Cervix
Cervical cancer is the third most common cancer among women worldwide and is entirely attributable to infection with the Human Papillomavirus (HPV). Global estimates indicate that 610,000 newly incident HPV-related cancers occurred in 2008, including cancers at several sites in the female and male anogenital and oral tracts. Of these, cervical cancer was estimated to account for 530,000 new cases and 275,000 deaths. Rates of cervical cancer are estimated to be at least four-fold higher in low resource countries with a “low” ranking for Human Development Index compared with those in developed countries in the “very high” category (1).
Although differences in HPV exposure in various countries may play some role in explaining the differences between cervical cancer rates (2), the higher incidence and death rates of cervical cancer in low resource settings are likely to result from the lack of organized cervical screening and inadequate access to treatment. In many developed countries, screening with cervical cytology has resulted in large-scale reductions in cervical cancer incidence and mortality over time (3,4).
The primary goal of cervical screening is to prevent cervical cancer. This is achieved by the detection, treatment, and follow-up of preinvasive cervical lesions (3,5,6). Modern understanding that almost all cervical cancers are caused by persistent infection with approximately 15 types of HPV has led to important new approaches to primary and secondary cervical cancer prevention via prophylactic HPV vaccination and primary HPV-based screening.
Classification of Preinvasive Cervical Disease
The proposal that invasive squamous carcinoma of the cervix arises through progression of a preinvasive lesion, as opposed to a de novo event, was initially postulated by Schauenstein in 1908 (7). The term “carcinoma in situ” was later introduced to describe cancerous changes confined to the epithelium (8). It is now understood that precancerous lesions arise from persisting HPV infection of the cervix, even though the majority of HPV infections regress (9).
The Dysplasia Terminology
Although referred to earlier by Papanicolaou and Traut (10), in 1956 Reagan and Hamonic (11) described cytologic differences between “carcinoma in situ” and a group of “less anaplastic” lesions, for which they introduced the term dysplasia (12). In 1975, the World Health Organization defined dysplasia as a “lesion in which part of the epithelium was replaced by cells showing varying degrees of atypia.” Dysplastic changes were graded as mild, moderate, and severe, but precise guidelines for these subdivisions were not defined and grading always remained highly subjective (13–16).
A dual terminology developed, leading to inconsistencies in treatment policies. If a diagnosis of “dysplasia” was made, this was considered a nonspecific change and the patient was subjected to a cone biopsy. If the diagnosis of “carcinoma in situ” was made, this was considered a “preinvasive cancer” and the patient underwent an obligatory hysterectomy (15–17).
Cervical Intraepithelial Neoplasia
Invasive squamous cell carcinoma of the cervix was demonstrated to be the end result of progressive intraepithelial dysplastic atypia occurring within the metaplastic epithelium of the cervical transformation zone (18). The classification of lesions from mild dysplasia to carcinoma in situ did not truly reflect either the morphologic or biologic continuum of preinvasive cervical disease. The diagnosis was highly subjective and was not reproducible.
After pioneering research into the natural history of cervical cancer precursors, Richart (19) proposed the term “cervical intraepithelial neoplasia” (CIN) in 1973 to describe the biologic spectrum of cervical preinvasive squamous disease. Three grades of CIN were described, CIN 1 (mild dysplasia), CIN 2 (moderate dysplasia), and CIN 3 (severe dysplasia/carcinoma in situ). This system was consistent with biologic evidence that strongly implied a single process of cervical squamous carcinogenesis (19–25).
Forty years’ experience with the CIN terminology, coupled with recent advances in the understanding of the role of HPV in the causation of cervical neoplasia (24–30), has led to a critical reappraisal of this model and to further reclassification of the terminology for reporting cytologic abnormalities consistent with preinvasive disease (31–38).
The CIN grading is also very subjective. No reproducible cytologic or histologic distinction at the lower end of the CIN continuum exists between CIN 1 and HPV infection alone. Both interobserver and intraobserver consistency in diagnosis are poor (37–40). Separating CIN 2 from CIN 3 is often not reproducible (26,41–43). In reality, the two critical questions in the assessment of the cervical epithelium are: (i) do the changes represent a cancer precursor; and (ii) is the lesion invasive cancer?
In histologic terms, CIN 3 is clearly established as a bona fide cancer precursor, although there are few identified risk factors for progression of CIN 3 to cancer other than time (44). CIN 3 is a reliable and more highly reproducible morphologic diagnosis, with undifferentiated cells having fixed genetic abnormalities replacing almost the full thickness of the cervical epithelium (40,41). It is reliably distinguished from recently acquired HPV infection and is a genuine surrogate marker of subsequent cancer risk. Uncertainty still exists in relation to the progressive potential of less severe dysplastic lesions (45,46).
CIN 1 is increasingly viewed as an insensitive histologic marker of HPV infection. The diagnosis includes errors of processing and interpretation of colposcopically directed biopsies (40). Standardized for positivity of a given high-risk HPV type, a diagnosis of CIN 1 does not predict a meaningfully higher risk of CIN 3 than does a negative biopsy (44). By contrast, longitudinal outcomes after HPV infection have demonstrated higher risk of CIN 2+ and CIN 3+ in HPV-positive versus HPV-negative women, with follow-up times up to 18 years now reported (47). These studies have demonstrated that the risk of subsequent high-grade CIN is dependent on the initial HPV type, with types 16 and 18 being associated with a higher risk than the other oncogenic types (47,48).
There appears to be considerable heterogeneity in the microscopic diagnosis, biology and clinical behavior of CIN 2 lesions (49). CIN 2 can sometimes be produced by noncarcinogenic HPV types and is equivocal in its cancer potential (49). Some CIN 2 lesions represent acute HPV infection with a more severe microscopic appearance that is destined to regress. Others are incipient precancer (CIN 3) that will persist and progress with an attendant high risk of future invasion if left untreated.
The risk factor profiles (49–51) and HPV genotype distributions (52) in CIN 2 and CIN 3 are different. CIN 2 is more likely to spontaneously regress than CIN 3 (53), but current clinical management of CIN 2 and CIN 3 is similar in most settings. The clinical dilemma remains the inability to reliably predict those lesions less severe than CIN 3 that are at greatest risk of progression to cancer.

Figure 7.1 Changes to the terminology and number of tiers used to describe cervical precancer over time with corresponding management options (procedure). CKC, cold-knife conization; Cryo, cryotherapy; Rx, treatment. Modified with permission. Courtesy of J. Thomas Cox. Reproduced from Darragh TM, Colgan TJ, Cox JT, et al.; for members of the LAST Project Work Groups. The Lower Anogenital Squamous Terminology Standardization Project for HPV-associated Lesions: Background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136:1266–1297.
New molecular markers hold promise in this regard (54–58) and there is emerging evidence that technologies such as dual immunohistochemical (IHC) staining for the markers Ki-67 and p16 can improve histologic classification of CIN 3 abnormalities and resolve CIN 2 diagnoses (58). The Lower Anogenital Squamous Terminology Standardization Project (LAST nomenclature) relies on p16 IHC staining to triage CIN 2 (58); p16 is a biomarker for disruption of the Rb pathway by HPV (57,59). CIN 2 that is p16 positive is combined with CIN 3 as a high-grade lesion. CIN 2 that is p16 negative is combined with CIN 1 as a low-grade lesion, the histologic diagnosis of HPV infection (Fig. 7.1).
Cervical Precancer—Modern Concepts
HPV infection is a necessary precursor of true precancer. A defined precancerous lesion remains the target of screening and preventive treatment programs and represents a genuine surrogate for cancer risk (60–62). CIN 3 is the most certain histologic surrogate marker of cancer risk. CIN 3 lesions demonstrate the same aneuploid DNA (63) content and genetic instability (64) as seen in invasive cancer. Some CIN 3 lesions are small and a proportion may regress, particularly after biopsy, but at this time, all CIN 3 lesions should be managed as definite precancer lesions.
CIN 2 has demonstrated greater heterogeneity in biology and definition and has a greater potential for regression when compared to CIN 3 (49,50,65). Despite the emergence of biomarkers such as p16 IHC staining, it is recommended that CIN 2 lesions should be treated to provide a further safety margin against the development of cancer.
A histologic diagnosis of a low-grade cervical intraepithelial lesion (HPV infection/CIN 1) is increasingly viewed as not representing precancer. However, infection with oncogenic HPV types is strongly predictive of future risk of high-grade abnormalities/precancer (47,48,67). HPV infection might not be associated with any microscopic abnormality, while most low-grade abnormalities will regress (68,69), particularly among young women (70).
High-grade lesions are commonly found within a broader field of low-grade disease, suggesting that CIN 3 may develop in high-risk HPV-infected epithelium independent of, and within a CIN 1 lesion, rather than as a classical stepwise progression. The reported progressive potential of histologically confirmed low-grade lesions varies from 12–33% (17,18,71–73).
Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions (LAST Project)
HPV interacts with genital tract squamous epithelia in two basic ways. Firstly, HPV infection may produce transient lesions, which support virion production. Such lesions have been variously described as low-grade lesions, intraepithelial neoplasia grade 1, mild dysplasia, or condyloma. Such lesions may be undetected clinically. Secondly, HPV-epithelial interaction may produce lesions classified as precancerous. Viral oncogenic overexpression drives cell proliferation to produce a clonal expansion of undifferentiated cells, characterized clinically by persistent viral detection, persistent and advancing colposcopic abnormalities, and increasing risk of malignant transformation. These precancerous lesions are not reliably distinguishable by routine histology, regardless of the site of the lesion or the sex of the individual (74–77).
On the basis of specified principles of HPV-associated disease (Table 7.1) and issues related to terminology, a consensus process was sponsored by the College of American Pathologists (CAP) and the American Society for Colposcopy and Cervical Pathology (ASCCP). In 2012, the Lower Anogenital Squamous Terminology (LAST Project) published a comprehensive reevaluation of the terminology of HPV-associated lesions of the lower anogenital tract including the cervix, vagina, vulva, perianal area, anus, penis, and scrotum (59).
Table 7.1 General Principles Underlying the LAST Project
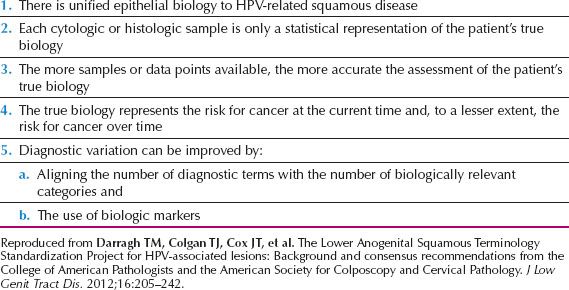
The LAST Project recommendations include the following:
1. There should be a unified histopathologic nomenclature with a single set of diagnostic terms. A two-tiered nomenclature was recommended for noninvasive HPV-associated squamous proliferations of the lower anogenital tract, which may be further qualified with the appropriate –IN terminology. (–IN refers to the generic intraepithelial neoplasia terminology without specifying location.)
2. HPV-associated squamous lesions of the lower anogenital tract should be classified as low-grade squamous intraepithelial lesion (LSIL) and high-grade squamous intraepithelial lesion (HSIL), which may be further classified by the –IN classification.
3. The biomarker p16 immunohistochemical (IHC) staining should be used when the hematoxylin and eosin (H&E) morphologic diagnosis is between precancer (–IN 2 or –IN 3) and a mimic of precancer (e.g., processes known to be unrelated to neoplastic risk, such as immature squamous metaplasia, atrophy, reparative epithelial changes, tangential cutting). Strong and diffuse block-positive p16 results would support a categorization of precancerous disease.
4. If the pathologist is entertaining an H&E morphologic interpretation of –IN 2 (under the old terminology, which is a biologically equivocal lesion falling between the morphologic changes of HPV infection and precancer), p16 IHC is recommended to help clarify the situation. Strong and diffuse block-positive p16 results support a categorization of precancer. Negative or nonblock-positive staining strongly favors an interpretation of low-grade disease or a non–HPV-associated pathology.
5. p16 IHC should be used as an adjudication tool for cases in which there is a professional disagreement in histologic specimen interpretation, with the caveat that the differential diagnosis should include a precancerous lesion (–IN 2 or –IN 3).
6. p16 IHC should not be used as a routine adjunct to histologic assessment of biopsy specimens with morphologic interpretations of negative, –IN 1, and –IN 3.
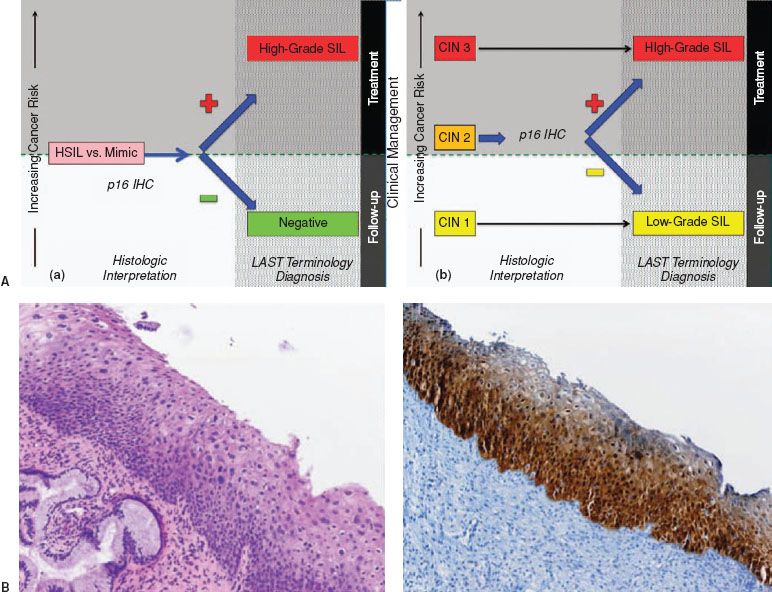
Figure 7.2 A: Pathologic diagnoses using p16 and potential clinical management options for cervical biopsies. (a) Use of p16 to evaluate the differential diagnosis of HSIL versus a mimic, such as immature squamous metaplasia and atrophy. (b) Use of p16 to evaluate morphologic CIN 2. The choice of clinical management for HSIL depends on the entire clinical scenario including patient’s age, colposcopic findings, and biopsy diagnosis. (Modified with permission. Courtesy of Philip E. Castle.) B: Cervical biopsy with SIL showing partial maturation, and a CIN lesion that is challenging to classify as low-grade or high-grade SIL. H&E morphology at medium power shows atypical parabasal-like cells extending into the middle third of the epithelium. Corresponding p16 IHC stains reveals diffuse strong staining meeting the definition of p16 strong diffuse block-positive. This case is best interpreted as HSIL. Reproduced from: Darragh TM, Colgan TJ, Cox JT, et al.; for members of the LAST Project Work Groups. The Lower Anogenital Squamous Terminology Standardization Project for HPV-associated lesions: Background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136:1266–1297.
Table 7.2 LAST Terminology and the Three-tiered CIN System in Cytologic and Histologic Diagnoses
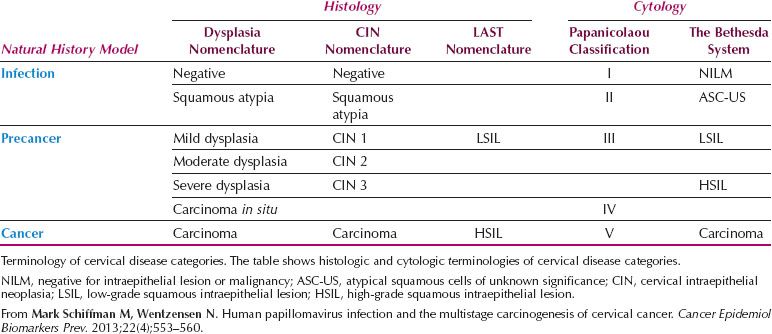
The LAST terminology for squamous HPV-associated lesions and associated p16 biomarker usage reflects modern clinical practice (Fig. 7.2A,B). If the LAST terminology is broadly accepted, squamous histology and cytologic reporting should be indistinguishable and consistent in the United States. Potential reconciliation of LAST terminology and the three-tiered CIN system in cytologic and histologic diagnoses is represented in Table 7.2.
Understanding the Cervical Transformation Zone
Embryogenesis
The cervix and vagina are derived from the müllerian ducts and are initially lined by a single layer of müllerian-derived columnar epithelium. At 18 to 20 weeks of gestation, the columnar epithelium lining the vaginal tube is colonized by the upward growth of stratified squamous epithelium derived from cloacal endoderm.
Original Squamo-columnar Junction
The junction in fetal life between the stratified squamous epithelium of the vagina and ectocervix, and the columnar epithelium of the endocervical canal is called the original squamocolumnar junction (78). Original squamous epithelium extends from Hart’s line or the mucocutaneous, vulvovaginal junction to the original squamocolumnar junction. The position of the original squamocolumnar junction is variable, lying on the ectocervix in 66%, within the endocervical canal in 30%, and on the vaginal fornices in 4% of female infants (79). The position of the original squamocolumnar junction determines the extent of cervical squamous metaplasia (79,80). Embryogenesis, in determining the distribution of native squamous and columnar epithelia, is an important early influence in determining future risk of neoplastic transformation (Fig. 7.3), although sexual behavior and subsequent exposure to HPV infection are the primary determinants of overall risk.

Figure 7.3 Location of squamocolumnar junction at various times in a woman’s life. CE, columnar epithelium; SE, squamous epithelium; SCJ, squamocolumnar junction.
New Squamocolumnar Junction
Increased estrogen secretion, particularly with puberty and with the first pregnancy, causes an increase in cervical volume and an eversion of endocervical columnar epithelium to an ectocervical location (79). This eversion of columnar epithelium onto the ectocervix is called an ectropion. An “ectropion” is often mistakenly referred to as an “erosion.”
The estrogen surge of puberty results in the establishment of lactobacilli as part of the normal flora of the vagina. These microorganisms produce lactic acid, reducing the vaginal pH to 4 or less (79). Everted endocervical columnar epithelium is exposed in the postpubertal years to the acidity of the vaginal environment. Damage to the everted columnar epithelium caused by vaginal acidity results in proliferation of a stromal reserve cell underlying the columnar epithelium. This replaces the columnar epithelium with an immature, undifferentiated, stratified, squamous, metaplastic epithelium (79,80). Immature squamous metaplasia then undergoes a maturation process, producing a mature, stratified squamous metaplastic epithelium, distinguishable only with difficulty from the original squamous epithelium.
The original linear junction between squamous and columnar epithelium is replaced by a zone of squamous metaplasia at varying degrees of maturation. At the upper or cephalad margin of this zone is a sharp demarcation between epithelium, which appears morphologically squamous, and villous epithelium, which appears colposcopically columnar. This colposcopic junction is called the new squamocolumnar junction.
The Transformation Zone
The transformation zone is defined as that area lying between the original squamocolumnar junction and the colposcopic new squamocolumnar junction (20,21). The initial clinical assessment for most women is in the postpubertal years, when mature squamous metaplastic epithelium has often replaced the distal or caudad limit of the columnar epithelium. As the transformation zone matures, the original squamocolumnar junction becomes impossible to delineate, and only the presence of nabothian follicles and gland openings hint at the original columnar origin of mature squamous metaplasia.
Cervical neoplasia almost invariably originates within the transformation zone (Fig. 7.4). For reasons that are poorly understood, persistent HPV infection causes cancers mainly at the transformation zone between different kinds of epithelia (e.g., cervix, anus, and oropharynx) (81). Carcinogenic HPV infection is equally common in the cervical and vaginal epithelia (82). However, cervical cancer is the third most common cancer among women worldwide, while vaginal cancer is rare. This reflects the pivotal importance of the metaplastic epithelium of the transformation zone in cervical carcinogenesis (83).

Figure 7.4 The anatomy of the transformation zone.
Research from Harvard Medical School has described an embryonic cell population within the transformation zone with specific morphologic and molecular features that may represent the cells of origin for most cervical precancers and cancers (84,85). Early in life, embryonic cervical epithelial cells are seen throughout the cervix. These cells subsequently diminish in number and concentrate at the SC junction in the adult. These cuboidal embryonic/SC junction cells have been demonstrated to give rise to subjacent metaplastic basal/reserve cells. This downward or basal (rather than upward or apical) evolution from progenitor cell to metaplastic progeny has been termed reverse or “top down” differentiation.
Figure 7.5 Colposcopy of immature squamous metaplasia. Note the tongues of pale grey squamous metaplastic epithelium growing over the fronds of native columnar epithelium.
A similar pattern has been noted in high-grade squamous intraepithelial lesions (HSILs), suggesting that HPV infection of the cuboidal SC junction cells may initiate outgrowth of basally-oriented neoplastic progeny. Most low-grade SILs are SC junction-negative, implying infection of metaplastic progeny rather than the original SC junction cells. This “model of “top down” differentiation may resolve the mystery of how SC junction cells remodel the cervix and participate in neoplasia. The model defines an alternative population of metaplastic progeny (including basal and reserve cells), the infection of which is paradoxically less likely to produce a biologically aggressive precursor. It also provides new targets in animal models to determine why the SC junction is uniquely susceptible to carcinogenic HPV infection” (85).
Squamous metaplasia is a permanent process but is not continuous. It occurs in “spurts,” with greatest activity during puberty and the first pregnancy. During the maturation phase, the columnar villi fuse, losing the distinctive appearance of columnar epithelium (Fig. 7.5) and producing a myriad of cytologic, colposcopic, and histologic appearances. The process fluctuates in response to hormonal influences, but ultimately produces a mature, glycogenated squamous epithelium. The presence of a subepithelial inflammatory infiltrate in biopsy specimens of immature squamous metaplasia may lead to a histologic misdiagnosis of chronic cervicitis (Fig. 7.6). The presence of such inflammatory white cells is a normal part of the metaplastic process and is not a response to an infectious organism. A histologic diagnosis of “chronic cervicitis” is often misleading and should not be accepted as a satisfactory explanation for an abnormal Papanicolaou (Pap) smear.
If the new squamocolumnar junction is seen in its entirety in the absence of premalignant disease, the incidence of squamous disease above the new squamocolumnar junction is very low and the colposcopic examination of the cervix is described as adequate in the current ASCCP Guidelines (86). If the new squamocolumnar junction is not seen in its entirety, the colposcopic examination is described as inadequate. The transformation zone further defines the distal limit of high-grade glandular intraepithelial neoplasia, which is the precursor lesion to invasive adenocarcinoma of the cervix.
Figure 7.6 Histology of immature squamous metaplasia (chronic cervicitis).
Upper Limit of Squamous Metaplasia
The new squamocolumnar junction is an unstable boundary. Serial colposcopic assessments of the cervix frequently show the new squamocolumnar junction to have moved cephalad. Careful colposcopic assessment of columnar villi immediately above the new squamocolumnar junction reveals opaque, opalescent tips, and early villous fusion (Fig. 7.5). Histologic study of colposcopically directed biopsy specimens reveals reserve cell hyperplasia and early immature squamous metaplasia occurring in epithelium, which appears colposcopically columnar. This early immature squamous metaplasia can extend as far as 10 mm above the new squamocolumnar junction.
The immature metaplastic epithelium cephalad to the new squamocolumnar junction is not included in the modern definition of the transformation zone, but represents the epithelium at greatest risk for future neoplastic transformation. During dynamic phases of metaplasia, occurring particularly with puberty and the first pregnancy, the immature metaplastic cells are actively phagocytic (79). The most critical phase is the initiation of squamous metaplasia at puberty and in early adolescence.
2011 IFCPC Colposcopic Terminology of the Cervix, Vagina, and Vulva
The International Federation for Colposcopy and Cervical Pathology (IFCPC) has recently released the latest colposcopic nomenclature for cervical and vulvar disease (Table 7.3), attempting to bring greater clarity to terminology in colposcopy practice (87,88). The most recent nomenclature has introduced a classification of transformation zone distribution, which shapes treatment of CIN lesions. It also includes vulvar and vaginal terminology for the first time.
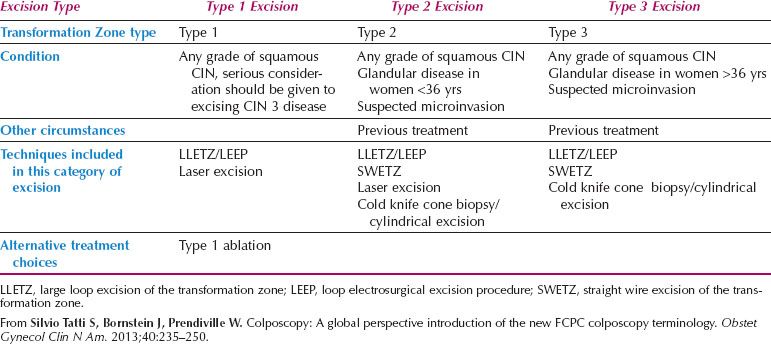
The formalization of a classification of the cervical transformation zone according to its distribution and location of the new squamocolumnar junction is of clinical value (89). A Type 1 Transformation Zone is fully visible with the new SCJ on the ectocervix (Fig. 7.7A). A Type 2 Transformation Zone is partially or totally endocervical but the new squamocolumnar junction is fully visible usually in the distal millimeters of the endocervical canal (Fig. 7.7B). A Type 3 Transformation Zone is partially or completely endocervical with the new squamocolumnar junction not fully visible as a result of its extension into the endocervical canal or the tightness of the canal (Fig. 7.7C). The location of the transformation zone, and whether the new SCJ is seen in its entirety, influences the diagnostic completion of the colposcopic examination and the method of treatment (Table 7.4). In the current nomenclature, three transformation zone excision types have been introduced (Fig. 7.8A–C). A fully visible small, ectocervical transformation zone is easy to assess and simple to treat either by destruction or simple excision. A large Type 3 Transformation Zone is not possible to completely assess colposcopically, as all or part of it is situated within the canal beyond colposcopic view. Treatment is more difficult and the risks of long-term morbidity and treatment failure are increased.
Table 7.3 2011 IFCPC Terminology of the Cervix
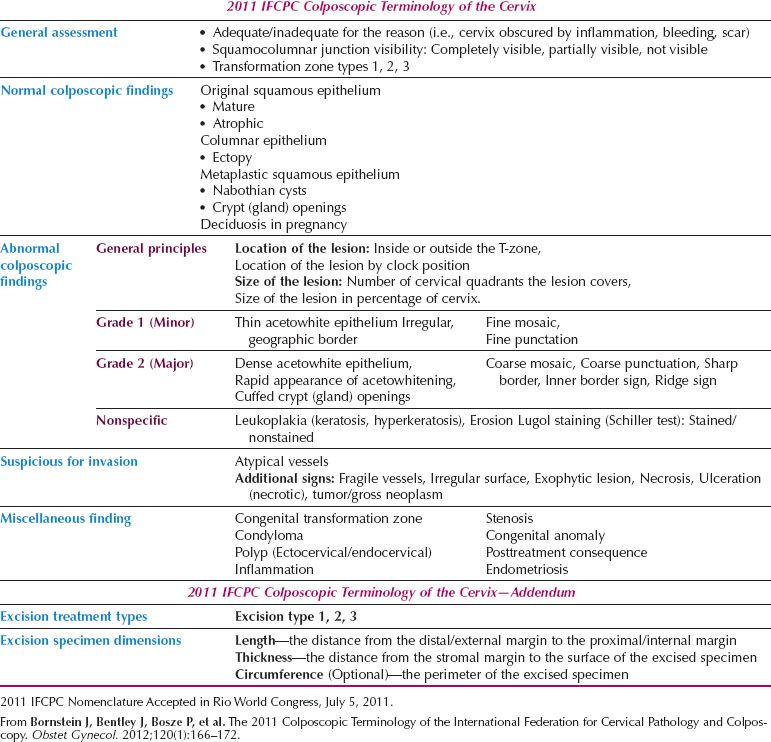
The current nomenclature emphasizes the importance of the colposcopic findings of an internal margin (Fig. 7.9) (90,91) and the raised, rolled margin described as a “ridge-sign” (92) (Fig. 7.10). Each is an integral component of the assessment of the lesion margin in the discrimination of high-grade disease within the Reid Colposcopic Index (90) and they are highly sensitive colposcopic predictors of HSIL.
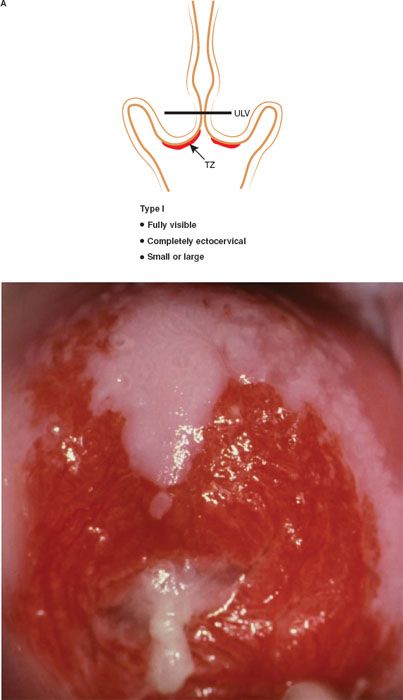
Figure 7.7 A: The Transformation Zone Classification—Type 1 TZ.
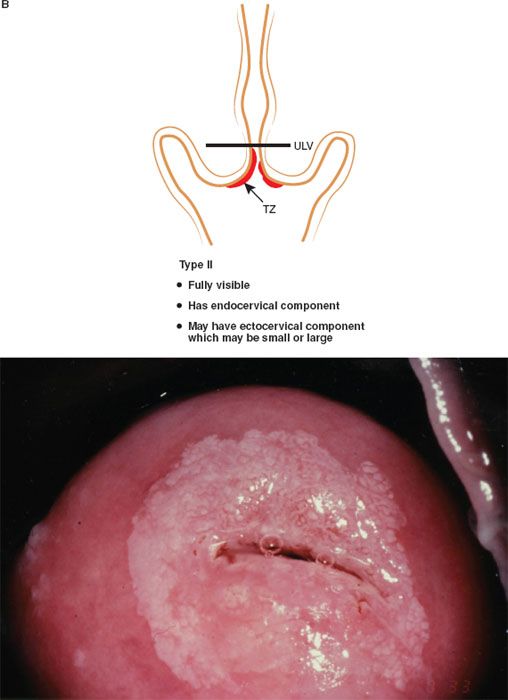
Figure 7.7 B: The Transformation Zone Classification—Type 2 TZ.
Human Papillomaviruses and Cervical Neoplasia
Extensive molecular biologic and epidemiologic research has confirmed certain HPV types to be carcinogenic in humans (9,64,65,77,93–99). The four major steps in the development of cervical cancer are (i) infection of the metaplastic epithelium of the transformation zone with one or more carcinogenic HPV types; (ii) viral persistence rather than clearance; (iii) progression of persistently infected epithelium to cervical precancer (CIN 3) (potentially reflecting the host immune response and/or exposure to the confirmed cofactors for HPV progression, which include multiparity, age at first full-term pregnancy, use of oral contraceptives, and current tobacco exposure) (51,100–103); and (iv) invasion.
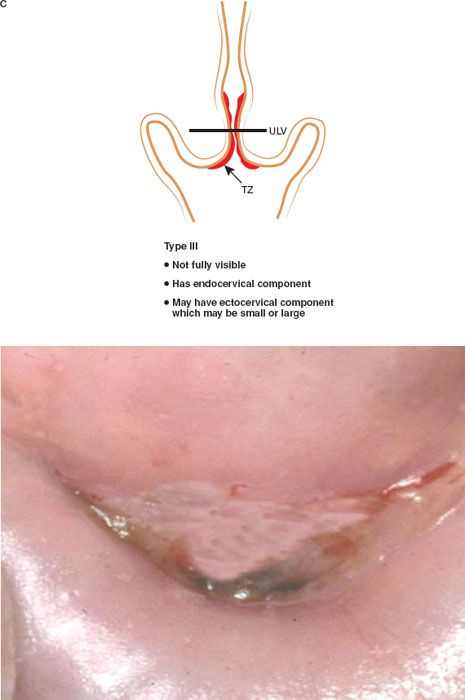
Figure 7.7 C: The Transformation Zone Classification—Type 3 TZ.
Table 7.4 2011 IFCPC Classification of Types of Excisional Procedures for Cervical Disease Based on Type of Transformation Zone
Taxonomy and Biology
Papillomaviruses are small, nonenveloped, double-stranded DNA viruses encased in a 72-sided icosahedral protein capsid. The HPV genome consists of circular, double-stranded DNA of approximately 7,900 nucleotide base pairs. Papillomaviruses are a divergent group of evolutionarily related viruses with similar biologic characteristics but enormous differences in species specificity, site of predilection, and oncogenic potential (104,105).
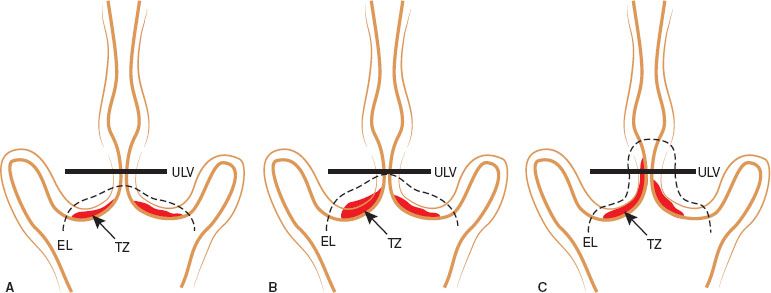
Figure 7.8 A: Type 1 Excision resects a completely ectocervical or type 1 Transformation Zone. The large loop excision of the TZ (LLETZ) procedure need not encroach the endocervical canal nor be greater than 8 mm thick throughout the resection. The excision margin is depicted by a dashed line. B: A Type 2 excision resects a Type 2 Transformation Zone (has an endocervical component but is fully visible). The excision margin is depicted by a dashed green line. C: A Type 3 excision resects a Type 3 Transformation Zone. A longer and larger amount of tissue of tissue is resected. The excision margin is depicted by a dashed green line. Adapted from Silvio Tatti S, Bornstein J, Prendiville W. Colposcopy: A global perspective introduction of the new IFCPC colposcopy terminology. Obstet Gynecol Clin N Am. 2013;40(2):235–250.

Figure 7.9 Colpophotograph of cervix of 25-year-old women with low-grade ectocervical SIL, an internal margin with a high-grade central lesion revealing an atypical vessel at a focus of microinvasive cancer.
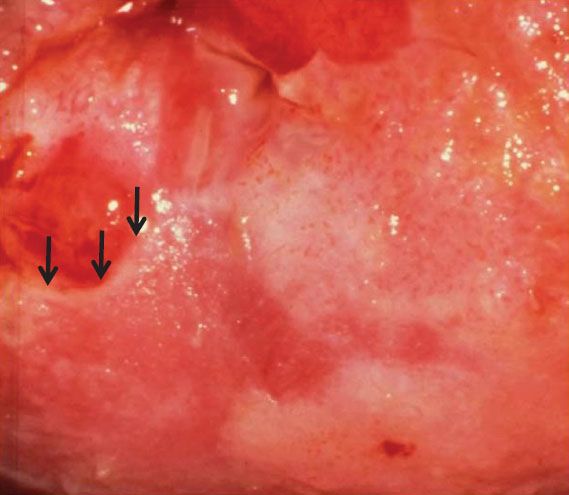
Figure 7.10 Colpophotograph of cervix of a 33-year-old woman with a high-grade SIL showing a “ridge” sign or raised, rolled, peeling margin at an area of early invasion.
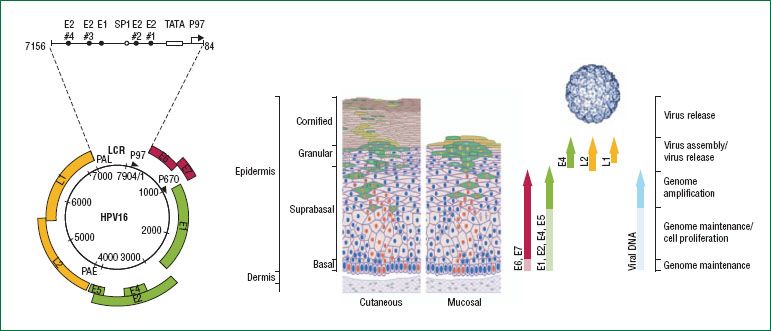
Figure 7.11 The HPV genome and its expression within the epithelium. The HPV genome consists of approximately 8,000 base pairs of single-stranded, circular DNA. HPV genes are designated as E or L according to their expression in early or late differentiation stage of the epithelium: E1, E2, E5, E6, and E7 are expressed early in the differentiation, E4 is expressed throughout, and L1 and L2 are expressed during the final stages of differentiation. The viral genome is maintained at the basal layer of the epithelium, where HPV infection is established. Early proteins are expressed at low levels for genome maintenance (raising the possibility of a latent state) and cell proliferation. As the basal epithelial cells differentiate, the viral life cycle enters successive stages of genome amplification, virus assembly and virus release, with a concomitant shift in expression patterns from early genes to late genes, including L1 and L2, which assemble into viral capsid. (Reproduced with permission from Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370;890–907.)
More than 100 types of HPV have been fully sequenced. HPV types are divided into phylogenetic trees, based on their DNA sequence and protein homologies. This assists in understanding HPV classification and behavior (106). The oncogenic HPV types infect the epithelium of the anogenital and oral tracts and are generally acquired through sexual contact.
Genetic sequencing has defined clades of viruses, which produce similar pathology (105). Viruses of clades alpha-7 and alpha-9 are most commonly associated with anogenital cancers and HPV 16 in particular (an alpha-9 species) appears to be a “uniquely powerful human carcinogen” (106), which is implicated in approximately 50% of cervical cancers and the majority of HPV-related cancers at other anogenital and oropharyngeal sites.
The HPV genome is usually maintained as a stable viral episome, independent of the host cell genome, in the nucleus of infected cells. It codes for only eight genes (107). In some high-grade CIN lesions, and more frequently in cervical cancer, HPV genomes are covalently bonded or integrated into the host chromosomes (108–112). This integration event involves the E1 and E2 genes with important consequences for regulation of viral gene expression (Fig. 7.11) (113). The late genes, L1 and L2, the sequences of which are highly conserved among all papillomaviruses, encode the common capsid proteins. These viral proteins reflect late viral gene expression and are exclusively present in well-differentiated keratinocytes (114). Both proteins play an important role in mediating efficient virus infectivity.
The proteins encoded by the E6 and E7 genes of high-risk HPV types, particularly HPV 16 (clade alpha-9) and 18 (clade alpha-7), are directly involved in cellular transformation in the presence of an active oncogene (115). E6 and E7 are the primary HPV oncoproteins with numerous cellular targets (116,117). Both E6 and E7 proteins can immortalize primary keratinocytes from cervical epithelium and influence transcription from viral and cellular promoters (118). The activity of these viral oncoproteins results in genomic instability, leading to the malignant phenotype. E6 proteins of high-risk HPV types bind the tumor suppressor protein p53 (119,120). This induces ubiquitination and degradation of p53, removing the p53-dependent control of the host cell cycle (121–123). The role of E6 as an antiapoptotic protein is of key significance in the development of cervical cancer.
E6 increases telomerase activity in keratinocytes through increased transcription of the telomerase catalytic subunit gene (hTERT) via induction of c-myc (124,125). Telomerase activity is usually absent in somatic cells, leading to shortening of telomeres with successive cell divisions and to eventual cell senescence. E6 mediation of telomerase activity may predispose to long-term infection and the development of cancer. E6 and E7 viral oncogenes have been shown to antagonize BRCA-mediated inhibition of the hTERT promoter (126).
The E7 gene product is a nuclear phosphoprotein that associates with the product of the retinoblastoma gene (pRb), which is a tumor suppressor gene important in the negative control of cell growth (127–129). E7 is the primary transforming protein. Degradation of p53 by E6 and the functional inactivation of pRb by E7 represent the main mechanisms whereby expression of HPV E6 and E7 oncoproteins subverts the function of the negative regulators of the cell cycle (130–132). Deregulated expression of the viral oncogenes is a predisposing factor to the development of HPV-associated cancers.
The products of the E2 gene are involved in transcriptional regulation of the HPV genome. The process of HPV integration into the cellular genome, which occurs in some high-grade CIN lesions and most invasive cervical cancers, disrupts the E2 gene (133). This results in increased levels of E6 and E7 expression, correlating with increased immortalization activity (133–136).
Aberrant expression of high-risk viral oncogenes can predispose to the development of cervical cancer, but their expression alone is not sufficient (107). HPV-mediated oncogenesis requires accumulation of additional genetic mutations over time. The median age of women with invasive cervical cancer is approximately 45 to 50 years in an unscreened population, whereas the median age for women with CIN 3 is often under 30 years. This suggests a long precancerous state, which allows the accumulation of secondary genetic mutations. These mutations can occur randomly but may also reflect the influence of cofactors such as tobacco carcinogens and exogenous and endogenous hormonal influences (65).
Human Papillomavirus Type–Specific Disease Pattern
Over 100 HPV types have been identified, but 15 anogenital types are often termed “oncogenic”; these include HPV16, the most frequently involved, HPVs 18, 45, 31, 33, 35, 52, and 58, which are the next most commonly identified in cancer, and a further seven types with lower level and less certain contributions (HPVs 51, 56, 39, 59, 68, 73, and 66) (106) (Fig. 7.12).
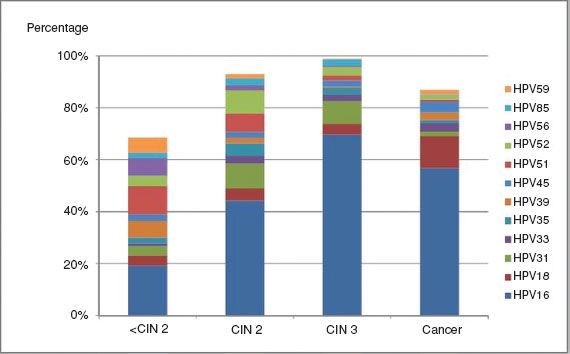
Figure 7.12 Attribution of carcinogenic HPV types to cervical disease categories. Expanded from the work of Wentzensen et al. The type attribution is based on the hierarchical attribution model for carcinogenic genotypes present in multiple infections. HPV genotyping is based on concurrent cytologic specimens, not on tissue specimens. From Mark Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(4):553–560.
Low-risk HPV types, particularly HPVs 6 and 11 (clade alpha-10), are associated with condylomata acuminata of the genital tract in both sexes. HPVs 6 and 11 are detected alone in low-grade cervical lesions (exophytic condylomata acuminata, subclinical HPV infection, CIN 1, and some CIN 2 lesions). These viruses are unable to integrate into the human genome. The E6 and E7 proteins of “low-risk” HPV types only weakly bind p53 and pRb, and thus do not immortalize keratinocytes in vitro.
Human papillomavirus 16 is the HPV type universally detected with greatest frequency in HPV-related invasive cancers. HPV16 is associated with 50% of cervical squamous cancers and about the same proportion of adenocarcinomas (137–140). It is present in a high proportion of high-grade cervical, vaginal, vulvar, perianal, and penile preinvasive lesions.
HPV18 is the second most common (20–25%) HPV type in invasive cervical cancer and is associated with the development of a substantial proportion of cervical adenocarcinomas. Organized cytologic screening programs have not been as effective against adenocarcinomas, rates of which have remained stable in some settings, while squamous cancers have declined (141).
Human Papillomavirus and Cervical Cancer: A Causal or Casual Association?
In the prevaccination era, most sexually active women were exposed to HPV infection (142) but the majority of exposed women cleared a specific HPV-type within 2 years (143,144). Humoral and cellular immune responses to natural infection with genital HPV types are inconsistently detected, possibly because the virus is nonlytic to infected cells and does not spread systemically. Secondary peaks of HPV infection in older and postmenopausal women have suggested the possibility of reactivation of a latent viral reservoir caused by senescence of cell-mediated immunity, although this could also potentially be explained by sexual behavior (of women or partners).
The longer a specific HPV type persists in the epithelium, the lower the probability of clearance within a defined period, and the greater the risk of precancer development (144). HPV type is the strongest factor affecting risk of viral persistence (66). A number of longitudinal studies have documented the long term risk of cervical precancer and cancer according to HPV type at baseline (47,48,67,145,146). Women exposed to HPV 16 are consistently documented to be at elevated risk. One of the longest reported follow-up periods has been for the Kaiser Permanente cohort in the USA, where the 16-year risk of developing CIN 3+ in women aged under 30 years was 14.6% (95% CI: 10 to 20.9) for women with HPV 16 at baseline; 7% (4.2 to 11.4), for women with other oncogenic HPV types, and 1.8% (1.2 to 2.5) for women with no HPV infection. For women over 30 years, the risks were 8.5% (95% CI: 4.1 to 17.2) for HPV 16, 3.1% (1.6 to 6.1) for other oncogenic types, and 0.7% (0.5 to 0.9) for HPV-negative women (47).
High viral loads do not generally imply an increased risk of progression, except for HPV16 (147,148). Recently acquired low-grade cervical lesions contain some of the highest viral loads, analogous to condylomata acuminata, and frequently regress (149). In general terms, viral load measurement is not clinically useful (9).
The median time from HPV infection to CIN 3 is short, often within 5 years (150). Infection occurs in the late teens or early twenties after the initiation of sexual activity (in unvaccinated populations) and the diagnosis of CIN 3 peaks at 25 to 30 years (138,150) or 20 to 24 years in some populations (141). CIN 3 has been diagnosed within 2 years of coitarche, and CIN 2 to 3 has been documented to rapidly develop within several months of an incident HPV infection (151,152). The biologic significance and risk of invasion associated with these early CIN 3 lesions is uncertain, but cytologic screening in women under 25 years is of limited effectiveness (153), and the International Agency for Research on Cancer (IARC) recommends starting screening at 25 years (3). The transit time from CIN 3 to invasive cancer is variable, but long-term follow-up from an unethical experiment in women managed only by punch or wedge biopsy has suggested that about 30% of cases of CIN 3 will progress to invasive cancer over 30 years (61). The major steps in the development of cervical cancer are summarized in Figure 7.13.
Cofactors in the Progression of Cervical Human Papillomavirus Infection
The established cofactors in progression of HPV infection to invasive cancer are the use of tobacco, multiparity, age at first full term pregnancy, and use of oral contraceptives (100–103). Host genetic factors influencing HPV infection control exist, but are poorly understood. There is a consistent human leucocyte antigen (HLA) association, reflecting the importance of T-cell responses in control of HPV infection and cervical cancer precursors (154). An earlier association between condom use and decreased persistence of high-risk and low-risk HPV types among high-risk HPV-positive women has been confirmed (155,156). Increased HPV clearance and low-grade CIN regression with condom use has been reported (157).
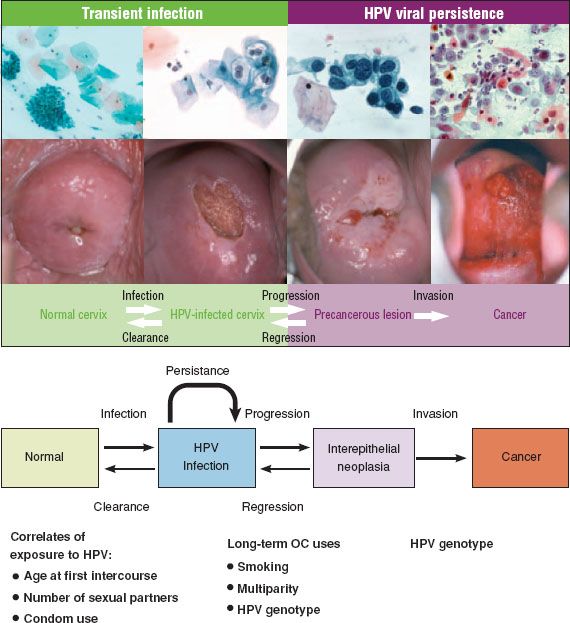
Figure 7.13 Major steps in the development of cervical cancer. Incident HPV infection is best measured by molecular tests. Most HPV infections show no concurrent cytologic abnormality. Approximately 30% of infections produce concurrent cytopathology, usually nonclassical (equivocal) changes. Most HPV infections clear within 2 years. Ten percent persist for 2 years and are highly linked to development of precancer. Top image reproduced with permission from Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907; Bottom image reproduced with permission from Mark Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(4);553–560.
Tobacco Use
Cigarette smoking has been demonstrated to be a risk factor for squamous cervical and vulvar carcinoma (100,158–163). An increased risk of developing a high-grade squamous intraepithelial lesion (HSIL) has been demonstrated among high-risk HPV positive women who smoke. It is uncertain whether smoking acts via an immunosuppressing or genotoxic pathway. The detection of high levels of genotoxic breakdown products of cigarette smoke—including nicotine, cotinine, hydrocarbons, and tars—in cervical secretions of smokers and the demonstration of mutagenic activity of these products in cervical cells, similar to that observed in lung cells, point to an important role for these compounds in cervical carcinogenesis.
Cigarette smoking influences epithelial immunity by decreasing the numbers of antigen-presenting Langerhans cells in the genital epithelium (164,165). Cervical HPV infection and CIN are associated with diminished numbers of intraepithelial Langerhans cells. Such local immunologic depletion could favor viral persistence, contributing to malignant transformation. Cigarette smoke concentrates have been demonstrated in vitro to transform HPV 16–immortalized endocervical cells (160). However, though squamous cervical cancer and adenocarcinoma share hormonal risk factors (increasing parity, younger age at first full-term pregnancy, and increasing duration of hormonal contraceptive use), smoking does not appear to be a risk factor for adenocarcinoma (103).
Sex Hormonal Influences
Condylomata acuminata may increase rapidly in size and number in pregnancy. This could suggest that maternal estrogen status is permissive for HPV replication, although it may reflect the immunosuppressive effect of pregnancy. Increased detection of HPV DNA in cervical cytologic samples in pregnancy, including detection of oncogenic HPV types in up to 27% of pregnant women, suggests hormonally induced active viral replication (166,167).
A pooled analysis of worldwide data has found that the relative risk of invasive cervical cancer for first full-term pregnancy under 17 years compared with 25 years or older is 1.77 (95% CI: 1.42 to 2.23). Independently of age at first full term pregnancy, parity has been shown to be a significant factor in the development of cervical cancer, the relative risk among parous women being 1.76 (95% CI: 1.53 to 2.02) for seven or more full-term pregnancies, compared with one or two (101).
The International collaboration of Epidemiologic Studies of Cervical Cancer has pooled the worldwide data and identified an increase in the relative risk of cervical cancer in current users of oral contraceptives, which declines after use ceases. Use for 10 years between the ages of 20 and 30 increased the cumulative incidence by age 50 from 7.3 to 8.3 per 1,000 women in less developed countries, and from 3.8 to 4.5 in more developed countries (102).
Exogenous and Endogenous Immunosuppression
Iatrogenic induction of immunosuppression in renal transplant recipients increases the rate of CIN to 16 times that of the general community (168). The risk of CIN and cervical cancer is increased in human immunodeficiency virus (HIV)–infected women and failure rates of treatment for preinvasive lesions are increased (169–173). Systemic immune suppression from diseases such as Hodgkin disease, leukemia, and collagen vascular diseases are associated with an increased incidence of HPV-associated disease (172,174).
Human Papillomavirus Vaccines
Over the last several years, prophylactic vaccination against HPV in young females has been introduced in most developed countries. The introduction of this intervention has been supported by evaluations of its cost-effectiveness, even in the context of cervical screening (175). Two vaccines are currently available—quadrivalent vaccine- Gardasil (Merck, USA) and bivalent vaccine- Cervarix (GSK, Belgium). These protect against HPV 16/18, together responsible for about 70% of invasive cervical cancers (176). HPV (predominantly HPV 16) has also been identified in varying fractions of vulval, vaginal, anal, penile, and oropharyngeal cancers (1) and thus the vaccines have the potential to prevent a proportion of these cancers. The quadrivalent vaccine also protects against HPV6/11 which is found to be associated with approximately 90% of anogenital warts.
HPV vaccination has been shown to be effective in preventing persistent infection and high-grade precancerous cervical intraepithelial neoplasia (CIN 2–3) in females naïve to HPV vaccine types (177) and at preventing persistent infection, external genital lesions, and anal intraepithelial neoplasia in males (178,179). In most countries, immunization programs have adopted the quadrivalent vaccine. Because the number of HPV-related cancers are lower in males than in females and heterosexual males benefit to some extent from female vaccination via herd immunity, inclusion of young males in vaccination programs is associated with a lower return on investment than female-only vaccination, especially if coverage in females is over 50%, because this increases the herd-immunity–induced protection to males (175,180,181).
Male vaccination has now been recommended in a few settings, including Australia, where publicly funded vaccination of 12- to 13-year-old boys commenced in 2013, with a 2-year catch-up to 14 to 15 years. It has been recommended in the USA, Canada, and Austria, but is not recommended in most other countries (175).
The HPV vaccine is a major scientific and public health advance in the prevention of HPV related cancer (182,183). HPV prophylactic vaccines, designed to prevent HPV infection, are based on virus-like particle (VLP) technology developed through the pioneering research of Zhou and Fraser in Brisbane, Australia, by Schiller and Lowy at the National Institutes of Health USA and by others (182–185). These DNA-free VLPs are empty capsids and contain no oncogenic or infectious materials. VLPs resemble the virus immunologically, and induce HPV type-specific antibody on administration (186,187). The immunogenicity of HPV involves presentation of the major capsid protein L1 to the immune system. L1 VLP vaccines induce strong, cell-mediated and humoral immune responses (188–191).
Vaccine Efficacy
Clinical trials have demonstrated that HPV vaccines are effective and safe (177,186–189,192,193). For ethical and scientific reasons, surrogate end points in efficacy trials have consisted of prevention of HPV acquisition and of persistent infection, development of high-grade precancerous lesions (CIN 2–3), and development of genital neoplasia and genital warts (as opposed to development of cervical cancer). Studies have been largely undertaken among sexually active women 16 to 25 years of age, although some studies have been extended to include women up to 45 years. Immunogenicity bridging studies have been carried out in young females and males aged 10 to 15 years, where antibody levels produced are substantially higher than in 16- to 23-year olds. A larger proportion of this population has been previously exposed to HPV, so that at a population level, effectiveness is lower. For population effectiveness, and thus for cost-effectiveness reasons, vaccination of women aged over 26 years has not been routinely recommended within vaccination programs. However, novel “screen-and-vaccinate” strategies might be possible in settings where a transition to primary HPV based screening is taking place, because women negative for particular HPV types could potentially be offered vaccination after screening. The cost-effectiveness of these new options requires detailed consideration.
In strict protocol studies, where only women naïve to the HPV types of interest have been considered, both the bivalent and quadrivalent vaccines have demonstrated efficacy approaching 98–100% in preventing high-grade cervical lesions among young sexually active women where the disease endpoints are associated with the vaccine-included types (177,182,183,186,192–199). In trial analyses that have included less strenuously defined criteria, such as including women with known infection or with disease associated with vaccine types prior to vaccination (and which are thus more likely to reflect actual population exposure in the relevant age group), vaccine efficacy is reduced. For example, the quadrivalent vaccine has been shown to be 44% effective in preventing CIN 2–3 associated with HPV 16 or HPV 18, in women aged 15 to 26 years (177).
No therapeutic effect has been demonstrated in women with vaccine-type existing HPV infection or HPV-related disease, with lesions regressing or progressing at similar rates in vaccine and placebo recipients (200). Follow-up studies of women have demonstrated sustained efficacy for up to 10 years. Three doses of vaccine induce peak antibody levels many times higher than those seen with natural HPV infection (182). Antibody levels fall significantly in the first 2 years after immunization, but remain above those stimulated by natural infection (201). A modeling study has suggested that antibody levels will remain above those associated with natural infection for 12 years or more (202). Immunologic memory is retained, and a single booster dose given 60 months postcompletion of the HPV vaccination protocol has been shown to produce a strong anamnestic increase in antibody titers, not seen in nonimmune subjects, with continued sustained efficacy typical of many vaccines (201). There are indications that sustained vaccine-induced protection may be maintained with fewer than three doses (203–205), and if confirmed, this has potential to reduce the cost of HPV vaccination (and thus to increase its cost-effectiveness), which will be of considerable importance in low resource settings. A recent Canadian trial found that the immunogenicity (geometric mean titres) for HPV 16 and HPV 18 one month after the last vaccine dose of a two-dose schedule at 0 and 6 months in girls 9 to 13 years was statistically noninferior to the immunogenicity in women receiving three doses (205). However, longer-term follow-up, more data on protection against disease endpoints for all HPV-related disease, and more extensive data in different age groups, will likely be needed before a recommendation to decrease the number of doses in routine vaccination schedules can be made (206). Immunization with HPV 16 and 18 VLPs may provide some protection against other high-risk HPV types and development of associated disease, although the duration and degree of any cross-protection is not yet clear.
A next generation nonavalent vaccine from Merck is scheduled for market release (207). It will be based on the VLP technology used for the quadrivalent vaccine and will provide broad spectrum protection against HPV types responsible for 89% of cervical cancers by adding VLPs for types 31, 33, 45, 52, and 58 (208).
The nonavalent vaccine is currently in Phase III trials. There is uncertainty about whether high levels of efficacy against HPV 16/18 will be maintained, since increasing the valency may result in decreased effectiveness of the vaccine to individual HPV types through immune interference (209).
Vaccine Safety
No serious adverse effects attributable to vaccination have been seen in placebo controlled trials (177,192). Local reactogenicity at the immunization site, systemic malaise and fever have been slightly more common than with placebo, but have not led to discontinuation of the vaccination schedule. Since vaccine licensure, over 170 million doses of HPV vaccine have been given to young women. The product labeling has been revised to address issues for monitoring for anaphylaxis and syncope, outcomes previously identified as concerns (210).
A recent large scale data linkage study of approximately 300,000 girls vaccinated with quadrivalent vaccine in Sweden and Denmark compared them with almost 700,000 unvaccinated girls born from 1988 to 2000. It concluded that there was no evidence supporting associations between exposure to the vaccine and autoimmune, neurologic, or venous thromboembolic adverse events, and that although associations for three autoimmune events were initially observed, on further assessment these were weak and not temporally related to vaccine exposure (211).
A 2013 review of the worldwide experience by the WHO’s Global Advisory Committee on Vaccine Safety (GAVCS) involved review of updated information about the safety of HPV vaccines. It concluded, “In summary, 4 years after the last review of HPV vaccine safety and with more than 170 million doses distributed worldwide and more countries offering the vaccine through national immunization programs, the Committee continues to be reassured by the safety profile of the available products” (210). Although vaccination in pregnancy is not recommended, HPV vaccination does not appear to be associated with increased rates of spontaneous pregnancy loss or adverse fetal outcomes (210,212,213).
Vaccine Deployment
The greatest public health benefit is achieved by vaccination of individuals prior to sexual initiation, as the vaccines are prophylactic (214). The US Advisory Committee on Immunization Practices (ACIP) has recommended routine vaccination of all 11- to 12-year-old girls with the quadrivalent HPV vaccine, and “catch-up” vaccination of all 13- to 26-year-old girls and women (215). Vaccination of 9- to 10-year-old girls should be at the providers’ discretion.
Vaccination of young sexually active women may still provide some protection. Routine cytology or HPV DNA testing prior to vaccination is not currently recommended, although such screening may be appropriate for sexually active women as part of cervical screening practices. Cervical cancer screening should continue for the immunized population, to screen for disease caused by nonvaccine HPV types, and to screen HPV infected women, as the vaccine is not therapeutic.
The optimal vaccination-dosing schedule is three doses at 0, 2, and 6 months. Accelerated delivery schedules over 4 months are being used in some countries. The US Advisory Committee on Immunization Practices (ACIP) has recommended (215):
1. First and second doses must be separated by at least 4 weeks.
2. Second and third doses must be separated by at least 12 weeks.
3. If the dosing schedule is interrupted, the vaccine series should not be restarted, but the required dose should be given as soon as possible.
The vast majority of studies examining the cost-effectiveness of vaccination of preadolescent females suggest that vaccinating 12-year-old girls against HPV is cost-effective, even in the context of cervical screening (175). Cost savings are achieved by reducing abnormal Pap smears, colposcopy referrals, cervical biopsies, treatment procedures, and cancer-related treatment costs, as well as reducing the costs of diagnosis and treatment of genital warts. Reductions in these outcomes also increase quality of life, measured in cost-effectiveness analyses as Quality-Adjusted Life-Years Saved (QALYS). Vaccination will potentially reduce the costs and morbidity associated with the complications of treatment procedures among young women (216,217), although the association between cervical treatment and obstetric complications has not been confirmed in all studies and settings (218).
Some clinical trials have shown high levels of effectiveness of HPV vaccination in men (179). The decision regarding vaccination of men is more complex, because HPV causes fewer cancers in men, and vaccinating women offers some protection to men via herd immunity. Therefore male HPV vaccination may not represent the best “value for money” compared to vaccinating more females or making other investments in health. This determination in a particular setting will depend on vaccine price, coverage in females, achievable coverage in males, rates of HPV-related disease in males, and other factors.
HPV Vaccine Experience
Coverage rates achieved for vaccination of young females vary widely between countries. The highest coverage rates have been achieved where national publicly funded school-based vaccination programs have been introduced, including England (80%), Scotland (90%), and Australia (73%) (175). By contrast, coverage rates in countries that have implemented practitioner-based vaccination programs are lower; for example, in preadolescent girls in the USA, reported rates have been less than 50% (219).
Australia was the first country to introduce a national HPV vaccination program and it has achieved relatively high coverage rates in adolescent girls (220). In Australia, a 2-year vaccination catch-up was performed to age 26 years. The recommended age of first screening is 18 to 20 years in sexually active women, and participation rates in cervical screening are relatively high. This overlap between vaccinated and screened groups has led to early effects on the number of high-grade lesions identified through screening. An early postvaccination ecologic analysis identified a decreasing incidence of high-grade disease in women less than 18 years (221). For women under age 20 years, a decrease in CIN 2–3 from 7.8 to 7.1 per 1,000 women screened (9%) was observed between 2010 and 2011, corresponding to a total decrease from 13.2 to 7.1 (46%) between 2006 and 2011 (141). For women 20 to 24 years, rates were stable until 2010, and then decreased from 19.7 to 17.4 (12%) between 2010 and 2011 (141). For the first time since the introduction of organized screening more than 20 years previously, the peak age for confirmed CIN 2–3 in Australia has shifted from 20 to 24 to 25 to 29 years.
Australia uses the quadrivalent vaccine in its national program and a number of studies have documented substantial decreases in anogenital warts in Australia in cohorts of females who were eligible for HPV vaccination. Studies have documented lesser, but still substantial, reductions in anogenital warts in heterosexual males in the same age group, presumably as a result of herd immunity from female vaccination (222,223). By contrast, no substantial postvaccination changes in rates of anogenital warts in young men who have sex with men (MSM) or older women have been documented, which is consistent with the expected effect of female HPV vaccination.
In low resource settings, the Global Alliance for Vaccines and Immunization (GAVI) has announced that it will make the HPV vaccine available to females for under $5 per dose. It is hoped that this will drive increased vaccine utilization in some of those settings with the greatest need because of the high burden of HPV-related disease and the lack of organized cervical screening programs.
Screening for Cervical Neoplasia
Incidence and mortality rates for cervical cancer in the United States have steadily decreased since the 1950s (224,225). Although the overall incidence of cervical cancer in Western countries was beginning to decline before the introduction of screening efforts, the significant decreases in cervical cancer incidence and mortality can be largely attributed to the success of widespread screening (3,4,226–228).
A single Pap smear has limited sensitivity for the detection of cervical cancer and precancer. Strategies used in the past to compensate for this limited sensitivity include repeat screening at short intervals (such as annual Pap smears) and a low threshold for repeat smears or referral for colposcopy (Fig. 7.14). This approach is costly and generates many unnecessary follow-up examinations and tests. Prior to the introduction of 2012 changes in the recommendations, expenditure in the United States totaled $6 billion annually for more than 50 million screening tests and for the ongoing clinical care of women with mainly minor cytologic abnormalities (229).

Figure 7.14 Sensitivity and specificity of screening test as reciprocal ratios.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree