Abstract
Aplasia is a pathologic term that is broadly defined as the absence or near-absence of one or more haematopoietic lineages in the bone marrow (BM). Clinically, BM aplasia affecting more than one lineage is referred to as aplastic anaemia (AA), despite the fact that this group of disorders often results in pancytopaenia rather than anaemia alone. Cytopaenias can be seen in a number of different conditions, and new-onset pancytopaenia in children and adults requires an extensive work-up, including a BM core biopsy (BMB) for confirmation of haematopoietic aplasia/hypoplasia and exclusion of an infiltrative marrow process or fibrosis. Bone marrow aplasia develops as a result of injury to multipotent haematopoietic stem cells, which can occur in the context of constitutional (primary aplasia) or acquired (secondary aplasia) disorders (Table 4.1). This chapter will discuss the diagnostic criteria and pathophysiology of specific disorders presenting with aplasia and demonstrate an algorithmic approach to the diagnostic evaluation of patients presenting with this common and non-specific finding (Table 4.2).
Introduction
Aplasia is a pathologic term that is broadly defined as the absence or near-absence of one or more haematopoietic lineages in the bone marrow (BM). Clinically, BM aplasia affecting more than one lineage is referred to as aplastic anaemia (AA), despite the fact that this group of disorders often results in pancytopaenia rather than anaemia alone. Cytopaenias can be seen in a number of different conditions, and new-onset pancytopaenia in children and adults requires an extensive work-up, including a BM core biopsy (BMB) for confirmation of haematopoietic aplasia/hypoplasia and exclusion of an infiltrative marrow process or fibrosis. Bone marrow aplasia develops as a result of injury to multipotent haematopoietic stem cells, which can occur in the context of constitutional (primary aplasia) or acquired (secondary aplasia) disorders (Table 4.1). This chapter will discuss the diagnostic criteria and pathophysiology of specific disorders presenting with aplasia and demonstrate an algorithmic approach to the diagnostic evaluation of patients presenting with this common and non-specific finding (Table 4.2).
Table 4.1 Constitutional and acquired causes of bone marrow aplasia.
| Single lineage aplasia | Trilineage aplasia/aplastic anaemia |
|---|---|
| Constitutional disorders | |
| Diamond–Blackfan anaemia Congenital amegakaryocytic thrombocytopaenia Severe congenital neutropaenia Thrombocytopaenia with absent radii | Fanconi anaemia Dyskeratosis congenita Shwachman–Diamond syndrome |
| Acquired disorders | |
| Transient erythroblastopaenia of childhood PRCA associated with LPD – Large granular lymphocytic leukaemia – Chronic lymphocytic leukaemia – Monoclonal gammopathy/plasma cell neoplasm PRCA associated with MDS PRCA associated with thymoma PRCA associated with anti-EPO antibodies PRCA associated with autoimmune disorders – Rheumatoid arthritis – Systemic lupus erythematosus – ABO-incompatible HSCT PRCA associated with infectious disorders – Parvovirus B19 – Epstein–Barr virus – Hepatitis A and C viruses – Human immunodeficiency virus PRCA associated with drugs and toxins Idiopathic PRCA | Idiopathic (autoimmune) AA AA associated with acquired clonal abnormalities – Paroxysmal nocturnal haemoglobinuria – Myelodysplastic syndrome AA associated with drugs, toxins, radiation AA associated with viral infection AA associated with anorexia nervosa AA associated with pregnancy |
PRCA – pure red cell aplasia; LPD – lymphoproliferative disorder; MDS – myelodysplastic syndrome; EPO – erythropoietin; HSCT– haematopoietic stem cell transplantation; AA – aplastic anaemia.
Table 4.2 Diagnostic evaluation of patients presenting with cytopaenia(s) and suspicion for aplasia.
| Testing modality: | Evaluate for: |
|---|---|
| Clinical history |
|
| Physical examination |
|
| Laboratory tests |
|
| Bone marrow aspirate and biopsy |
|
| Flow cytometry |
|
| Cytogenetic tests |
|
| Molecular tests (single gene or NGS-based) |
|
| Additional specific tests associated with selected underlying diagnoses | |
| Dyskeratosis congenita |
|
| Diamond–Blackfan anaemia |
|
| Severe congenital neutropaenia |
|
| Large granular lymphocytic leukaemia |
|
| Plasma cell neoplasms |
|
| Rheumatoid arthritis Systemic lupus erythematosus |
|
| Parvovirus B19 |
|
| Paroxysmal nocturnal haemoglobinuria |
|
a For complete list see Table 4.4.
LPD – lymphoproliferative disorder; PNH – paroxysmal nocturnal haemoglobinuria; TCR – T-cell receptor; NGS – next generation sequencing.
Clinical and Morphologic Findings of Aplasia
Patients typically present with non-specific symptoms associated with low peripheral blood counts, such as recurrent infections due to neutropaenia, bleeding due to thrombocytopaenia, or fatigue and cardiopulmonary symptoms due to anaemia.
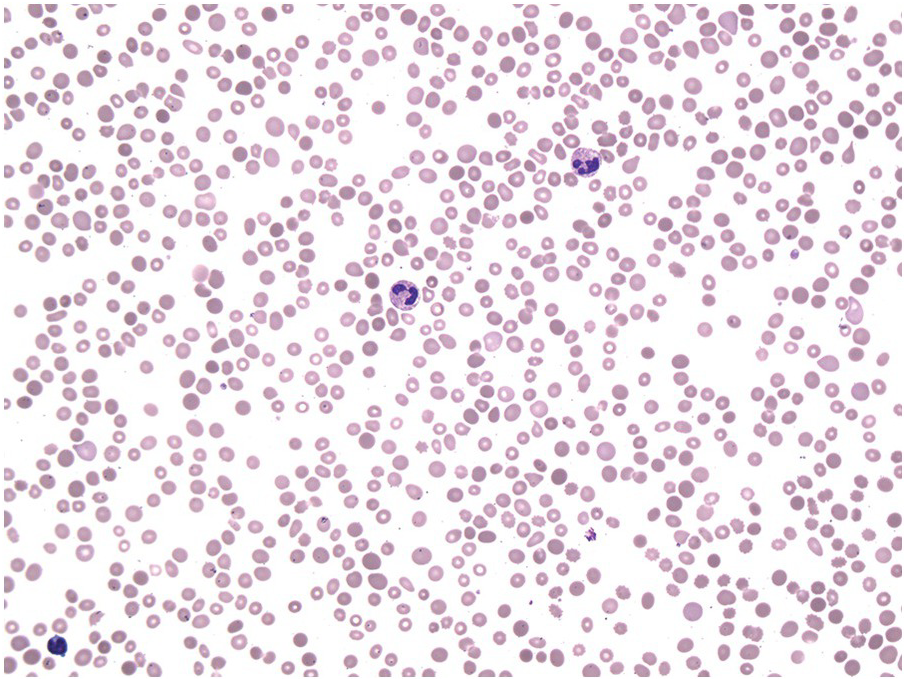
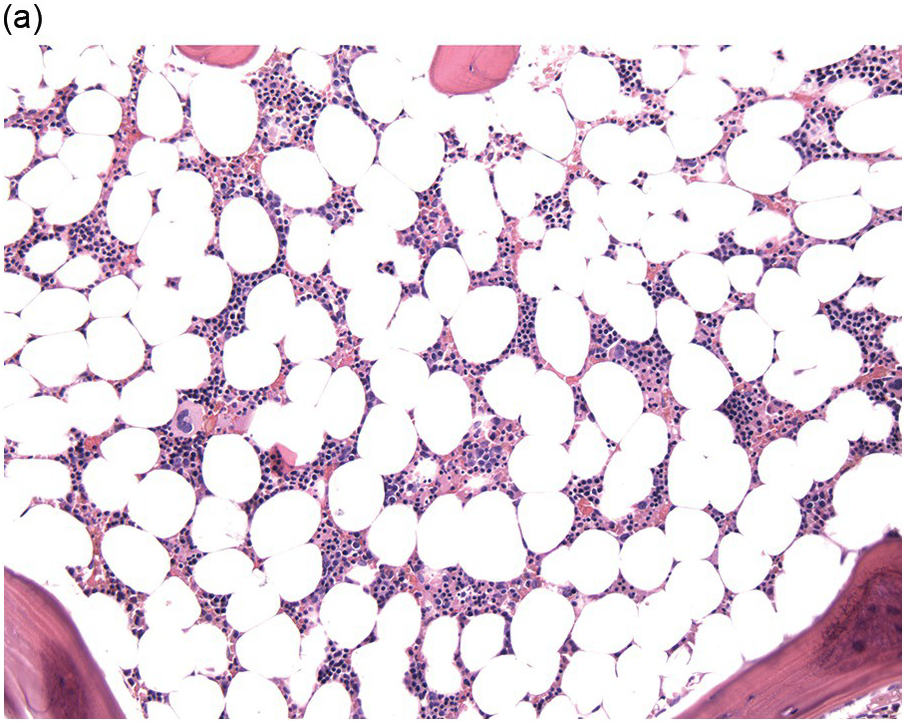
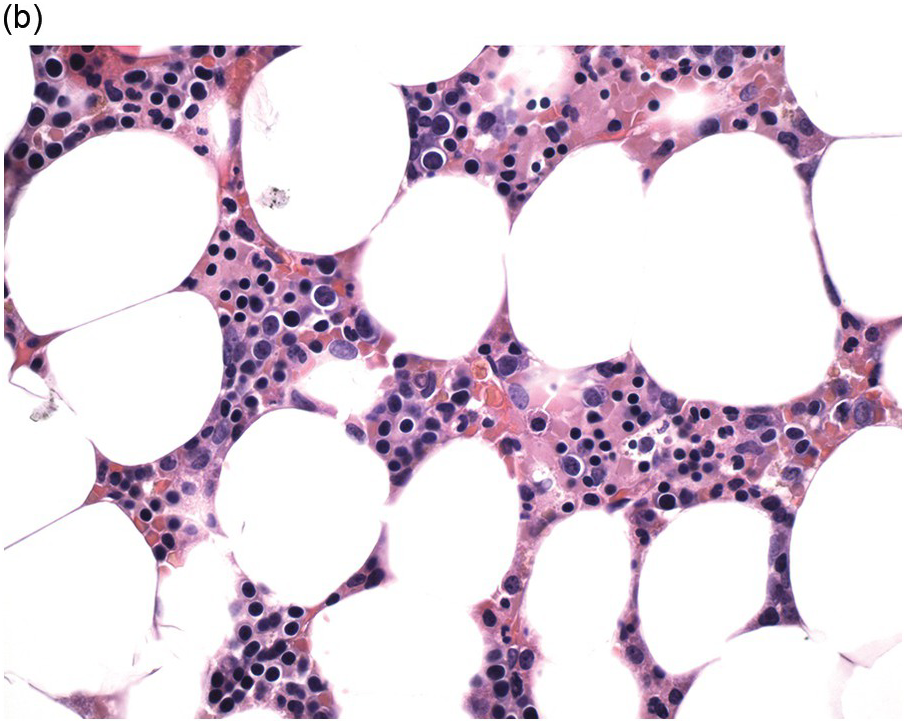
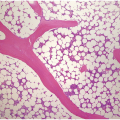
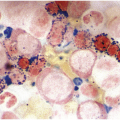
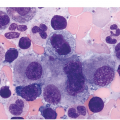
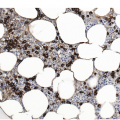
The complete blood count (CBC) reveals varying degrees of pancytopaenia, typically with normal cell morphology on a peripheral blood smear (Figure 4.1); the presence of abnormal cells, such as blasts, atypical lymphocytes or myelodysplasia, indicates cytopaenias secondary to an underlying clonal haematologic disorder. A diagnosis of aplastic anaemia is made if at least two of the following CBC criteria are met: (1) haemoglobin <10 g/dL; (2) platelet count <50 × 109/L; and (3) absolute neutrophil count <1.5 × 109/L [1, 2]. Bone marrow evaluation is required to confirm aplasia and exclude other causes. The BMB from a pancytopaenic patient will typically show a hypocellular marrow with marked trilineage aplasia/hypoplasia with normal cell morphology (Figure 4.2). In the case of single lineage aplasia, such as pure red cell aplasia, the biopsy will often demonstrate a normocellular for age BM with marked hypoplasia of the lineage affected and preservation of unaffected lineages (see ‘Acquired Pure Red Cell Aplasia’). Based on peripheral blood counts and BM findings, aplastic anaemia is categorized as non-severe, severe and very severe (Table 4.3). This classification is used for therapy selection and risk stratification.
(a) Low magnification shows an erythroid-predominant, markedly hypocellular for age marrow with markedly reduced megakaryocytes.
(b) High magnification demonstrates a predominance of red cell precursors with a markedly hypoplastic and left-shifted myeloid lineage, with only rare neutrophils.
Table 4.3 Aplastic anaemia classification based on peripheral blood counts and bone marrow cellularity [1].
| Category | ANC | Platelet count | Reticulocyte count | BM cellularity |
|---|---|---|---|---|
| Non-severe | 500–1500 μL | 20,000–50,000/μL | >20,000/μL | <25% |
| Severe | <500/μL | <20,000/μL | <20,000/μL | <25% |
| Very severe | <200 μL | <20,000/μL | <20,000/μL | <25% |
ANC – absolute neutrophil count.
In the majority of cases, the clinical and morphologic findings are non-specific as to the aetiology of BM aplasia, and further investigation into underlying causes of BM failure is required. Decisions regarding what additional testing should be performed are influenced by the clinical setting. For example, the presence of additional clinical findings, such as short stature, microcephaly or developmental delay, especially when seen in children, should prompt genetic evaluation for specific inherited syndromes (see ‘Constitutional Aplastic Disorders’). The work-up of adult patients should include evaluation for infectious and autoimmune disorders as well as for acquired clonal abnormalities, which may be seen in the context of paroxysmal nocturnal haemoglobinuria and myelodysplastic syndrome (MDS) (see ‘Acquired Aplastic Disorders’). Despite extensive evaluation, in many patients the underlying cause(s) of aplasia remain(s) unclear, and such cases are classified as idiopathic.
Constitutional Aplastic Disorders
Patients with constitutional bone marrow failure (BMF) syndromes typically demonstrate clinically significant cytopaenias affecting one or more lineages. In a subset of patients, associated congenital anomalies are present as well. This group of disorders includes Fanconi anaemia (FA), dyskeratosis congenita (DC), Shwachman–Diamond syndrome (SDS), congenital amegakaryocytic thrombocytopaenia (CAMT), Diamond–Blackfan anaemia (DBA), severe congenital neutropaenia (SCN) and thrombocytopaenia with absent radii (TAR) (Table 4.1). While these all represent constitutional disorders, age at presentation can range from infancy to adolescence or adulthood, depending on the disorder. Inherited BMF syndromes are all rare diseases and quantitative epidemiological data are largely lacking. However, the most common constitutional aplastic disorders appear to include FA, DBA, SDS and DC [3].
Fanconi Anaemia (FA)
Fanconi anaemia is a heterogeneous inherited disorder characterized by congenital anomalies, BMF and a predisposition to malignancy (including haematologic and solid tumours) due to defects in DNA repair. Although FA occurs worldwide [4], certain ethnic groups show an increased prevalence of FA due to the presence of founder mutations (Ashkenazi Jews, Spanish Gypsies, and Black and Afrikaner South Africans) [4]. Fanconi anaemia is also relatively more common in populations with increased consanguinity, due to its predominantly autosomal recessive pattern of inheritance.
The median age of diagnosis for FA is 6.5 years, but diagnoses may be made at any time from birth to adulthood [3]. Patients classically show a spectrum of congenital abnormalities, including short stature, microcephaly and abnormalities involving the skin (café au lait spots, hypopigmentation), upper limbs (hypoplastic or absent thumbs +/– radii), eyes and genitourinary system [3, 5]. However, physical findings may be subtle or absent in up to 30% of patients [5, 6]. For many patients, FA may be suspected only after signs of BMF emerge, with the onset of marrow aplasia peaking at approximately 7 years of age [3, 7]. The risk of developing BMF, MDS, acute myeloid leukaemia (AML) or solid tumours by 50 years of age is approximately 50%, 40%, 10–15% and 20–30% respectively [7, 8].
Fanconi anaemia is caused by mutations in one of at least 20 different genes involved in the repair of DNA interstrand crosslinks (Table 4.4) [9], which form upon exposure to radiation, chemotherapeutic drugs and endogenous aldehydes generated during demethylation reactions, lipid peroxidation and ethanol metabolism [10]. The increased sensitivity to DNA crosslinking agents is exploited in diagnostic testing, which involves evaluating for chromosomal breakage after treatment of cells with diepoxybutane or mitomycin C [11]. Positive chromosomal breakage studies are typically followed by complementation studies and/or single gene sequencing or, more recently, next generation sequencing analysis, to identify specific genetic lesions [11].
Table 4.4 Molecular and clinical characteristics of inherited bone marrow failure syndromes [3, 9, 31, 40].
| Disease | Inheritance | Gene mutated (approximate % of patients with mutation) | Pathway affected | Clinical findings |
|---|---|---|---|---|
| Fanconi anaemia | AR XLR | FANCA (60%), FANCC (14%), FANCD1 (BRCA2) (3%), FANCD2 (3%), FANCE (3%), FANCF (2%), FANCG (10%), FANCI (1%), FANCJ (2%), FANCL (<1%), FANCM (<1%), FANCN (PALB2) (<1%), FANCO (RAD51C) (<1%), FANCP (SLX4) (<1%), FANCQ (ERCC4) (<1%), FANCR (RAD51) (<1%), FANCS (BRCA1) (<1%), FANCT (UBE2T) (<1%), FANCU (XRCC2) (<1%), MAD212 (REV7) (<1%) FANCB (2%) | DNA interstrand crosslink repair | Pancytopaenia Short stature, microcephaly, and abnormalities involving the skin, upper limbs, eyes, and GU tract Predisposition to MDS/AML Predisposition to solid tumours (SCC of head and neck, GI tract, and anogenital area) |
| Dyskeratosis congenita | XLR AD AR AD & AR | DKC1 (35%) TINF2 (10-20%), TERC (5%) ACD (<1%), CTC1 (<1%), NOP10 (<1%), NHP2 (<1%), WRAP53 (<1%) TERT (5%), RTEL1 (5-10%), PARN (<1%) | Telomere maintenance | Pancytopaenia Triad of abnormal skin pigmentation, nail dystrophy, and leukoplakia Pulmonary fibrosis, oesophageal stenosis Predisposition to MDS/AML Predisposition to solid tumours (SCC of head and neck, GI tract, and anogenital area) |
| Shwachman–Diamond syndrome | AR | SBDS (>90%) | Ribosome biogenesis | Neutropaenia, exocrine pancreatic insufficiency, and skeletal abnormalities Progression to pancytopaenia in ~20% Predisposition to MDS/AML |
| Congenital amegakaryocytic thrombocytopaenia | AR | MPL (60-100%) | Thrombopoietin signaling | Isolated severe thrombocytopaenia at presentation Progression to pancytopaenia |
| Diamond–Blackfan anaemia | AD XLR | RSP19 (25%), RPL11 (6-7%), RPS26 (6%), RPS10 (2-3%), RPL35A (3%), RPS24 (2%), RPS17 (1%), RPS7 (<1%), RPS19 (<1%), RPS20 (<1%), RPS28 (<1%), RPS29 (<1%), RPL5 (<1%), RPL15 (<1%), RPL17 (<1%), RPL19 (<1%), RPL26 (<1%), RPL31 (<1%) GATA1 (<1%), TSR2 (<1%) | Ribosome biogenesis | Anaemia at presentation; additional cytopaenias may develop in 20–40% Short stature; additional congenital anomalies in 25–50% (craniofacial, upper limb, cardiac, GU) Predisposition to MDS/AML Predisposition to solid tumours (colon cancer, sarcoma, female genital cancers) |
| Severe congenital neutropaenia | AD AR XLR | ELANE (30-60%), GFI1 (1-30%) HAX1 (1-5%), G6PC3 (<1%) WAS (<1%) | ?Unfolded protein response | Isolated persistent neutropaenia HAX1 and G6PC3 mutations may be associated with extra-haematopoietic abnormalities Predisposition to MDS/AML |
| Thrombocytopaenia with absent radii | AR | 1q21.1 microdeletion (including RBM8A) and RBM8A SNP on remaining allele (>90) | RNA processing | Thrombocytopaenia and bilateral radial hypoplasia/aplasia Platelet count increases over time Skeletal, cardiac, GU anomalies Cow’s milk intolerance Case reports of MDS, AML and ALL |
AR – autosomal recessive; XLR – X-linked recessive; AD – autosomal dominant; MDS – myelodysplastic syndrome; AML – acute myeloid leukaemia; SCC – squamous cell carcinoma; GI – gastrointestinal; GU – genitourinary; ALL – acute lymphoblastic leukaemia.
Fanconi anaemia patients typically develop cytopaenias within the first decade of life, often initially as isolated thrombocytopaenia, with progression to pancytopaenia in the majority of patients [6, 12]. Anaemia is typically macrocytic. Patients with FA are at a markedly increased risk of developing MDS (6,000-fold higher risk than the general population) and AML (700-fold higher risk) [7], which most commonly occur before the age of 30 [12]. In addition to hypoplasia, BM examination in patients with FA will often demonstrate dyserythropoiesis (e.g. megaloblastoid change, irregular nuclear contours, nuclear budding), which should not by itself be considered evidence of MDS [13]. The most reliable morphologic criteria for making a diagnosis of MDS include the presence of dysgranulopoiesis or increased blasts, which are both highly correlated with the presence of a clonal cytogenetic abnormality [13]. The most common cytogenetic abnormalities seen in FA patients include gains of 1q and 3q, deletion of chromosome 7/7q and abnormalities involving the RUNX1 locus at 21q [13–15]. In one study examining BM samples from 57 patients with FA, gain of 3q, 7/7q–, and RUNX1 abnormalities were seen exclusively in patients with MDS/AML, whereas gain of 1q was also seen in approximately 40% of patients with normal BMB, suggesting that gain of 1q may occur early, prior to malignant transformation [15]. Limited sequencing analysis of FA-associated MDS/AML has suggested that mutations seen recurrently in de novo AML are relatively rare in the context of FA [15]. Due to the relative rarity of 1q+ and 3q+ in de novo AML, it has been suggested that the identification of these cytogenetic abnormalities in young patients with MDS/AML should prompt evaluation for underlying FA [13–15].
Dyskeratosis Congenita (DC)
Dyskeratosis congenita is a heterogeneous constitutional disorder characterized by mucocutaneous lesions, including the classic triad of abnormal skin pigmentation, nail dystrophy and leukoplakia, as well as BMF and cancer predisposition (MDS/AML and solid tumours) due to defects in telomere maintenance. Patients may also develop lacrimal duct, oesophageal or urethral stenosis, early greying and pulmonary fibrosis. Mucocutaneous lesions are typically the first signs of disease and usually become apparent within the first decade of life, although only approximately 50% of patients will demonstrate all three aspects of the classic triad [5]. Bone marrow failure typically develops during adolescence; by age 50, the cumulative incidence of BMF in DC patients is 50% [8, 16]. The median age at diagnosis for DC is 14 years, with a range of 0–75 years [3].
Several severe forms of DC are recognized as distinct syndromes [3, 9, 17]. Patients with Hoyeraal–Hreidarsson (HH) syndrome develop severe AA at a very early age and demonstrate cerebellar hypoplasia and associated ataxia, immunodeficiency, intrauterine growth retardation and developmental delay. Revesz syndrome is characterized by bilateral exudative retinopathy (also known as Coats disease of the retina) and CNS calcifications in addition to features of classic DC. In patients with Coats plus syndrome, bilateral exudative retinopathy and CNS calcifications are seen along with retinal telangiectasias, growth retardation, bone abnormalities and gastrointestinal vascular ectasias.
Dyskeratosis congenita is due to pathogenic germline mutations in one of at least 11 genes involved in telomere maintenance and can show X-linked recessive, autosomal recessive or autosomal dominant inheritance patterns, depending on the gene mutated (Table 4.4) [9]. The most commonly mutated gene in DC is the DKC1 gene; DKC1 mutations are present in approximately 35% of DC patients and show X-linked recessive inheritance [3]. Many patients with HH syndrome harbour DKC1 mutations [17]. Five of the known DC genes (DKC1, TERC, TERT, NOP10 and NHP2) encode protein subunits of the telomerase enzyme, which functions to maintain telomere length by replacing telomere DNA normally lost during cell division [3]. Additional DC genes encode proteins involved in telomerase trafficking (WRAP53, encoding the TCAB1 protein) and stability (RTEL1) [9]. The TINF2 gene encodes a protein subunit of the shelterin complex, which binds to telomere ends to prevent shortening [3]; TINF2 mutations are associated with Revesz syndrome [17]. Coats plus syndrome is caused by mutations in CTC1, which encodes a telomere capping protein [9]. In approximately 50% of patients with classic clinical findings and proven short telomere length, no mutations are identified in the known DC genes, suggesting the presence of additional, as of yet undiscovered, causative mutations [16]. Diagnosis relies on a combination of clinical findings, telomere length analysis using flow cytometry with fluorescence in situ hybridization (flow FISH) and, in some cases, genetic testing [17–19].
Patients with DC often show peripheral blood macrocytosis with anaemia and/or thrombocytopaenia, with eventual progression to pancytopaenia [17]. Bone marrow findings in cytopaenic patients are similar to those seen in FA and other aplastic processes, with hypocellularity +/– mild dyspoiesis [3, 6]. A propensity to developing unbalanced chromosomal rearrangements may be seen by cytogenetic analysis [5]; however, recurrent clonal cytogenetic abnormalities have not been described in the literature [3]. Dyskeratosis congenita patients show a cumulative incidence of MDS and AML by age 50 of 30% and 10%, respectively [8].
Shwachman–Diamond Syndrome (SDS)
Shwachman–Diamond syndrome is characterized by exocrine pancreatic insufficiency, failure to thrive, BM dysfunction (manifesting most commonly as profound neutropaenia), recurrent infections and skeletal abnormalities, including short stature and metaphyseal dysostosis [5, 6, 16]. Patients classically present in infancy (median age at diagnosis of 2 weeks, range birth to 11 years) with malabsorption and failure to thrive due to pancreatic insufficiency, with neutropaenia also seen in 80% of patients at presentation [3, 20]. However, the use of genetic testing in diagnosing SDS has uncovered patients with atypical phenotypes who present later in childhood [20]. Patients with SDS are at increased risk of developing MDS and AML but the development of solid tumours is uncommon [3, 16]. Reported rates of transformation to MDS/AML range from 0 to 36%, with the variation likely due, in part, to differences in the median age of the various study populations [16]. The development of AML typically occurs in early adulthood (median age 19, range 2–43 years) and is approximately three times more common in males than females for unknown reasons [3].
Shwachman–Diamond syndrome is an autosomal recessive disorder in which >90% of patients harbour biallelic mutations in the SBDS gene located on chromosome 7q11. SBDS gene mutations commonly arise from gene conversion events involving an SBDS pseudogene [21] and are thought to cause markedly decreased SBDS protein levels. Interestingly, patients with homozygous null alleles have not been reported, suggesting that at least some low-level protein production is necessary for life [16]. The SBDS protein is involved in ribosomal maturation, with disease-associated mutations thought to cause altered mRNA translation, and may play a role in multiple additional cellular functions as well [3, 22]. Diagnosis of SDS is based on clinical and laboratory findings, with confirmatory genetic testing involving sequencing of the SBDS gene [18, 19, 23]. Notably, lack of SBDS mutations does not exclude a diagnosis of SDS, as up to 10% of patients may be negative for SBDS mutations.
Intermittent or chronic neutropaenia is present in the majority of patients. 60–80% of patients exhibit anaemia, which may be normocytic or macrocytic and is commonly associated with an elevated fetal haemoglobin; thrombocytopaenia is less common. Approximately 20% of patients progress to pancytopaenia at a median age of 3 years (range birth to 35 years) [3, 18, 23, 24]. The BM is typically hypocellular and may show mild trilineage dysplastic changes, which may be transient and are not considered diagnostic of MDS [6]. Bone trabeculae show features of osteoporosis [6]. Clonal cytogenetic abnormalities are common, particularly isochromosome 7q (i(7)(q10)) and deletion of 20(q11) (del(20)(q11)). Similar to morphologic dysplastic changes, these cytogenetic findings may be transient and are not indicative of neoplasia [3, 16, 25, 26]. Interestingly, i(7)(q10) leads to duplication of the SBDS gene locus and is thought to be associated with increased expression of the SBDS protein [26]. Del(20)(q11) leads to loss of the EIF6 locus, and decreased eIF6 protein levels are thought to enhance ribosomal biogenesis [27]. Therefore both of these common cytogenetic abnormalities are thought to abrogate the effect of biallelic mutation of the SBDS gene.
Congenital Amegakaryocytic Thrombocytopaenia (CAMT)
Congenital amegakaryocytic thrombocytopaenia is a rare, autosomal recessive inherited BMF syndrome characterized by isolated severe thrombocytopaenia in infancy with eventual progression to AA. Congenital anomalies characteristic of other inherited BMF syndromes are not present in CAMT. Patients typically present with signs of marked thrombocytopaenia, including petechiae, purpura or epistaxis, within hours of birth; white blood cell count and haemoglobin levels are typically within normal limits [28, 29]. Rare patients with CAMT may present later in childhood [28]. In a subset of patients, platelet counts may spontaneously improve for an extended period of time but this improvement is typically transient, with eventual recurrence of thrombocytopaenia [29].
The majority of cases of CAMT are due to biallelic mutation of the MPL gene, which encodes the thrombopoietin (TPO) receptor [30]. Nonsense and frame shift mutations lead to complete loss of function, while missense and splice site mutations may lead to generation of a hypomorphic MPL protein with a low level of residual activity [30, 31]. The presence of the latter may be associated with a milder disease phenotype and/or with transient improvement in platelet counts, although this is not always the case [3, 30]. With this absence or near absence of a functional TPO receptor, patients with CAMT accordingly show markedly elevated serum TPO levels [30]. In addition to playing an essential role in megakaryopoiesis, TPO is also an important regulator of multipotent haematopoietic progenitor cells, which likely explains the progression to aplastic anaemia with loss of all haematopoietic lineages seen in the majority of patients with CAMT [3]. Diagnosis relies on a combination of clinical and laboratory findings, including sequencing analysis of the MPL gene.
BM core biopsy at diagnosis shows a normocellular marrow with markedly decreased or absent megakaryocytes and normal myeloid and erythroid maturation [28]. Megakaryocytes that are present may be immature and small in size. In rare cases, normal numbers of megakaryocytes may be present in the initial BMB; however, subsequent biopsies demonstrate progressive loss of megakaryocytes [32]. Over time, patients with CAMT are at significant risk for progression to pancytopaenia and marrow hypoplasia. In one study of 20 CAMT patients, 75% progressed to pancytopaenia at a median age of 38 months (range 6–82 months) [29]. Whether CAMT is associated with an increased risk of MDS or AML remains to be determined. Rare cases of MDS and AML developing in patients with CAMT have been described; however, in none of these patients was the presence of a MPL mutation confirmed [28]. In a series examining five patients with confirmed MPL mutations, two patients developed clonal cytogenetic abnormalities, one showing monosomy 7 and another showing trisomy 8. The clonal abnormalities developed after progression to AA but there was no evidence of BM dysplasia in either case [33]. A separate report described a third patient with a confirmed MPL mutation who also developed monosomy 7 in the context of severe AA without evidence of marrow dysplasia [34]. Of these three patients, follow-up data was reported for two patients, both of whom underwent haematopoietic stem cell transplant due to marrow aplasia, as is frequently the case for patients with CAMT. Therefore it is possible that the use of haematopoietic stem cell transplant at a relatively young age may obscure the true risk of malignant transformation associated with CAMT.
Diamond–Blackfan Anaemia (DBA)
Diamond–Blackfan anaemia is a congenital pure red cell aplasia due to mutations in ribosomal proteins. While patients with DBA present with isolated anaemia, neutropaenia and/or thrombocytopaenia may eventually develop in 20 to 40% of patients [35]. In addition to haematologic abnormalities, short stature is commonly seen, and 25 to 50% of patients show associated congenital anomalies, most commonly craniofacial, upper limb, cardiac and genitourinary abnormalities [3, 16, 36]. The median age at diagnosis is 3 months, with greater than 90% of patients being diagnosed within the first year; however, the diagnosis may be made in adult patients and in some patients with known pathogenic mutations clinical findings may be extremely subtle, presumably due to variable penetrance of disease-associated mutations [16, 37, 38]. Patients with DBA are at increased risk of developing malignancies, including MDS and AML as well as solid tumours, particularly colon cancer, sarcoma and female genital cancers; however, the risk of malignancy, while elevated over the normal population, appears to be lower than that seen in FA and DC [39]. Interestingly, spontaneous remission, with stable physiologically acceptable haemoglobin levels persisting without treatment, has been reported in approximately 25% of patients [3, 40].
Approximately 45% of cases of DBA are familial and show autosomal dominant inheritance [37]. Approximately 65% of DBA patients harbour heterozygous mutations or deletions in one of at least 18 different genes encoding protein components of the 60S large and 40S small ribosomal subunits, with mutations in the RPS19 gene being most common and present in approximately 25% of patients (Table 4.4) [9, 40, 41]. Additional rare cases have been described with mutations in TSR2, whose protein product binds RPS26, as well as GATA1 [40].
Diamond–Blackfan anaemia is characterized by the presence of macrocytic anaemia without polychromasia (reticulocytopaenia), increased fetal haemoglobin and elevated erythrocyte adenosine deaminase. The BM is normo- to somewhat hypocellular with markedly decreased or absent red cell precursors; the granulocytic and megakaryocytic lineages are typically unaffected [3, 40]. Significant marrow aplasia may develop over time, with 75% of patients developing trilineage hypoplasia in one study of patients with steroid-refractory disease [35]. Interestingly, while DBA is classically considered a pure red cell aplasia, in vitro studies using DBA patient samples have demonstrated a proliferative defect in more primitive, multipotent haematopoietic cells, suggesting the presence of a multilineage defect, which may explain why patients can develop pancytopaenia and BM hypoplasia later in the course of disease [35]. Clonal cytogenetic abnormalities have not been described in the literature [3]. Given the genetic heterogeneity of DBA, diagnosis continues to rely heavily on clinicopathologic findings, including isolated macrocytic anaemia presenting before 1 year of age with associated reticulocytopaenia and a normocellular marrow with a paucity of red cell precursors; however, genetic testing also plays a role in ‘non-classical’ cases [19, 42].
Severe Congenital Neutropaenia (SCN)
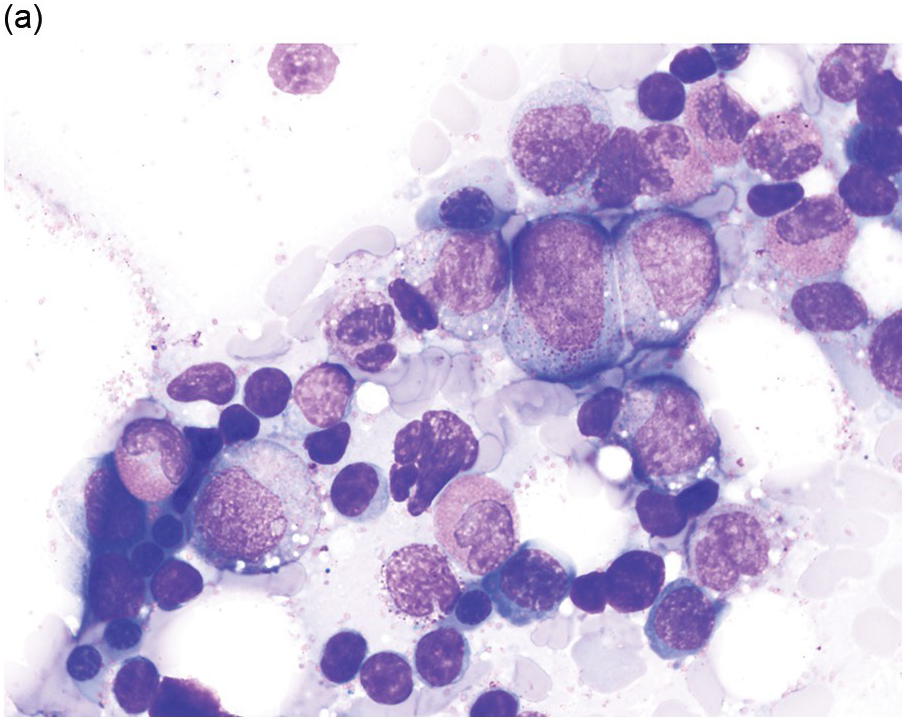
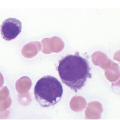
Severe congenital neutropaenia (sometimes referred to as Kostmann syndrome) is a heterogeneous group of inherited disorders characterized by the presence of persistent profound neutropaenia, with absolute neutrophil counts (ANC) <200/μl, and a predisposition to MDS/AML [43]. Patients typically present in infancy with severe bacterial infections. Neutrophil levels may increase transiently in the context of infection but generally do not reach the normal range. Additional peripheral blood findings may include mild anaemia, thrombocytosis, monocytosis and eosinophilia. Severe congenital neutropaenia must be distinguished from cyclic neutropaenia, a distinct congenital neutropaenic disorder characterized by episodes of neutropaenia occurring on an approximately 21-day cycle, followed by count recovery, which is not associated with an increased risk of malignant transformation. Within the BM of SCN patients, myeloid elements are hypoplastic and show maturation arrest at the promyelocyte/myelocyte stage [43]. Promyelocytes may show atypia, including binucleation and cytoplasmic vacuolization. Erythroid and megakaryocytic lineages are unaffected (Figure 4.3).