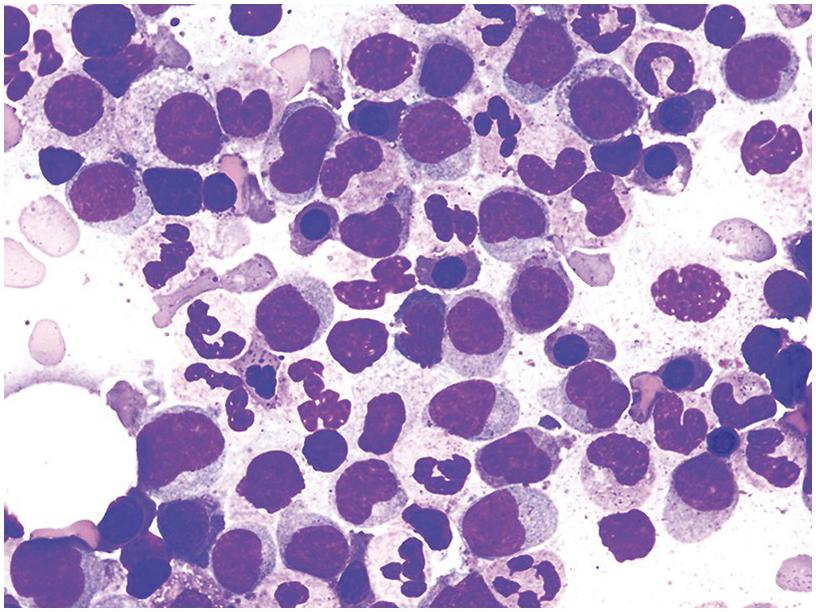
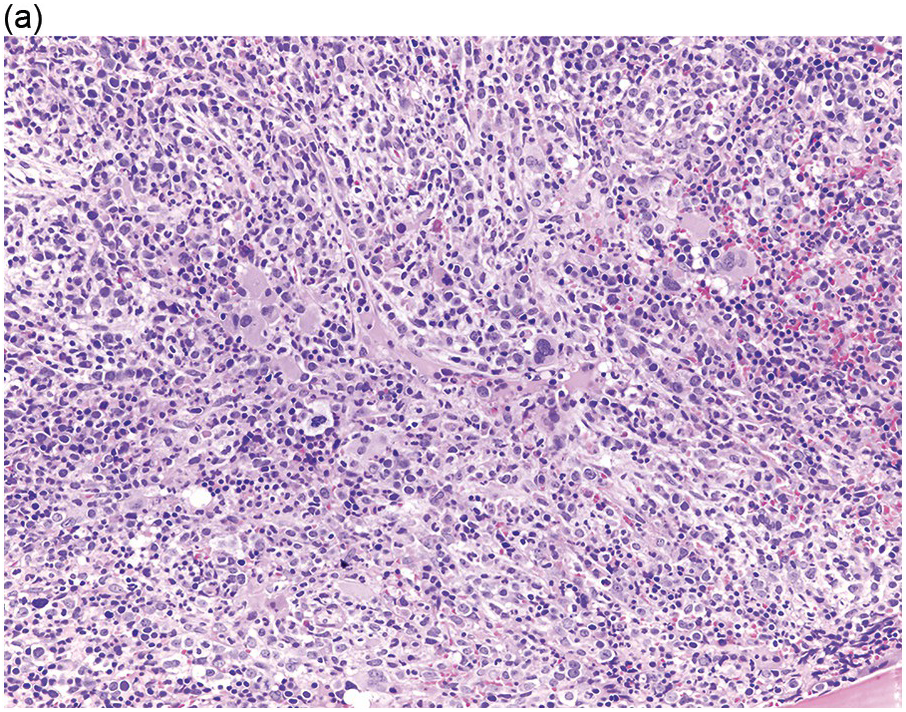
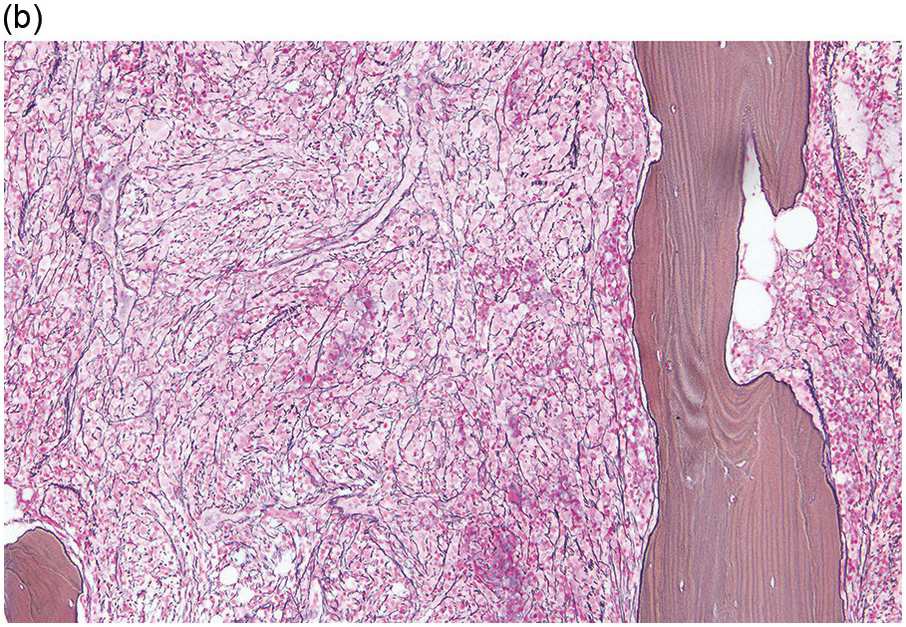
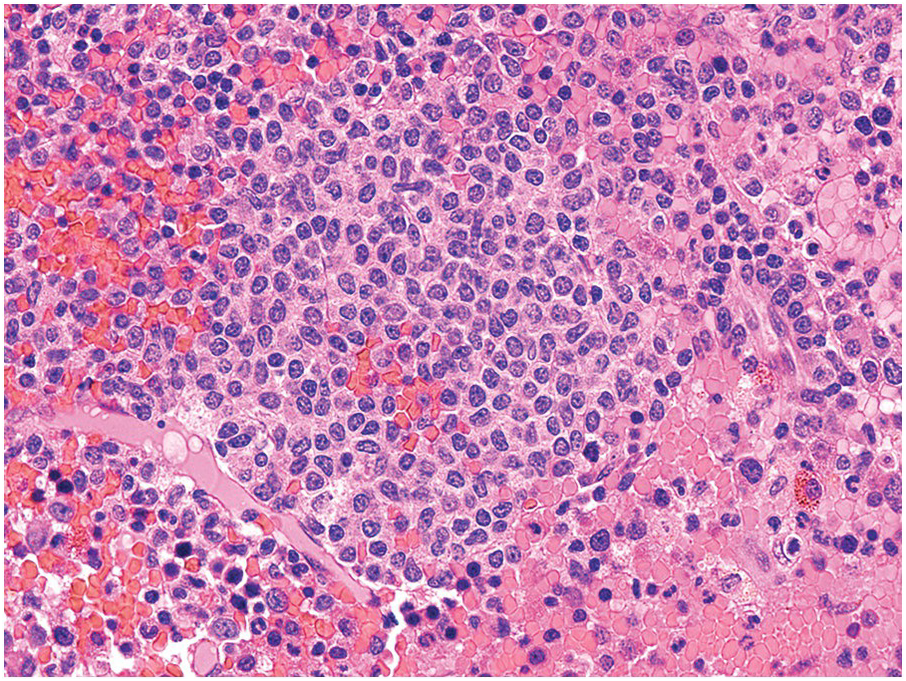
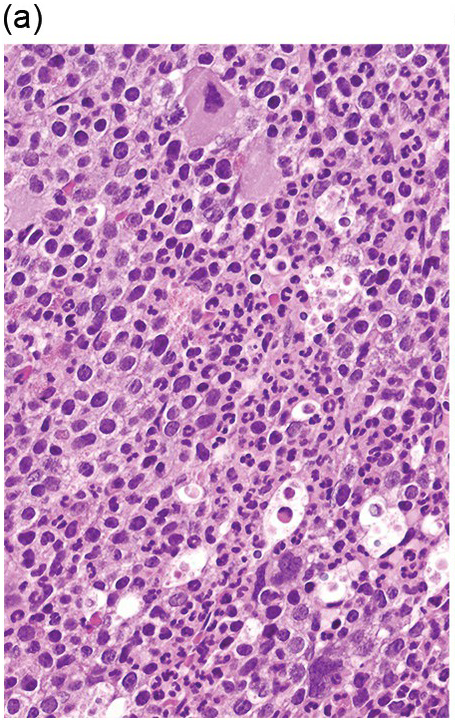
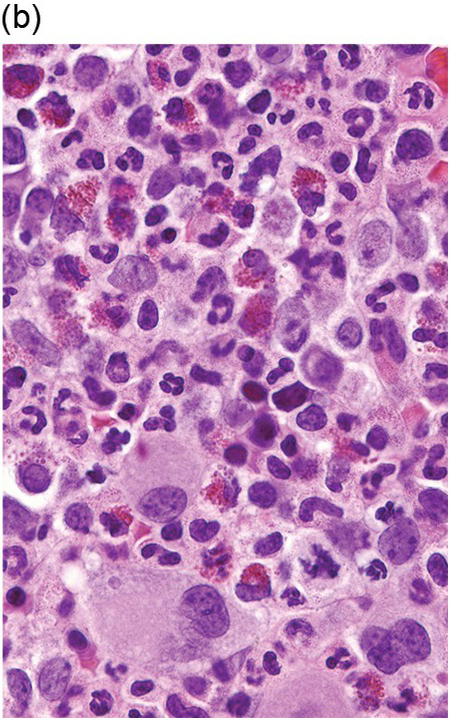
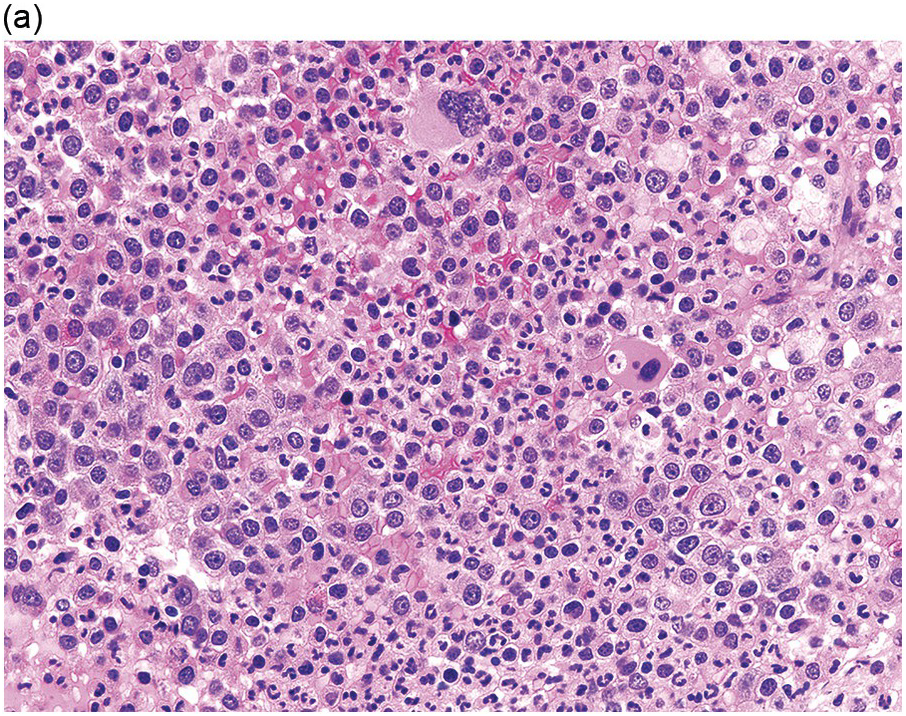
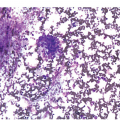
The BM is hypercellular in over 75% of cases, usually due to a granulocytic and monocytic proliferation (Figure 11.2) [10]. Most cases have dysgranulopoiesis and a large subset demonstrates dyserythropoeisis (Figure 11.3) [10]. Dysplastic megakaryocytes are present in up to 75% of the cases (Figure 11.4) [10]. Megakaryocytes are typically small and hypolobated (micromegakaryocytes) and do not form clusters [10]. Up to 30% of the cases may have significant (MF2–3) fibrosis (Figure 11.5). Nodules of plasmacytoid dendritic cells have been reported in up to 20% of the cases (Figure 11.6) [11]. The plasmacytoid dendritic cells are closely related to monocytes, i.e. are mature forms different from the blastic plasmacytoid dendritic cells found in blastic plasmacytoid dendritic neoplasm and therefore should not be considered as blast equivalents [11]. In addition, an increased number of lymphocytes and lymphoid aggregates may be seen.
(a) BMB demonstrates a hypercellular BM with myeloid predominance, immature monocytosis and dysmegakaryopoiesis.
(b) CMML-2 with increased number of immature myeloid elements, including blasts.
Figure 11.2 Chronic myelomonocytic leukaemia.
Figure 11.3 Chronic myelomonocytic leukaemia. Bone marrow aspirate is hypercellular with immature monocytosis and trilineage dysplasia.
The updated WHO Classification divides CMML into three categories: CMML-0, CMML-1 and CMML-2, based on the percentage of myeloid blasts (including promonocytes) in peripheral blood and BM (Table 11.3). The finding of ≥20% blasts (including promonocytes) in the blood or the BM indicates acute myeloid leukaemia (AML) rather than CMML.
Spleen
Splenomegaly is due to leukaemic infiltration of the red pulp. Morphologic examination of splenectomy specimens demonstrated the presence of extramedullary haematopoiesis in splenic red pulp and expansion of splenic cords by a myelomonocytic infiltrate [12–14].
Skin
Cutaneous involvement is frequently seen in CMML. Skin lesions, typically multiple, include papules, pigmented nodules, indurated plaques or tumours. They often occur late in the course of the disease, as a possible early manifestation of transformation to acute leukaemia, and indicate poor prognosis [15, 16]. In one study of 108 patients with CMML, 11 patients (10.2%) had skin involvement. Four of these patients developed AML within four months. The overall survival from diagnosis of the skin involvement to the last follow-up was significantly shorter than in patients with leukaemia cutis [17]. Another study of 42 CMML patients identified four clinicopathologic profiles of skin involvement [18]. The most common group (43%) comprised myelomonocytic cell tumours with a proliferation of granulocytic or monocytic cells. The second group comprised mature plasmacytoid dendritic cell tumours (38%), denoted by a proliferation of mature plasmacytoid dendritic cells. The third group comprised blastic plasmacytoid dendritic cell tumours (10%). The fourth group consisted of blastic indeterminate dendritic cell tumours (10%), distinguished by a proliferation of large blast cells that not only exhibited monocytic markers but also the dendritic markers CD1a and S100. These four groups showed distinctive outcomes [18].
Immunophenotype/Cytochemistry
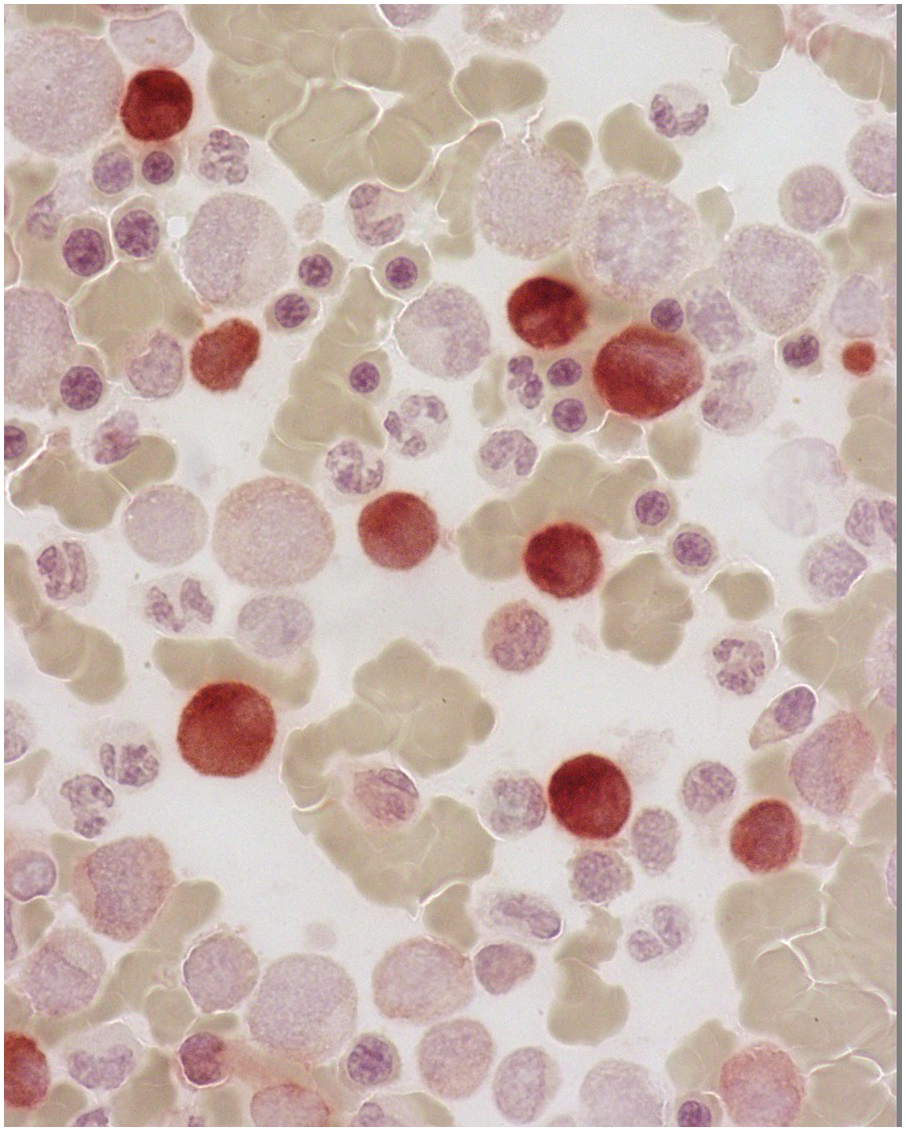
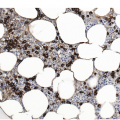
On the BM aspirate, the monocytic proliferation is confirmed by cytochemical staining of the BM aspirate smears with non-specific esterase such as α-naphthyl butyrate esterase (Figure 11.7). With flow cytometry, the monocytes express CD13, CD33 and CD14 [19]. In CMML, the peripheral blood and BM monocytes usually have aberrant phenotypes with two or more of these abnormalities. They may have aberrant expression of CD56, CD2 and CD23 or decreased expression of HLA-DR, CD13, CD15, CD64 or CD36 [19]. A characteristic repartition of peripheral blood monocyte subsets due to an increase of ‘classical’ CD14(+)/CD16(–) cells and a consequent relative reduction of CD14(+)/CD16(+) ‘intermediate’ and CD14(low)/CD16(+) ‘non-classical’ monocytic cells has been proposed as a possible diagnostic signature of CMML, that may help distinction from monocytosis occasionally observed in other haematological malignancies or reactive forms of monocytosis [20]. Granulocytes may also show abnormal scatter properties and aberrant phenotypic features (such as loss of CD10 or overexpression of CD56). Immunohistochemistry can be performed on the BM biopsy (BMB) to identify and quantify the monocytic cells. CD14 is the most sensitive and specific marker (Figure 11.8), followed by CD68 (PGM-1) and CD163 [19]. An increased number of CD34 positive blasts is associated with disease progression [21]. The most useful marker for plasmacytoid dendritic cells is CD123. Plasmacytoid dendritic cells may also express CD14, CD43, CD68, CD45RA, CD33 (weak staining), CD4 and granzyme B.
Figure 11.8 Chronic myelomonocytic leukaemia. Increased monocytes in the bone marrow biopsy are highlighted by CD14 immunostain.
Differential Diagnosis and Work-Up
The most clinically relevant differential diagnoses of CMML include MDS, AML and reactive monocytosis [21b, 21c]. To this end, numerous recent studies have contributed to advancing the understanding of molecular pathogenesis of CMML (see section on genetic/molecular mechanisms below). Thus in problematic cases a characteristic molecular profile may help support the diagnosis of CMML, as well as provide prognostic information. Cases of reactive monocytosis typically do not have cytogenetic or molecular abnormalities. However, a subset of older patients may display clonal haematopoiesis with myeloid-type mutations in the absence of defining features of CMML or other myeloid neoplasm (so-called ‘clonal haematopoiesis of indeterminate potential’ or CHIP) [8b]. Thus the presence of a myeloid-type mutation alone may be an incidental finding and does not necessarily exclude a reactive process. Promonocytes and monoblasts are not conspicuous in normal or reactive BM samples. By flow cytometry, abnormal antigen expression may be present in reactive monocytes, including co-expression of CD2 and CD56 or underexpression of HLA-DR or CD13 [22, 23]. However, aberrant expression is usually limited to one marker. The presence of two or more abnormally expressed antigens is significantly more frequent in myeloid neoplasms compared to reactive monocytosis [23].
NPM1 mutation has been described in a small percentage (3 to 5%) of cases of CMML [24–27]. In these cases careful review of the blast count is indicated to exclude the alternative diagnosis of AML associated with NPN1 mutation [28]. Close follow-up of these patients is recommended, since presence of NPM1 mutation or 11q23 rearrangement may herald rapid progression to acute leukaemia [29, 30]. The distinction between CMML and AML with monocytic differentiation can be challenging. Monoblasts are large cells with abundant, moderately to intensely basophilic cytoplasm, which may demonstrate pseudopod formation, scattered fine azurophilic granules and vacuoles. Nuclei are round with delicate lacy chromatin and one or more prominent nucleoli [30b]. Promonocytes are large cells with intermediate features between monoblasts and immature monocytes. They have less basophilic and sometimes more obviously granulated cytoplasm with occasional large azurophilic granules and vacuoles. The nucleus is irregular with a delicately convoluted configuration. A nucleolus may be present [30b]. However, the distinction between promonocytes and other abnormal marrow elements, such as dysplastic myeloid precursors or immature monocytes, is notoriously subjective and may be controversial. There appears to be a significant association between AML with monocytic differentiation and NPM1, DNMT3A, TET2 and KRAS mutations [31, 32].
Chronic myelomonocytic leukaemia may show fibrosis in a variable proportion of cases [33–35]. While the results of these retrospective studies are not yet conclusive, the presence of marrow fibrosis in CMML may be of prognostic importance and can cause diagnostic difficulties. The incidence and significance of this finding is worthy of more study.
Primary myelofibrosis (PMF) may show monocytosis at the time of diagnosis or may develop monocytosis during the course of the disease [36, 37]. These cases should not be called CMML but rather considered within the spectrum of PMF, particularly if the BM morphology is diagnostic of the latter [36, 37]. However, rare ‘grey zone’ cases with truly overlapping PMF and CMML features have been reported [33].
Genetics/Molecular Mechanisms
Clonal cytogenetic abnormalities are found in 20 to 40% of patients with CMML, but none is specific. The most frequent recurring abnormalities include +8, –7/del (7q) and structural abnormalities of 12p. As many as 40% of patients exhibit point mutations of RAS genes at diagnosis, or during the course of disease. An acquired RAS mutation has been documented in cases of CMML evolving from the myelodysplastic to the myeloproliferative variant [38]. The presence of mutations in both TET2 and SRSF2 has been found to be strongly associated with the diagnosis of CMML [39–41]. In fact, either TET2, SRSF2 or ASXL1 gene mutations are present in the vast majority of patients with CMML [41]. The presence of TET2, RAS and ASXL1 mutations seem to be capable of imparting a more aggressive course independently of the cytogenetic abnormalities [27, 38, 41].
Postulated Cell of Origin
A haematopoietic stem cell.
Prognosis and Predictive Factors
The general prognosis of CMML patients is poor with an expected median overall survival of approximately 30 months. Progression to AML occurs in 15 to 30% of the cases. The most important prognostic factor appears to be the blast count in peripheral blood and BM [3]. Thus the value of subdividing CMML into three subgroups based on the blast count (CMML-0, CMML-1 and CMML-2) is based on the different survival of these patients (see Table 11.4) [21, 42]. The more recent sequencing studies noted that ASXL1 mutations, age, haemoglobin, WBC and platelet counts defined prognostically distinct patient subsets with varied overall survival [27]. A ‘CMML-specific prognostic scoring system’ (CPSS-mol) has been proposed based on genetic mutations in RUNX1, NRAS, SETBP1 and ASXL1 (all independently associated with inferior outcome), cytogenetics (high risk defined as trisomy 8, abnormalities of chromosome 7 and complex karyotype) and red blood cell transfusion dependence [43, 44]. CPSS-mol divides patients into four risk groups with markedly different median overall survival (ranging from >144 to 18 months) and cumulative incidence of leukaemic evolution (ranging from 0 to 48% at four years). Another CMML prognostic model from the Mayo Clinic identified increased absolute monocyte count (>10 × 109/L), presence of circulating immature myeloid cells, anaemia and thrombocytopaenia as independent variables for survival [45]. The risk groups included low (OS = 32 months), intermediate (OS = 18.5 months), and high (OS = 10 months) risk.
Table 11.4 Characteristics of survival in CMML patients (data from MDS Registry Düsseldorf) [42].
| n | Median survival (months) | P value | |
|---|---|---|---|
| • CMML-0 | 101 | 31 | |
| CMML-0 dysplastic | 61 | 48 | |
| CMML-0 proliferative | 40 | 17 | 0.03 |
| • CMML-1 | 204 | 19 | |
| CMML-1 dysplastic | 105 | 29 | |
| CMML-1 proliferative | 99 | 15 | 0.008 |
| • CMML-2 | 81 | 13 | |
| CMML-2 dysplastic | 38 | 17 | |
| CMML-2 proliferative | 43 | 10 | 0.09 |
Atypical Chronic Myeloid Leukaemia, BCR-ABL1-negative (aCML)
Epidemiology
aCML is very rare. Patients with aCML are usually elderly. Male-to-female ratio is approximately 1:1.
Clinical Features
aCML is characterized by an increase in the WBC (median values 35 × 109/L to 96 × 109/L) and presence of immature granulocytes, including blasts, promyelocytes and myelocytes, that account for 10 to 20% of peripheral blood white cells (Table 11.5). Anaemia and thrombocytopaenia are frequently present. Some patients have symptomatic splenomegaly.
| • Leukocytosis (WBC ≥13 × 109/L) due to increased numbers of neutrophils and their precursors, which comprise ≥10% of leukocytes |
| • Dysgranulopoiesis, which may include abnormal chromatin clumping |
| • Minimal absolute basophilia (usually <2% of leukocytes) |
| • No or minimal absolute monocytosis (always ≤10% of leukocytes) |
| • Hypercellular bone marrow with granulocytic proliferation and dysplasia, with or without dysplasia in the erythroid and megakaryocytic lineages |
| • Less than 20% blasts in the blood and in the bone marrow |
| • No BCR-ABL1, PDGFRA, PDGFRB, FGFR1 or PCM1-JAK2 gene rearrangements |
| • Not meeting WHO criteria for BCR-ABL1-positive CML, PMF, PV or ET |
Morphology
Peripheral Blood
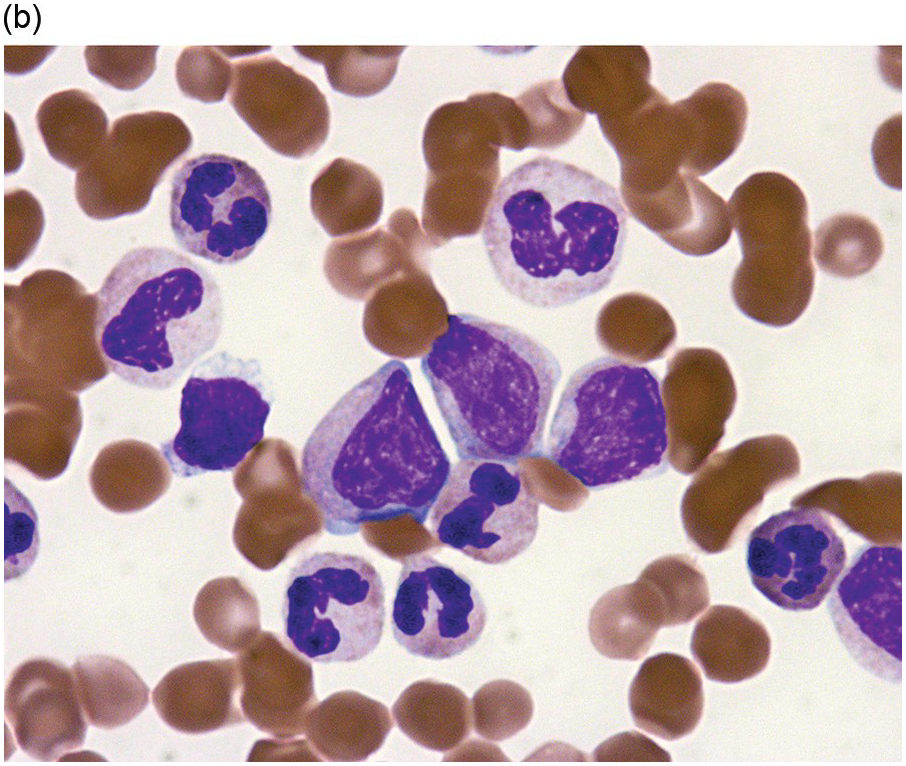
Dysplastic neutrophils are typically seen. These may show pseudo-Pelger–Huet changes, nuclear hypolobation or condensation, cytoplasmic hypogranularity or hyperlobulated bizarre-appearing nuclei often with abnormally clumped chromatin (Figure 11.9). Blasts are usually <5% of the cellularity. Neutrophil precursors constitute ≥10% of the leukocytes. The percentage of the monocytes should be <10% of all cells.
Bone Marrow
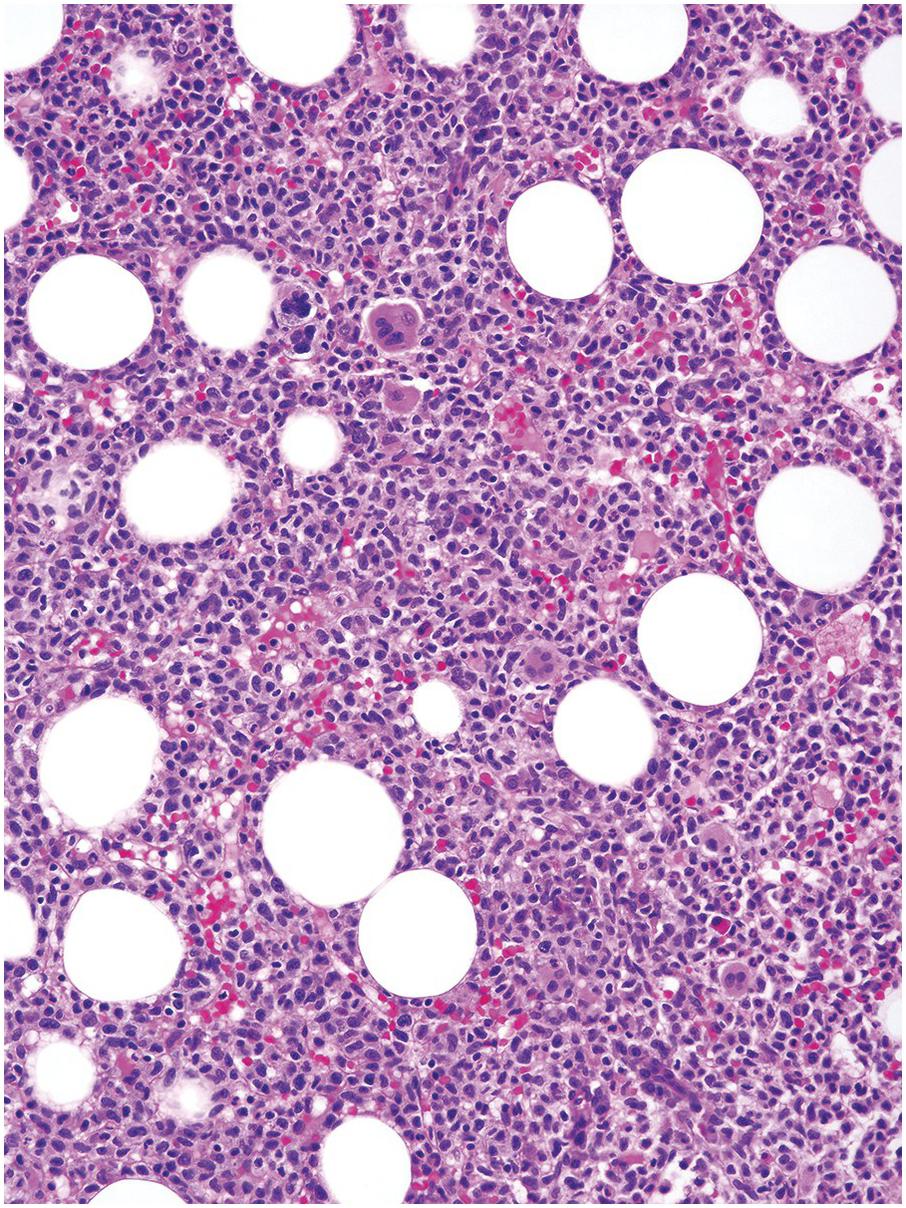
The BM is hypercellular, with an elevated myeloid-to-erythroid ratio (usually >10:1), due to granulocytic proliferation with invariable myeloid dysplasia (Figure 11.10). In contrast, the amount of erythropoiesis and megakaryopoiesis varies from case to case. Megakaryocytes are often reduced in number, particularly in cases of aCML associated with thrombocytopaenia. A subset of the cases is characterized by dyserythropoeisis and/or dysmegakaryopoiesis.
(a) Bone marrow biopsy demonstrates a hypercellular BM with myeloid predominance, including blasts, numerous neutrophils and pleomorphic-appearing megakaryocytes.
(b) High magnification showing a different case with dysmegakaryopoiesis and increased eosinophils, neutrophils and blasts.
Figure 11.10 Atypical chronic myeloid leukaemia.
Spleen
There is one case report of a patient with likely aCML who underwent spelenectomy due to massive splenomegaly and increasing blood transfusion requirements. The splenectomy specimen showed extramedullary haematopoiesis with myeloid precursor cells and some hypolobulated megakaryocytes [46].
Skin
No available information.
Immunophenotype/Cytochemistry
There are no specific immunophenotypic or cytochemical abnormalities.
Differential Diagnosis and Work-Up
The main differential diagnosis is with CML, BCR-ABL1-positive and other MPNs. Myeloid dysplasia is not a feature of CML, while basophilia and eosinophilia are consistently present in CML but only occasionally seen in aCML. BCR-ABL1 gene rearrangement should be interrogated in all cases of suspected aCML. Its presence is diagnostic of CML.
Other MPNs, in particular advanced stage polycythaemia vera and post-essential thrombocythaemia (ET) myelofibrosis, can develop marked neutrophilia that resembles aCML [47]. All these cases have previous history of MPN. Bone marrow biopsy shows characteristic features of MPN with increased enlarged and atypical megakaryocytes. Dysgranulopoiesis is only rarely present in cases of advanced neutrophilic MPNs and might be due to prior treatment. The molecular profile of MPNs is usually very different from aCML with the presence of JAK2/MPL/CALR mutations and absent SETBP1/ETNK1 mutations.
Chronic neutrophilic leukaemia (CNL) is a very rare subtype of MPN, characterized by sustained neutrophilia, BM hypercellularity due to granulocytic proliferation and hepatosplenomegaly in the absence of an identifiable cause of physiologic neutrophilia (Figure 11.11). The updated WHO classification guidelines require presence of ≥25 × 109/L WBC with ≥80% segmented neutrophils/bands and <10% immature granulocytes in peripheral blood (Table 11.6). Cases of CNL lack granulocytic dysplasia. Toxic granulation can be seen. Somatic activating CSF3R gene mutation is present in 90 to 100% of the cases of CNL and is currently considered a disease-defining mutation [48, 49]. CSF3R mutations occur almost exclusively in CNL [50, 51].
(a) Bone marrow biopsy is markedly hypercellular with increased granulopoiesis and a proliferation of mature neutrophils.
(b) Bone marrow aspirate highlights the markedly increased myeloid-to-erythroid ratio with evidence of full neutrophilic maturation. There is no significant dysplasia.
Figure 11.11 Chronic neutrophilic leukaemia.
| • Leukocytosis (WBC ≥25× 109/L) due to increased neutrophils and band forms that comprise ≥80% of leukocytes | |
| o Neutrophil precursors comprise <10% of leukocytes | |
| o Absent dysgranulopoiesis | |
| o Monocyte count <1 × 109/L | |
| o Myeloblasts rarely observed | |
| • Hypercellular bone marrow with neutrophilic proliferation, normal neutrophil maturation and myeloblasts <5% of nucleated cells | |
| • Not meeting WHO criteria for BCR-ABL1 CML, PV, ET or PMF | |
| • No BCR-ABL1, PDGFRA, PDGFRB, FGFR1 or PCM1-JAK2 gene rearrangements | |
| • Presence of CSF3R T618I or other activating CSF3 mutation | |
| or, | |
| in the absence of a CSFR3R mutation, persistent neutrophilia (at least three months), splenomegaly and no identifiable cause of reactive neutrophilia including absence of a plasma cell neoplasm or, if present, demonstration of clonality of myeloid cells by cytogenetic or molecular studies | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree