Cervical cancer is the third most common cancer in women worldwide, after breast and colorectal cancer. Annual global estimates for the year 2008 were 530,000 new cases and 275,000 deaths (1). It is the most common cancer in women in Eastern Africa, South Central Asia, and Melanesia. In the United States, 12,340 new cases with 4,030 deaths were anticipated in 2013 (2). The median age at diagnosis is 48 years, and the majority of cases are diagnosed between the ages of 35 and 55 years, when women are in the prime of their lives; 0.2% are diagnosed under the age of 20 (3).
Cervical cancer progresses slowly from preinvasive cervical intraepithelial neoplasia (CIN) to invasive cancer, and screening asymptomatic women with regular Papanicolaou (Pap) smears allows diagnosis of the readily treatable preinvasive phase. Hence, appropriate screening programs are an important public health issue. In developed countries, most cases of cervical cancer occur in women who have not had regular Pap smear screening.
In low-resource countries, facilities for screening asymptomatic women are not readily available. In addition, cultural attitudes and lack of public education discourage early diagnosis. Hence, most patients in developing countries present with advanced disease that may have already invaded the bladder, rectum, pelvic nerves, or bone. Because radiation therapy and palliative care facilities are also usually inadequate in these countries, many of these women die as social outcasts, with severe pain and a foul-smelling vaginal discharge. Most of these women have dependent children, so the social devastation caused by this disease is enormous.
Molecular biology has firmly established a causal relationship between persistent infection with high-risk human papilloma virus (HPV) genotypes and cervical cancer. In a study of almost 1,000 cases of cervical cancer worldwide, the prevalence of HPV infection was 99.7% (4). This causal relationship has led to the opportunity for primary prevention through the development of vaccines. The quadrivalent vaccine Gardasil and the bivalent vaccine Cervarix have been available for almost a decade, and one or other has been introduced into the school immunization program in many countries. Secondary prevention, by screening directly for carcinogenic HPV, is likely to replace Pap smears for primary screening in older women. Sankaranarayanan et al. (5) reported a large randomized trial from India which showed that a single round of HPV testing dramatically reduced the incidence of advanced cervical cancer and cervical cancer mortality within 8 years, when compared to traditional screening methods.
Nearly 9% of women in the United States have been estimated to receive no therapy for their disease. This neglect is more common in older, unmarried women, who present with late-stage disease. Obstacles to treatment include lack of access to treatment facilities, inability to pay, and inadequate social support (6).
Diagnosis
Early diagnosis of cervical cancer can be extremely challenging because of three factors:
1. The frequently asymptomatic nature of early stage disease, particularly in women who are not sexually active
2. The origin of some tumors from within the endocervical canal or beneath the epithelium of the ectocervix, making visualization on speculum examination impossible
3. The significant false-negative rate for Pap smears, even in women having regular screening
Symptoms
Abnormal vaginal bleeding is the most common presenting symptom of invasive cancer of the cervix. This may include postcoital, intermenstrual, or postmenopausal bleeding. Unlike endometrial cancer, which usually bleeds early, cervical cancer often is asymptomatic until quite advanced in women who are not sexually active. Large tumors commonly become infected and a vaginal discharge, sometimes malodorous, may occur before the onset of bleeding. In very advanced cases, pelvic pain, pressure symptoms pertaining to the bowel or bladder, and occasionally vaginal passage of urine or feces may be presenting symptoms.
In a review of 81 patients diagnosed with cervical cancer in southern California, Pretorius et al. (7) reported that 56% presented with abnormal vaginal bleeding, 28% with an abnormal Pap smear, 9% with pain, 4% with vaginal discharge, and 4% with other symptoms.
Signs
Physical examination should include palpation of the liver, supraclavicular, and groin nodes to exclude metastatic disease. On speculum examination, the primary lesion may be exophytic, endophytic, ulcerative, or polypoid. If the tumor arises beneath the epithelium or in the endocervical canal, the ectocervix may appear macroscopically normal. Direct extension to the vagina is usually grossly apparent, but the infiltration may be subepithelial and suspected only on the basis of obliteration of the vaginal fornices or the presence of apical stenosis. In the latter situation, it may be difficult to visualize the cervix. On palpation, the cervix is firm (except during pregnancy) and usually expanded. The size of the cervix is best determined by rectal examination, which is also necessary for the detection of any extension of disease into the parametrium.
Cytology
The presence of malignant cells in a background of necrotic debris, blood, and inflammatory cells is typical of invasive carcinoma (Fig. 8.1). Differentiation between squamous and glandular cells is usually possible except for poorly differentiated lesions. The Pap smear is basically a screening test for asymptomatic women, and the false-negative rate for Pap smears in the presence of invasive cancer may be up to 50% (8).
Biopsy
Any obvious tumor growth or ulceration should undergo office punch biopsy or loop excision for histologic confirmation. Any cervix that is unusually firm or expanded should also undergo biopsy and endocervical curettage (ECC).
If the patient has a normal-appearing cervix but is symptomatic or has an abnormal Pap smear, colposcopy should be performed. If a definitive diagnosis of invasive cancer cannot be made on the basis of an office biopsy, diagnostic conization may be necessary.
Colposcopy for Invasive Cancer
If a carcinoma is entirely within the endocervical canal, the ectocervix may be colposcopically normal. Ectocervical microcarcinomas are classically associated with atypical vessels, which are prone to bleed. Atypical vessels show a completely irregular and haphazard disposition, great variation in caliber, and abrupt changes in direction, often forming acute angles (Fig. 8.2). The intercapillary distance is increased and tends to be variable (9).
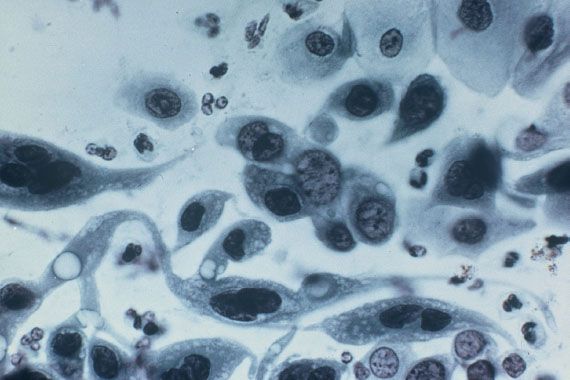
Figure 8.1 Pap smear of cervical squamous cell carcinoma. Malignant squamous cells, singly and in groups, show nuclear pleomorphism. A “tadpole” cell is present on the left. (Original magnification 165×.)
Frankly invasive cancers can usually be seen with the naked eye, but the colposcope highlights their surface irregularity and highly atypical, nonbranching, blood vessels (Fig. 8.3). Endophytic tumors may present as an “erosion,” the true nature of which can be recognized only by their papillary surface and atypical vessels. A keratotic surface may mask the colposcopic features of an endophytic lesion, so biopsy of areas of keratosis is mandatory.

Figure 8.2 Colposcopic appearance of microinvasive cervical cancer. Note the severe varicose vascular abnormality with course punctation and transitional forms to atypical vessels.
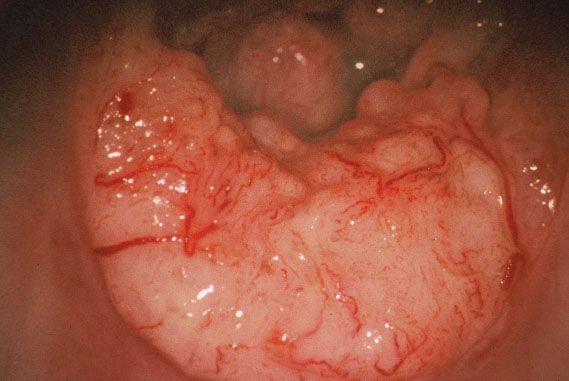
Figure 8.3 Colposcopic appearance of invasive cervical cancer. Note the surface irregularity and dilated atypical vessels.
Adenocarcinomas present no specific features. They often occur in association with squamous CIN, and all of the vascular changes described previously may be seen with these lesions.
Staging
Cervical cancer is staged clinically because most patients worldwide are treated only with radiation therapy.
In 2009, the FIGO Cancer Committee modified the staging (Table 8.1). The main changes from the 1994 FIGO staging were the removal of stage 0, and the division of stage IIA into IIA1 (tumor ≤4 cm) and IIA2 (tumor >4 cm). As expected, survival has been reported to be greater for IIA1 tumors (68% vs. 50%; p = 0.0015) (10).
Diagnostic imaging is now encouraged to assess tumor size. The ability of magnetic resonance imaging (MRI) to accurately determine not only tumor size, but also extension to surrounding organs, has made the need for examination under anesthesia (EUA), cystoscopy and sigmoidoscopy less frequent; they are now considered optional. A comparison of the FIGO staging and the TNM (tumor, nodes, metastasis) classification is shown in Table 8.2.
Clinical Staging
Clinical staging is often inaccurate in defining the extent of disease. The Gynecologic Oncology Group (GOG) (11), in a study of 290 patients with surgically staged cervical cancer, reported errors in FIGO clinical staging ranging from 24% in stage IB to 67% for stage IVA disease. Most patients were upstaged on the basis of surgical exploration, with the most likely sites of occult metastases being the pelvic and para-aortic lymph nodes. Other sites of occult disease were the parametrium, peritoneum, and omentum. As many as 14% of patients may be downstaged (12), typically because a benign pathologic process was discovered, such as pelvic inflammatory disease, endometriosis, or fibroids.
Noninvasive Diagnostic Studies
Because information about the extent of disease is critical for treatment planning, various imaging studies have been used to define more accurately the extent of disease.
Table 8.1 Carcinoma of the Cervix Uteri: FIGO Nomenclature (Capetown, 2009)

Computed Tomography
Computed tomography (CT) has been used to help stage pelvic cancers since the mid-1970s. In addition to the lymph nodes, a pelvic and abdominal CT scan allows an evaluation of the liver, urinary tract, and bony structures. A CT can detect only changes in the size of the nodes, those greater than 1 cm in diameter usually being considered positive. Normal-sized nodes containing microscopic deposits give false-negative results, whereas nodal enlargement from inflammatory or hyperplastic changes gives false-positive results. If nodes greater than 1.5 cm in diameter are considered positive, then the sensitivity of the test is improved at the expense of the specificity.
In a review of the literature, Hacker and Berek (13) reported that the overall accuracy for the detection of para-aortic lymph node metastases was 84.4%, with a false-positive rate of approximately 21% (9 of 41 positive readings) and a false-negative rate of approximately 13% (13 of 99 negative readings).
Magnetic Resonance Imaging
Because CT cannot discriminate between cancer and normal soft tissue of the cervix and uterus, it is limited in the evaluation of early cervical cancer. MRI, which has been used since the early 1980s, has high-contrast resolution and multiplanar imaging capability and is a valuable modality for determining tumor size, degree of stromal penetration, vaginal extension, corpus extension, parametrial extension, and lymph node status (Fig. 8.4).
Subak et al. (14) evaluated CT or MRI before surgical exploration in 79 patients with FIGO stage IB, IIA, or IIB cervical carcinoma. They reported that MRI estimated tumor size to within 0.5 cm of the surgical specimen in 64 of 69 patients (93%) and had an accuracy of 78% for measuring depth of stromal invasion. By contrast, CT was unable to evaluate tumor size or depth of invasion. For the evaluation of stage of disease, MRI had an accuracy of 90% compared with 65% for CT (p < 0.005), and it was more accurate in assessing parametrial invasion (94% vs. 76%; p < 0.005). Both modalities were comparable for the evaluation of lymph node metastases (each 86% accurate). Narayan et al. (15) from Melbourne reported that the cervical diameter determined by EUA correlated poorly with the MRI diameter, but the MRI diameter correlated strongly with the pathologic diameter after surgical removal of the specimen (p < 0.0001).
Table 8.2 Carcinoma of the Cervix Uteri: UICC Stage Grouping

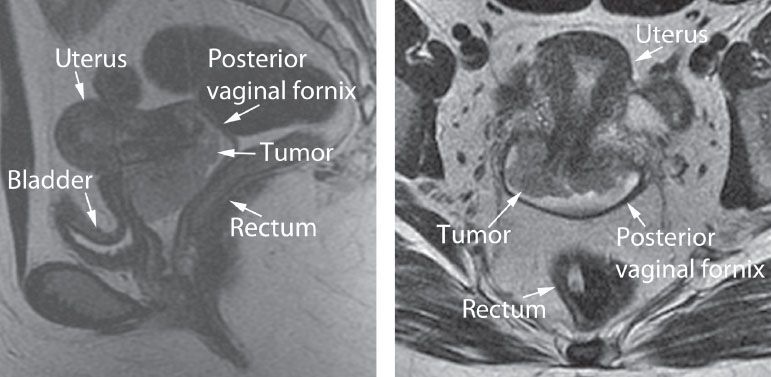
Figure 8.4 Pelvic MRI—6-cm cervical carcinoma with anterior vaginal extension and no uterine, bladder, or rectal involvement. Left: Sagittal view. Right: Coronal view. (Scans courtesy of Dr. Shreyas Vasanawala, Stanford University School of Medicine.)
The ability of MRI to more accurately determine tumor diameter and parametrial infiltration, particularly in patients with bulky cervical tumors, makes it an important adjunct to clinical evaluation in treatment planning. MRI is also important for the evaluation of pregnant patients because it poses no risk to the fetus (16).
A meta-analysis comparing the utility of lymphangiogram, CT, and MRI for the detection of pelvic and para-aortic lymph node metastases in patients with cervical cancer concluded that the three imaging modalities performed comparably (17).
Extension of cervical cancer into the uterus can be readily detected by MRI. In univariate analysis, Narayan et al. (18) reported a significant association between nodal involvement and both FIGO stage (p = 0.018) and uterine body involvement; in multivariate analysis, however, only uterine body extension was independently related to the risk of lymph node involvement. Positron emission tomography (PET)-documented pelvic node positivity was 75% (39 of 52) in patients with uterine extension compared to 11% (2 of 18) in those without (p < 0.001).
Positron Emission Tomography
The PET imaging technique has been available in some centers since the mid-1990s. It depends on metabolic, rather than anatomic, alteration for the detection of disease. PET uses radionuclides, which decay with the emission of positrons (positively charged particles). Because cancer cells are avid users of glucose, a radionuclide-labeled analogue of glucose, 2-[18F] fluoro-2-deoxy-d-glucose (FDG), can be used to detect sites of malignancy by identifying sites of increased glycolysis. The PET scan has the potential to delineate more accurately the extent of disease, particularly in lymph nodes that are not enlarged and in distant sites that are undetectable by conventional imaging studies (Fig. 8.5).
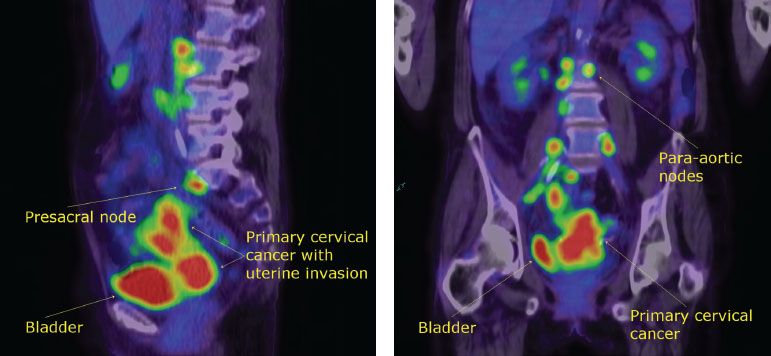
Figure 8.5 PET scan of a patient with a stage IB2 cervical cancer showing positive para-aortic nodes. Left: Coronal section. Right: Sagittal section. (Scan courtesy of Dr. Eva Wegner, Sydney, Australia.)
An Italian study evaluated the utility of PET scanning for preoperative staging in 159 patients with stages IB1–IIA1 cervical cancer undergoing radical hysterectomy and pelvic lymphadectomy (19). Sensitivity of the PET scan was only 32.1% and positive predictive value only 69.2%. The authors concluded that the PET scan had minimal clinical impact on the pretreatment planning of patients with early stage cervical cancer.

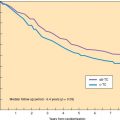
Figure 8.6 Survival curves for patients with a negative CT scan in relation to PET scan status of the para-aortic lymph nodes. (Reproduced with permission from Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol. 2001;19:3745–3749.)
In a study aimed at determining whether PET scanning could obviate the need for surgical staging in patients with locoregionally advanced cervical cancer, Narayan et al. (20) reported a sensitivity of 83%, specificity of 92%, positive predictive value of 91%, and negative predictive value of 85% in 24 patients evaluable for pelvic nodal status. However, PET detected only four of seven (57%) cases of positive para-aortic nodes. All histologically confirmed nodes not visualized on PET were <1 cm in diameter.
Grigsby et al. (21) retrospectively compared the results of CT lymph node staging with whole-body FDG–PET in 101 consecutive patients with cervical cancer who were referred for primary radiation therapy. CT demonstrated abnormally enlarged pelvic lymph nodes in 20 patients (20%) and para-aortic lymph nodes in 7 patients (7%). PET demonstrated abnormal FDG uptake in pelvic nodes in 67 patients (67%), in para-aortic nodes in 21 patients (21%), and in supraclavicular nodes in 8 patients (8%). For the 94 patients with negative para-aortic nodes on CT scan, the 2-year progression-free survival (PFS) was 64% in PET-negative patients and 18% in PET-positive patients (p < 0.0001) (Fig. 8.6). A multivariate analysis demonstrated that the most significant factor for PFS was the presence of positive para-aortic lymph nodes as detected by PET imaging (p = 0.025).
An analysis of 15 published PET studies in cervical cancer reported that the pooled sensitivity of FDG–PET for para-aortic lymph node metastases was 84% (95% confidence interval [CI], 68–94%), and the specificity 95% (95% CI, 89–98%) (22).
Fine-Needle Aspiration Cytology
If pelvic or abdominal masses or enlarged lymph nodes are detected during physical examination or imaging studies, fine-needle aspiration may be performed under CT or ultrasonic guidance. The procedure is performed under local anesthesia and is free of major complications, even in the presence of clotting problems or perforation of a hollow viscus. The reported accuracy for abdominopelvic nodes ranges from 74–95% (23,24). Only a positive cytologic diagnosis should be used as a basis for therapeutic decision making.
Surgical Staging
The inability of available noninvasive diagnostic tests to detect small lymph node metastases led many investigators in the 1970s to undertake pretreatment staging laparotomies to identify patients with positive para-aortic nodes. These patients were treated with extended-field radiation to encompass the involved nodes (11,25). In the 1990s, some investigators proposed laparoscopic staging (26).
In spite of the theoretical advantages of surgical staging, the benefits in terms of patient outcomes are controversial. Lai et al. (27), from Taipei, conducted a randomized trial to compare clinical with surgical staging for patients with locally advanced cervical cancer. Patients in the surgical arm were randomly allocated to either a laparoscopic or an extraperitoneal approach. Although para-aortic nodal metastases were documented in 25% of patients in the surgical arm, patient accrual was terminated after 61 patients were entered because interim analysis showed a significantly worse outcome in terms of PFS (p = 0.003) and overall survival (p = 0.024) for patients in the surgical arm.
A retrospective GOG review was more positive. Three phase III studies (GOG 85, 120, and 165) were retrospectively reviewed to compare the outcome for patients who had negative para-aortic lymph nodes by pretreatment surgical staging with that of patients who had only radiographic (CT or MRI) exclusion of para-aortic nodal disease before pelvic chemoradiation (28). There were 555 patients in the surgical staging group, and 130 in the radiographic group. In multivariate analysis, radiographic staging was associated with a poorer prognosis both for disease progression (hazard ratio [HR] 1.35; 95% CI, 1.01 to 1.81) and for death (HR 1.46; 95% CI, 1.08 to 1.99).
The results from the ongoing collaborative study of PET, MRI, and surgical staging being conducted by the American College of Radiology Imaging network and the GOG will help clarify whether or not there is any ongoing role for surgical staging in the PET scan era. By constructing a mathematical model from the available literature, Petereit et al. (29) predicted that surgical staging and tailored postoperative radiation would save 2.6, 6, and 7 lives per 100 patients treated, for stages IB, IIB, and IIIB, respectively.
At the Royal Hospital for Women in Sydney, PET/CT scanning is used prior to chemoradiation for staging patients with locally advanced disease. If positive nodes ≥1.5 cm diameter are identified below the inferior mesenteric artery, an extraperitoneal approach via a lower midline incision is used to resect the nodes before treatment begins. The peritoneum is stripped off the anterior and lateral abdominal walls to expose each pelvic sidewall. The round ligaments must be transected extraperitoneally to facilitate exposure. The dissection may be extended cephalad as far as necessary by extending the lower midline incision above the umbilicus (30).
Patterns of Spread
Cervical cancer spreads by the following means:
1. Direct invasion of the cervical stroma, corpus, vagina, and parametrium
2. Lymphatic permeation and metastasis
3. Hematogenous dissemination
Direct Infiltration
Invasive cervical cancer, whether squamous or glandular, arises from intraepithelial neoplasia. Malignant cells penetrate the basement membrane, and then progressively infiltrate the underlying stroma. They may progressively infiltrate laterally to involve the cardinal and uterosacral ligaments, superiorly to involve the uterine corpus, inferiorly to involve the vagina, anteriorly to involve the bladder, and posteriorly to involve the peritoneum of the pouch of Douglas and the rectum.
Lymphatic Spread
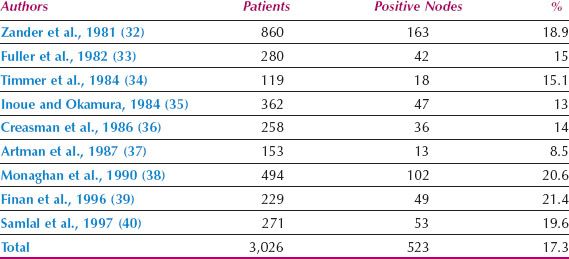
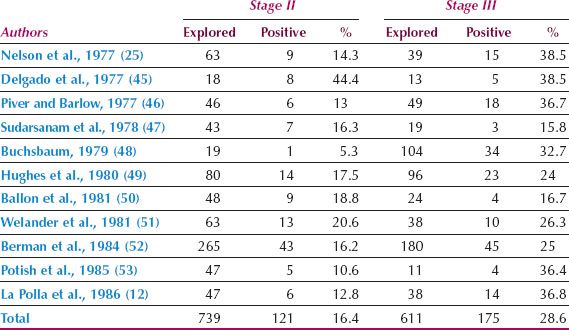
Cervical cancer can spread to all pelvic node groups, although the obturator nodes are most frequently involved. The parametrial nodes are not necessarily involved before the nodes on the pelvic sidewall. Although tumor cells can reach the common iliac and para-aortic nodes directly by the posterior cervical trunk (31), this is very uncommon, and lymph node spread in cervical cancer almost invariably occurs in an orderly fashion from the nodes on the pelvic sidewall to the common iliac and then the para-aortic group. From the para-aortic nodes, spread can occasionally occur through the thoracic duct to the left scalene nodes (41). The incidence of pelvic lymph node metastases in patients with stage IB cervical cancer treated by radical hysterectomy is shown in Table 8.3. The incidence of para-aortic nodal metastases in patients with stages II and III cervical cancer undergoing surgical staging is shown in Table 8.4.
Table 8.3 Incidence of Pelvic Lymph Node Metastases in Patients with Stage IB Cervical Cancer Treated by Radical Hysterectomy
Lymphatic invasion by tumor cells is commonly seen in the primary tumor, and tumor cells are also seen occasionally in lymphatic channels in the parametrium. Burghardt and Girardi (42) believe that tumor emboli may be held up in lymphatic vessels and grow to become foci of discontinuous parametrial involvement.
Ovarian involvement by cervical cancer is rare but most likely occurs through the lymphatic connection between the uterus and the adnexal structures (43). In a study of patients with clinical stage IB cervical cancer, the GOG reported ovarian spread in 4 of 770 patients (0.5%) with squamous carcinoma and in 2 of 121 patients (1.7%) with adenocarcinoma. All six patients with ovarian metastases had other evidence of extracervical spread (44).
Table 8.4 Incidence of Para-aortic Lymph Node Metastases in Patients Undergoing Surgical Staging for Stages II and III Cervical Cancer
Hematogenous Spread
Although spread to virtually all parts of the body has been reported, the most common organs for hematogenous spread are the lungs, liver, and bone.
Treatment
Treatment of invasive cancer involves appropriate management for both the primary lesion and potential sites of metastatic disease. Both surgery and radiation therapy may be used for primary treatment, although definitive surgery is usually limited to patients with stages I or early IIA disease. Some European and Asian centers also treat patients with stage IIB disease with primary surgery (54–56).
Microinvasive Carcinoma
The term microcarcinoma of the uterine cervix was first introduced by Mestwerdt (57) in the German literature in 1947. He suggested that 5 mm was the deepest penetration acceptable. Since then, both terminology and treatment have been the subject of much debate.
In 1961, the Cancer Committee of FIGO recommended that clinical stage I cervical cancer should be subdivided into stage IA and stage IB. Stage IA was vaguely defined as a preclinical cancer with early stromal invasion. This did little to clarify even the definition.
In 1974, the Committee on Nomenclature of the Society of Gynecologic Oncologists (SGO) in the United States proposed that microinvasive carcinoma should be defined as a lesion that invaded below the basement membrane to a depth of 3 mm or less, and in which there was no evidence of lymph-vascular space invasion (LVSI). Although this definition provided no horizontal dimension, patients whose disease fulfilled these criteria were shown to have virtually no risk of lymph node metastases and to be adequately treated by either hysterectomy or cone biopsy (58–60).
A precise definition of microinvasive carcinoma was not adopted by FIGO until 1995. Stage IA1 was defined as a tumor that invaded to a depth of 3 mm or less, whereas stage IA2 referred to a tumor that invaded to a depth greater than 3 mm and up to 5 mm. In both stages, the horizontal spread should not exceed 7 mm. LVSI was not included as part of the definition. The official FIGO staging remains unchanged.
Stage IA1 Squamous Carcinoma
Although stromal invasion can be seen in small punch biopsies, a definitive diagnosis of microinvasion can be made only in conization (or hysterectomy) specimens. The conization specimen must be thoroughly sampled to make the correct diagnosis and to be certain about the margins.
In an extensive review of the literature, Ostor (61) reported that of 2,274 squamous lesions with invasion of less than 1 mm, only three cases had lymph node metastases (0.1%); invasive recurrence developed in 8 cases (0.4%). Among 1,324 squamous lesions invading between 1 and 3 mm, there were 7 cases with lymph node metastases (0.5%) and 26 cases in which invasive recurrence developed (2%). No horizontal limitation was placed on these lesions, so they do not strictly fit the current FIGO definition of stage IA1 disease, and most of the cases were treated without lymph node dissection.
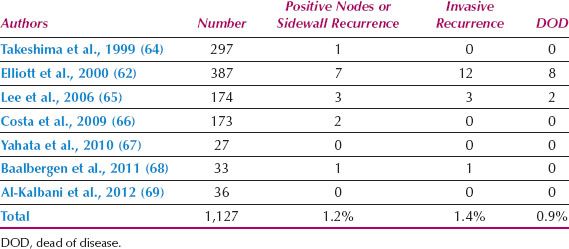
Studies of stage IA disease in patients meeting the 1995 FIGO definition are shown in Table 8.5. This table includes patients with squamous and adenocarcinomas. The largest single institution series was reported by Elliott et al. (62) from Sydney. They reported 476 such patients, of whom 418 (88%) had squamous and 58 (12%) glandular tumors. Of 180 patients undergoing lymphadenectomy, the incidence of positive nodes in patients with stage IA1 disease was 0.8% (1 of 121 cases). For the cumulative series of 1,127 patients, the overall incidence of positive nodes or pelvic side wall recurrence was 1.2%, and 0.9% of patients died of disease.
Roman et al. (63) reported 87 cases of microinvasive carcinoma diagnosed on cone biopsy and followed by either repeat cone biopsy or hysterectomy. Significant predictors of residual invasion included status of the internal margin (residual invasion present in 22% of women with dysplasia at the margin vs. 3% with a negative margin; p < 0.03) and the combined status of the internal margin and the postconization ECC (residual invasion 4% if both negative, 13% if one positive, and 33% if both positive; p < 0.015). Depth of invasion and the number of invasive foci were not significant. The researchers concluded that if either the internal margin or the postconization ECC contained dysplasia or carcinoma, then the risk of residual invasion was high and warranted repeat conization before definitive treatment planning.
Table 8.5 Lymph Node and Recurrence Status of patients with FIGO Stage IA1 Cervical Cancer
A study from Chang Mai, Thailand, confirmed these findings (70). Histopathology slides were reviewed from 129 patients who underwent hysterectomy following a cone biopsy that showed microinvasive squamous cell carcinoma. All had high-grade CIN or invasive carcinoma at the cone margins. Of the 129 patients, 77 (59.7%) had residual disease in the hysterectomy specimen, of whom 20 patients (15.5%) had residual invasive cancer: 18 were microinvasive and 2 were frankly invasive. Factors that significantly affected the risk of residual disease included positive postconization ECC (p = 0.001), positive cone margins for invasive cancer (p = 0.003), and depth of stromal invasion >1 mm (p = 0.014). They also recommended repeat conization to determine the exact severity of the lesion before planning definite treatment.
In view of these considerations, a cone biopsy with clear surgical margins and a negative ECC should be considered adequate treatment for a patient with stage IA1 squamous carcinoma of the cervix. If future childbearing is not required, then extrafascial hysterectomy may be considered. This should be the treatment of choice in postmenopausal women because stenosis of the endocervical canal is common after conization, which limits the ability to obtain endocervical cytology (71). If the cone margins or postconization ECC reveals high-grade dysplasia or microinvasive carcinoma, then a repeat conization should be performed before proceeding to simple hysterectomy because more extensively invasive disease may be present.
LVSI is uncommon in stage IA1 lesions. Elliott et al. (62) reported LVSI in 8.5% of tumors invading 1 mm or less, 19% between 1.1 and 2 mm, 29% between 2.1 and 3 mm, and 53% between 3.1 and 5 mm. Lee et al. (65) found a positive correlation between depth of invasion and the presence of LVSI but found no definite correlation between LVSI and lymph node metastases, only two of their four patients with positive nodes having LVSI. Its significance in microinvasive cervical cancer remains controversial, and it is not mentioned in the FIGO definition. It probably should be disregarded when planning treatment, unless it is extensive, although Rob et al. (71) recommend sentinel lymph node mapping, and removal of only the sentinel nodes, for patients with LVSI. A proposed algorithm for the management of microinvasive cervical cancer is shown in Figure 8.7.
Stage IA2 Squamous Carcinoma
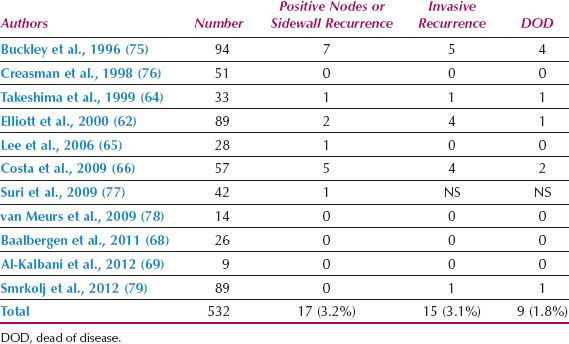
Reports of nodal status and outcome for patients with FIGO stage IA2 cervical cancer are shown in Table 8.6. There are a limited number of reported cases, but the incidence of positive nodes (3.2%) and death from disease (1.8%) are low when the horizontal spread is limited to 7 mm. Takeshima et al. (64) reported that, of 73 patients with depth of invasion between 3 and 5 mm, the incidence of lymph node metastasis was 3.4% for tumors with a horizontal spread of 7 mm or less and 9.1% for those with greater than 7 mm spread.
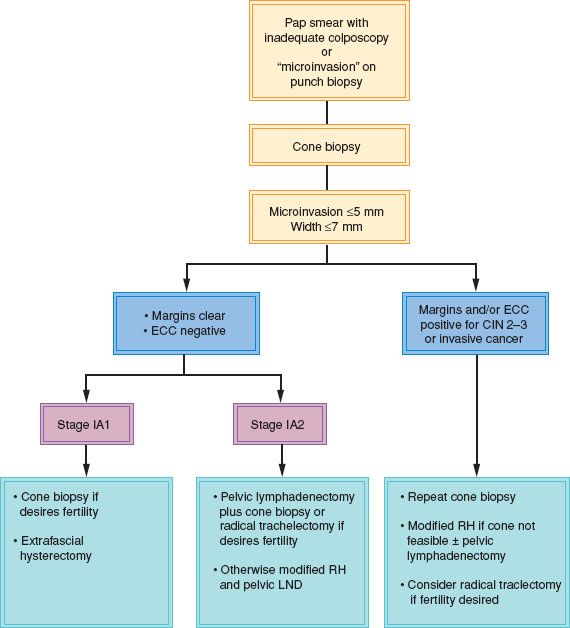
Figure 8.7 Algorithm for the management of patients with a high-grade Pap smear and inadequate colposcopy or with microinvasive cervical carcinoma on punch biopsy.
Many patients with early cervical cancer are young, and preservation of fertility is a major concern. Consequently, surgical approaches that remove the primary lesion and regional lymph nodes, while conserving the corpus for future childbearing, have been explored.
In 1994, Dargent et al. (72) pioneered the use of radical trachelectomy and laparoscopic pelvic lymphadenectomy. A nonabsorbable cervical cerclage was placed around the uterine isthmus at the time of the trachelectomy. Subsequent studies have confirmed that the operation is feasible in experienced hands, cure rates are high, and subsequent pregnancies can be carried to viability in many cases (see Table 21.3). Radical abdominal trachelectomy was first reported by Smith et al. (73) in 1997. One advantage of this approach is that the anatomy is more familiar to most gynecologic oncologists.
Less radical procedures, such as large cone biopsy or simple trachelectomy, combined with laparoscopic pelvic lymphadenectomy, are possible alternatives to radical trachelectomy, particularly in patients without vascular space invasion (74). The less radical procedures have less morbidity, but the small number of patients with stage IA2 cervical cancer makes it impractical to conduct prospective randomized trials to compare the two approaches.
There are some long-term morbidities associated with radical trachelectomy. In a retrospective review of 29 patients undergoing radical trachelectomy, the group from St. Bartholomew’s Hospital in London reported dysmenorrhea in 24%, irregular menstruation in 17%, recurrent candidiasis in 14%, cervical suture problems in 14%, isthmic stenosis in 10%, and prolonged amenorrhea in 7% of patients (80).
Table 8.6 Lymph Node and Recurrence Status of Patients with FIGO Stage IA2 Cervical Cancer
If the patient does not require fertility preservation, the recommended treatment for stage IA2 squamous carcinoma of the cervix is modified radical hysterectomy and pelvic lymph node dissection. In a medically unfit patient, intracavitary radiation may be used.
Microinvasive Adenocarcinoma
Although the concept of microinvasive squamous carcinoma has long been accepted, the concept for the glandular counterpart has been more controversial. Only recently have reports looked specifically at microinvasive adenocarcinoma as now defined by the FIGO staging (67–69,81), and the incidence of lymph node metastases appears to be comparable.
Most cases arise adjacent to the transformation zone, although Teshima et al. (82) reported that 3 of 30 cases (10%) arose outside the transformation zone. Adenocarcinoma in situ may extend up the entire endocervical canal, and invasion may occur at any point (82). Lee and Flynn (83), in a study of 40 cases of adenocarcinoma invasive to 5 mm or less, reported that in 78% of the cases, the midpoint of the invasive focus was in the region of the transformation zone. The endometrioid variant is particularly likely to arise higher in the canal (83), and this variant has been reported to be associated with late recurrence and a worse survival for both stages IA1 and IA2 disease (81).
Whereas squamous lesions are usually unifocal, glandular lesions are sometimes multifocal. Ostor et al. (84) reported that 21 of 77 cases (27.3%) were multicentric, meaning that both cervical lips were affected, without continuity around the “edges” at 3 and 9 o’clock. They reported no “skip” lesions, which they arbitrarily defined as separation between discrete microinvasive adenocarcinomas in the same lip of greater than 3 mm. More than one focus of invasive disease was present in 4 of 40 cases (10%) reported by Lee and Flynn (83).
Positive lymph nodes have rarely been reported in FIGO stage IA1 lesions, although Elliott et al. (62) reported a solitary nodal metastasis in a patient with <1 mm stromal invasion. Kaku et al. (85) reported recurrences at the vaginal vault in 2 of 30 patients (6.7%) with less than 5 mm of invasion. One patient had a tumor volume of 1,222 mm3, but the other had a tumor with a depth of 3.9 mm and a width of 4.9 mm (i.e., FIGO stage IA2). The only adenocarcinoma recurrence in the 77 patients reported by Ostor et al. (84) involved a patient whose tumor invaded to a depth of 3.2 mm but was 21 mm in length (i.e., stage IB1).
A study from Melbourne reported 29 patients with stage IA1 and 9 with stage IA2 microinvasive adenocarcinoma of the cervix (86). A variety of treatment methods were used. Cone biopsy of the cervix was performed in 18 patients, including 2 with stage IA2 disease. No recurrences were noted during an average follow-up of 72 months. In a literature review, the same authors noted positive nodes in 12 of 814 patients (1.5%) undergoing pelvic lymph node dissection for microinvasive adenocarcinoma of the cervix. LVSI was present in 25 patients (3%), all without lymph node involvement (86).
The Surveillance, Epidemiology, and End Results (SEER) database was used to identify 131 cases of stage IA1 and 170 cases of stage IA2 adenocarcinoma of the cervix treated between 1988 and 1997 (87). There was no histologic review, and patients were treated in a variety of ways from cone biopsy to radical hysterectomy and pelvic lymphadenectomy. Simple hysterectomy alone was used for 118 patients (39.2%). With a mean follow-up of 46.5 months, the censored survival was 99.2% for patients with stage IA1 disease and 98.2% for stage IA2.
In view of these observations, it seems reasonable to treat the disease in a similar manner to its squamous counterpart. For patients desiring fertility preservation, a cone biopsy with negative margins appears to be adequate treatment for stage 1A1 (88). Ideally, the cone biopsy should be a cold knife procedure (89), although loop excision procedures in expert hands may also be acceptable (90). For stage 1A2 disease, radical trachelectomy and resection of pelvic lymph nodes is desirable. Following childbearing, it seems reasonable to recommend hysterectomy because Pap smears and colposcopy are less reliable, and Poynor et al. (91) reported that ECC was positive before cervical conization in only 43% of patients with glandular lesions.
Stage IB1 and Early Stage IIA1 Cervical Cancer
In 1994, FIGO recognized the prognostic significance of tumor size by subdividing stage IB disease into stage IB1 (primary lesion ≤4 cm diameter) and stage IB2 (primary lesion >4 cm diameter). This same subdivision was applied to patients with stage IIA2 disease in 2009.
Patients with stage IB1 are universally regarded as being ideal candidates for radical hysterectomy and pelvic lymphadenectomy, although equal cure rates may be obtained with primary radiation therapy (92). The choice of modality should depend mainly on the availability of the appropriate expertise. Since the introduction of fellowship training in gynecologic oncology, expertise in radical pelvic surgery has become widely available in the United States and most developed countries. The Patterns of Care study in the United States suggests that the same may not be true for radiation oncology, particularly outside of tertiary referral units (93). If both surgical and radiotherapeutic expertise are available, radiation is usually reserved for the medically unfit patient. Chronologic age should not be considered a contraindication to radical surgery, because elderly patients experience morbidity similar to that of younger patients (94).
Primary surgery has the advantage of removing the primary disease and allowing accurate surgical staging, thereby allowing any adjuvant therapy to be more accurately targeted. In addition, it avoids possible chronic radiation damage to the bladder, small and large bowel, and vagina, which is difficult to manage. Surgical injuries to the same organs are more readily repaired because the blood supply is not compromised. Sexual dysfunction is, in general, underreported, but is a problem for many patients who have had both external-beam therapy and brachytherapy because of vaginal atrophy, fibrosis, and stenosis (95). Although the vagina is shortened by approximately 1.5 cm after radical hysterectomy, it is more elastic; in premenopausal patients, ovarian function can be preserved. In postmenopausal patients, the nonirradiated vagina responds much better to estrogen therapy.
Influence of Diagnostic Conization
The influence of previous cone biopsy on the morbidity of radical hysterectomy is controversial. Samlal et al. (96) reported no significant difference in morbidity, but the conization–radical hysterectomy interval in their study was 6 weeks. They believed that delaying the definitive surgery might allow the tissue reaction to subside, thereby decreasing morbidity. Others have found that the interval between the conization and radical hysterectomy has no influence on morbidity, and they recommend proceeding without delay (97). The primary author prefers to proceed immediately with radical hysterectomy if the surgical margins of the cone biopsy are involved, but to postpone surgery for 6 weeks if the cone margins are clear.
Types of Radical Hysterectomy
In 1974, Piver et al. (98) described the following five types of hysterectomy: extrafascial, modified radical, radical, extended radical, and partial exenteration.
Types I–V
Extrafascial Hysterectomy (Type I) This is a simple hysterectomy and is suitable for stage IA1 cervical carcinoma.
Modified Radical Hysterectomy (Type II) This is basically the hysterectomy described by Ernst Wertheim (99). The uterine artery is ligated where it crosses the ureter, and the medial halves of the cardinal ligaments and proximal uterosacral ligaments are resected. Piver et al. (98) described removal of the upper one-third of the vagina, but this is rarely necessary unless vaginal intraepithelial neoplasia (VAIN) 3 is extensive. The operation described by Wertheim involved selective removal of enlarged nodes rather than systematic pelvic lymphadenectomy. The modified radical hysterectomy is appropriate for stage IA2 cervical cancer.
Radical Hysterectomy (Type III) The most commonly performed operation for stage IB cervical cancer is that originally described by Meigs in 1944 (100). The uterine artery is ligated at its origin from the superior vesicle or internal iliac artery, allowing removal of the entire width of the cardinal ligaments. Piver (98) originally described resection of the uterosacral ligaments at their sacral attachments and resection of the upper half of the vagina. Such extensive dissection of the uterosacral ligaments and vagina is not required for stage IB cervical cancer.
Extended Radical Hysterectomy (Type IV) This differs from the type III operation in three aspects: (i) The ureter is completely dissected from the vesicouterine ligament, (ii) the superior vesicle artery is sacrificed, and (iii) three-fourths of the vagina is excised. The risk of ureteric fistula is increased with this procedure, which Piver (98) used for selected small central recurrences after radiation therapy.
Partial Exenteration (Type V) The indication for this procedure was removal of a central recurrence involving a portion of the distal ureter or bladder. The relevant organ was partially excised and the ureter reimplanted into the bladder. This procedure is occasionally performed if cancer is found to be unexpectedly encasing the distal ureter at the time of radical hysterectomy. Alternatively, the operation may be aborted and the patient treated with primary radiation.
Kyoto Classification
A new classification for radical hysterectomy was described by Querleu and Morrow (101) following a consensus meeting in Kyoto, Japan, which was arranged by Shingo Fujii in February 2007. The classification is based only on the lateral extent of the resection. Four basic types are described, A to D, adding when necessary a few subtypes that consider nerve preservation and paracervical lymphadenectomy. Lymph node dissection is considered separately, and four levels (1 to 4) are defined according to the corresponding arterial anatomy and the radicality of the procedure.
A three-dimensional anatomic template for the parametrial resection has been described by Cibula et al. (102), and is included in Chapter 20. A description of the procedures from the original paper by Querleu and Morrow (101) is given below.
Type A: Minimum Resection of Paracervix This is an extrafascial hysterectomy. The paracervix is transected medial to the ureter but lateral to the cervix. The uterosacral and vesicouterine ligaments are not transected at a distance from the uterus. Vaginal resection is generally at a minimum, routinely less than 10 mm, without removal of the vaginal part of the paracervix (paracolpos).
Type B: Transection of the Paracervix at the Ureter This type has two levels:
B1—Without removal of lateral paracervical lymph nodes
B2—With removal of lateral paracervical nodes
Partial resection of the uterosacral and vesicouterine ligaments is a standard part of this category. The ureter is unroofed and rolled laterally, permitting transection of the paracervix at the level of the ureteral tunnel. The neural component of the paracervix caudal to the deep uterine vein is not resected. At least 10 mm of the vagina from the cervix or tumor is resected.
The operation corresponds to the modified or proximal radical hysterectomy and is adapted to early cervical cancer. The radicality of this operation can be improved without increasing postoperative morbidity by lymph node dissection of the lateral part of the paracervix, thus defining two subtypes—B1 and B2.
The border between paracervical and iliac or parietal lymph node dissection is defined arbitrarily as the obturator nerve: Paracervical nodes are medial and caudal. The combination of paracervical and parietal dissections is simply a comprehensive pelvic node dissection.
The morbidity of type B2 does not differ from that of B1, although the combination of B1 with paracervical lymph node dissection may be equivalent to that of type C1 resection.
Type C In type C, the paracervix is transected at the junction with the internal iliac vascular system and has two types:
C1—With nerve preservation
C2—Without preservation of autonomic nerves
This type involves transection of the uterosacral ligament at the rectum and vesicouterine ligament at the bladder. The ureter is mobilized completely, and 15 to 20 mm of vagina from the tumor or cervix and the corresponding paracolpos is resected routinely, depending on vaginal and paracervical extent and on surgeon choice.
Type C corresponds to variants of classical radical hysterectomy. By contrast with types A and B, in which the autonomic nerve supply to the bladder is not threatened, the issue of nerve preservation is crucial. Two subcategories are defined: C1 with nerve preservation and C2 without preservation of autonomic nerves. In C1, the uterosacral ligament is transected after separation of the hypogastric nerves. The bladder branches of the pelvic plexus are preserved in the lateral ligament of the bladder (i.e., lateral part of bladder pillar). If the caudal part of the paracervix is transected, then careful identification of bladder nerves is needed.
For C2, the paracervix is transected completely, including the part caudal to the deep uterine vein.
Type D In type D, the entire paracervix is resected:
D1—Resection of the entire paracervix along with the hypogastric vessels
D2—Resection of the entire paracervix, along with the hypogastric vessels and adjacent fascial or muscular structure
This group of rare operations features additional ultraradical procedures, mostly indicated at the time of pelvic exenteration. Type D1 is resection of the entire paracervix at the pelvic sidewall along with the hypogastric vessels, exposing the roots of the sciatic nerve. There is total resection of the vessels of the lateral part of the paracervix. These vessels (i.e., inferior gluteal, internal pudendal, and obturator vessels) arise from the internal iliac system.
Type D2 is D1 plus resection of the entire paracervix with the hypogastric vessels and adjacent fascial or muscular structures. This resection corresponds to the LEER (laterally extended endopelvic resection) procedure (103).
Lymph Node Dissection
Lymph node dissection has four levels:
Level 1—External and internal iliac
Level 2—Common iliac (including presacral)
Level 3—Aortic inframesenteric
Level 4—Aortic infrarenal
This classification ignores the widely used pelvic versus para-aortic dissection, in which the limit of the pelvis dissection is around the midcommon iliac area.
Within every level, and independently from each other, several types of lymphadenectomy must be defined to describe adequately the radicality of the procedure: diagnostic (minimum sampling of sentinel node only, removal of enlarged nodes only, or random sampling), systematic lymphadenectomy, and debulking (resection of all nodes ≥2 cm) (104).
Technique for Radical Hysterectomy
The patient is given prophylactic antibiotics on induction of anesthesia, and pneumatic calf compressors are used during and after surgery until the patient is fully mobilized. Prophylactic anticoagulants are given for at least 5 days postoperatively.
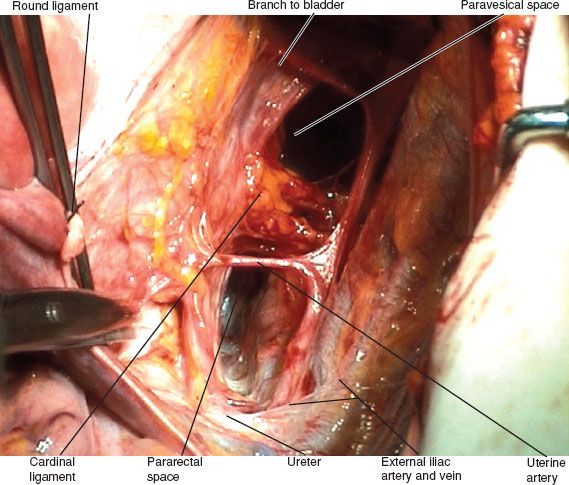
Figure 8.8 Paravesicle and pararectal spaces.
Incision
The abdomen may be opened either through a lower midline incision extending to the left of the umbilicus or through a low transverse Maylard or Cherney incision. The low transverse incision, which is described in Chapter 20, requires division of the rectus abdominus muscle, but provides excellent exposure of the primary tumor and pelvic sidewalls. The midline incision, which can be readily extended, provides better exposure of the para-aortic region if necessary.
Exploration
After entering the peritoneal cavity, all organs are systematically palpated, and any evidence of metastatic spread is documented by frozen section. The vesicouterine fold and pouch of Douglas peritoneum are examined for evidence of tumor infiltration, and the tubes and ovaries are examined for any abnormalities. Any bulky pelvic or para-aortic nodes are removed and frozen sections obtained to differentiate between inflammatory and malignant changes.
Radical Hysterectomy
With the uterus under traction, the retroperitoneum is entered through the round ligaments bilaterally. The ureter is identified as it crosses the pelvic rim, and the pelvic sidewall spaces are developed by a combination of sharp and blunt dissection.
The paravesicle space (Fig. 8.8) is bordered by the following:
1. The obliterated umbilical artery (a continuation of the superior vesicle artery) running along the bladder medially
2. The obturator internus muscle laterally
3. The cardinal ligament or paracervix posteriorly
4. The pubic symphysis anteriorly
The pararectal space is bordered by the following:
1. The rectum medially
2. The hypogastric artery laterally
3. The cardinal ligament or paracervix anteriorly
4. The sacrum posteriorly
The floor of the spaces is formed by the levator ani muscle.
Bladder Takedown
The vesicouterine fold of peritoneum is opened and the bladder dissected off the anterior cervix and upper vagina. This should be done before any blood supply is ligated, because occasionally tumor may infiltrate into the bladder base, making hysterectomy impossible. Rather than resecting the relevant section of the bladder in this situation, the abdomen is usually closed and the patient treated with primary chemoradiation.
Ligation of the Uterine Artery
The uterine artery usually arises from the superior vesicle artery, close to its origin from the hypogastric artery. The artery is ligated at its origin in a type III or type C radical hysterectomy, or at the point where it crosses the ureter in the modified or type B radical hysterectomy, then mobilized over the ureter by gentle traction and dissection. The superficial uterine veins must be identified and clipped or troublesome bleeding will occur.
Dissection of the Ureter
The roof of the ureteric tunnel is the anterior vesicouterine ligament. This can be taken down in a piecemeal fashion bilaterally (Fig. 8.9), thereby avoiding the troublesome venous bleeding that can occur by blindly advancing a right-angled forceps into the tunnel. Each ureter is mobilized off its peritoneal attachment fairly low in the pelvis to avoid unnecessary stripping from its peritoneal blood supply. It is also mobilized off the side of the uterus. This exposes the posterior or caudal vesicouterine ligament, which is also divided in a type III hysterectomy but not in a type II procedure. The anterolateral surface of the distal ureter is left attached to the bladder in a further effort to preserve the blood supply. If the caudal vesicouterine ligament is transected, it is desirable to identify and preserve the bladder branch from the inferior hypogastric plexus (105,106).

Figure 8.9 Piecemeal dissection of anterior vesicouterine ligament.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree