- Diabetes is a strong and independent risk factor for ischemic cerebrovascular disease with a relative risk of around two.
- Ischemic stroke in the patient with diabetes has worse outcomes, including a higher rate of mortality than in patients without diabetes.
- While transient ischemic attacks (TIA) appear to occur less frequently in those with diabetes, people with diabetes who experience a TIA are more likely to go on to have a completed stroke in the immediate period following.
- The patient with diabetes is predisposed to vascular events, including stroke, for a number of reasons including premature atherosclerosis, reduced response to nitric oxide and a general state of hypercoagulability.
- Prevention of stroke in the patient with diabetes is best accomplished through aggressive management of coexisting hypertension and hyperlipidemia, as well as lifestyle modification.
- Reduction of glycated hemoglobin as a proxy for good glycemic control is likely to be associated with a reduction of macrovascular events.
- Aggressive management of hyperglycemia in the acute stroke period may improve outcomes.
Epidemiology of stroke in general
Cerebrovascular disease is a leading cause of morbidity and mortality. It is a highly prevalent disease with approximately 700 000 strokes occurring each year in the USA. Of these, 500 000 are first time events, while 200 000 are recurrent [1].
Stroke is the third leading cause of death in the USA, following heart disease and all forms of cancer. Thus, a woman is more than twice as likely to die from a stroke than she is from breast cancer, and one and a half times as likely as from lung cancer [2]. Furthermore, it is the number one reason listed for discharge diagnosis for patients discharged from hospitals to chronic care facilities. In total, the cost of stroke to the health care system in the USA was $62.7 billion in the year 2007 [1].
Statistics in other countries are similar to those seen in the USA. The Oxford Vascular Study [3], which compiled stroke statistics for every person in the county of Oxfordshire, demonstrated an overall incidence of 1.62 strokes per 1000 patients per year. The WHO MONICA study looked at 21 populations in 11 countries, 10 European countries as well as China, and found an incidence of 125–361/100 000 patients in men,and 61–194/100000 in women [4].
Diabetes as a risk factor for stroke
There is strong evidence that diabetes mellitus, whether type 1 (T1DM) or type 2 (T2DM), is a strong risk factor for ischemic cerebrovascular disease (Table 42.1). Observational studies have demonstrated associations between the two diseases. A model created from data from the Framingham Heart Study showed that diabetes confers an increased relative risk of 1.4 in men and 1.72 in women [5]. The Honolulu Heart Study showed that diabetes increases the risk of thromboembolic stroke between two-to threefold over those without the disease in Japanese men living in Hawaii [6].
The effect of diabetes is stronger in ethnic minorities in the USA. The Greater Cincinnati and Northern Kentucky Stroke Study found that as a sole risk factor, diabetes increased the odds ratio for having an ischemic stroke by 2.1 in Caucasians; however, in African-Americans that odds ratio was increased by 2.7. These results held true across all age cohorts [7].
Similarly, the Northern Manhattan Study found that diabetes was a stronger risk factor for ischemic stroke among African-Americans and Caribbean Latinos than among Caucasians. In these ethnicities, diabetes increased stroke risk by 1.8 and 2.1, respectively, partly because of increased prevalence of the disease. The fraction of strokes that could be directly attributable to diabetes as a risk factor was 14% among African-Americans and 10% among Caribbean Latinos [8].
The Copenhagen City Heart Study found a difference in the effect of diabetes among men and women. Thus, while diabetes increased the relative risk of first stroke, incident stroke and hospital admission for stroke among men by 1.5–2, among women the same relative risks were increased by 2–6.5 [9].
Figure 42.1 Lacunar infarct in the left posterior thalamus. (a) Diffusion weighted imaging, showing hyperintensity in the area of restricted diffusion, corresponding to acute ischemia. (b) Apparent diffusion coefficient image corresponding to the slice seen in (a), demonstrating hypointensity in the same distribution, confirming the presence of acute ischemia.
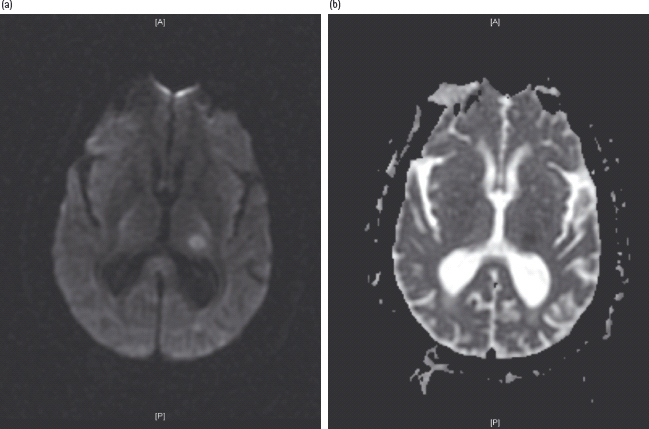
Table 42.1 Risk factors for stroke.
| Hypertension |
| Diabetes |
| Tobacco use |
| Hyperlipidemia |
| Atrial fibrillation |
| Carotid artery disease |
In addition to its effects as a sole risk factor, diabetes also exacerbates the effects of other risk factors. Thus, in patients with isolated systolic hypertension, diabetes confers additional risk of ischemic stroke or transient ischemic attack (TIA).
Stroke in patients with diabetes
In epidemiologic studies, ischemic stroke occurs at a younger age in patients who have diabetes. They are also more likely to be African-American. Among other risk factors, those with diabetes who have ischemic stroke are more likely to have hypertension, hyperlipidemia and to have experienced a myocardial infarction (MI) in the past [7].
Furthermore, diabetes increases the risk of death from stroke. In a prospective study in a Finnish cohort, men had an increased relative risk of 6 for mortality from ischemic stroke, while women had a relative risk of 8.2. These relative risks were higher than those for systolic blood pressure (BP), smoking or total serum cholesterol. In this cohort, the fraction of stroke deaths directly attributable to diabetes were 16% in men and 33.3% in women [10].
In terms of stroke subtype, diabetes is most commonly associated with lacunar infarcts. These are small deep infarcts in the territory of a single penetrating arterial branch. Conversely, the highest prevalence of diabetes is found in patients with demonstrable microvascular disease. Similarly, lacunar disease is more likely to be associated with diabetes than hemorrhages in the same location (Figure 42.1) [11–14].
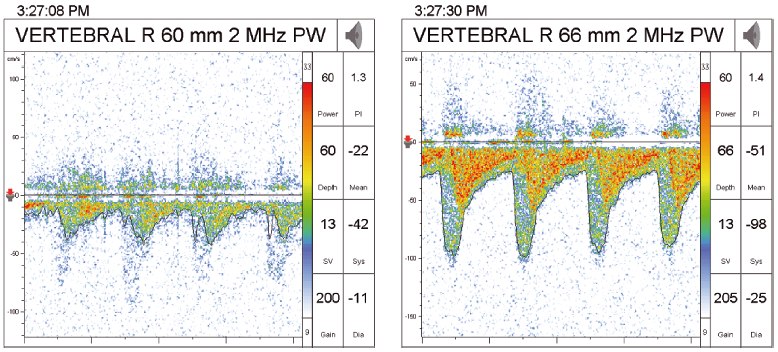
Diabetes is associated with both extracranial and intracranial stenosis. In one study of 510 patients referred for asymptomatic carotid bruits, only 200 had extracranial stenosis by Doppler examination. Sixty-six had asymptomatic intracranial stenosis, of whom 37 had concurrent extracranial stenosis. Of the patients with intracranial stenosis, 19 had diabetes (Figure 42.2) [15].
Figure 42.2 Intracranial vertebral artery stenosis as seen by transcranial Dopplers. The first image shows normal flow pre-stenosis, while the second image shows increased velocity of flow post-stenosis.
TIA is less common in those with diabetes than in those not having the disease. This may indicate that patients with diabetes are more likely to present with completed infarct rather than reversible ischemia [16]; however, patients with diabetes who do present with TIA are more likely to go on to full-blown ischemic stroke in the next 2 days as predicted by the ABCD2 score. This score stratifies patients with TIA by the following factors: age greater than 60 years, BP greater than 140/90 mmHg, clinical features of motor or speech involvement, duration greater than 60 minutes and diabetes [17].
Diabetes is a risk factor for coronary artery disease (CAD), and thus for MI and subsequent development of atrial fibrillation. In one large retrospective study of the relation between diabetes and atrial fibrillation or atrial flutter, diabetes was found after logistic regression to be a strong independent predictor for atrial arrhythmias in a review of over 850 000 charts over a 10-year period. The odds ratio found for diabetes was 2.13, 95% confidence interval (CI) 2.10–2.16 [18]. In turn, atrial fibrillation has been repeatedly demonstrated to be a strong risk factor for cardioembolic stroke, with an estimated 75 000 strokes per year attributable to the arrhythmia [19].
Furthermore, diabetes increases the risk of cardiac embolization. The CHADS2 score is a validated method of stratifying risk of cardioembolic stroke in patients with atrial fibrillation. It assigns one point each for the presence of congestive heart failure (CHF), hypertension, age greater than 75 years, and diabetes, and two points for previous stroke or TIA. Each point increase was associated with a 1.5-fold increased risk of stroke. Diabetes alone, then, would increase the risk of stroke in a patient with atrial fibrillation from 1.9 to 2.8 [20]. While the CHADS2 score is not perfect, and attempts have been made to make it more precise [21], it has the benefit of being very easy to use, thus guiding the non-stroke practitioner towards using anticoagulation in the appropriate section of the population.
In large observational studies, there has not appeared to be an association between diabetes and hemorrhagic strokes. By contrast, in the Hemorrhagic Stroke Project, a case–control study of young patients with intracerebral hemorrhage, diabetes conferred an adjusted odds ration of 2.4. The risk factor with the most impact was, as predicted, hypertension, which outweighed the contribution from diabetes by greater than twofold [22].
Intermediate hyperglycemia and other risk factors
While it is well established that diabetes is a strong risk factor for ischemic stroke, forms of intermediate hyperglycemia are not so clearly indicated as risk factors. Selvin et al. [23] looked at people with and without diabetes in the Atherosclerosis Risk In Communities (ARIC) trial and compared hemoglobin A1c (HbA1c) levels drawn at a specified visit, not necessarily related to time of incident stroke. With increasing tertiles of HbA1c across the normal distribution within each group, the risk of stroke increased in both those with diabetes and those without the disease, although it was only in those with diabetes that the difference achieved statistical significance [23].
In contrast, Myint et al. [24] abstracted data from the European Prospective Investigation into Cancer (EPIC) on HbA1c levels in patients without known diabetes and correlated these data with stroke risk. In this population, it was only after HbA1c levels were higher than 7.0% (53 mmol/mol) that an increased risk of stroke was demonstrated, compared to those patients with HbA1c less than 5.0% (31 mmol/mol). Given that these patients are most likely to have undiagnosed diabetes, this finding may not implicate chronic hyperglycemia alone as the primary risk factor for stroke [24].
Insulin resistance (IR), another forme fruste of diabetes, likewise has demonstrated conflicting evidence for an association with stroke. The ARIC study investigated hyperinsulinemia in those without diabetes and found a mild increase in risk of stroke of 1.19 with each increase of 50 pmol/L of fasting insulin. After adjustment for other risk factors such as age, systolic BP and smoking, the increase in risk was not as well defined [25].
Obesity, a proxy for insulin resistance and intermediate hyperglycemia, has also been linked to stroke through a number of epidemiologic studies. For example, the Copenhagen City Heart Study found that body mass index (BMI) was independently associated with increased risk of stroke [26]. Similarly, the Nurses’ Health Study found, as expected, an increasing risk of stroke with increasing BMI, with a relative risk of stroke of 2.37 (95% CI 1.60–3.50) seen in patients with BMI >32 kg/m2 [27]; however, ARIC was unable to demonstrate any relationship between BMI and stroke, or between waist : hip ratio, a better measurement of abdominal obesity, and stroke [25]. In addition, when adjustment for cardiovascular risks was performed, the relative risk of BMI for stroke was once again attenuated.
Although these individual forms of intermediate hyperglycemia have not conclusively been shown to predispose people to stroke, the constellation of diseases together called the metabolic syndrome has been. The combination of hypertension, hyperlipidemia, IR and abdominal obesity creates an environment that is highly susceptible to vascular damage and ischemic sequelae.
In a Finnish cohort, the metabolic syndrome in the absence of diabetes or cardiovascular disease (CVD) was associated with stroke with a relative risk of around 2, after adjustment for multiple other risk factors [28]. Similarly, a cohort from the ARIC study who likewise was free of diabetes, coronary heart disease (CHD) or stroke, had an increased risk of 1.5–2.0 for ischemic stroke. In addition, when separating out each risk factor, there appeared to be a synergistic effect from the combination over the relative risks inherent in each component [29].
Pathophysiology of ischemic stroke in diabetes
Diabetes predisposes patients to vascular thrombo-occlusive events in a number of ways. There is accelerated atherosclerosis in both large and medium sized vessels. There is disordered endothelial response and the blood of the patient with diabetes is hypercoagulable [30].
Carotid intima-media thickness (cIMT) is a useful proxy for early atherosclerosis, as it is easily measured by Doppler. Furthermore, cIMT is associated with primary stroke, with an increased relative risk per standard deviation (0.163 mm) of 1.57 in patients who had not had a previous stroke [31]. It also predicts recurrent stroke, with each 0.1 mm of increase in cIMT associated with an increased risk of 18% [32].
Diabetes is associated with increased cIMT. The IR Atherosclerosis Study demonstrated an increase in common cIMT in patients with chronic diabetes, but not in those with newly diagnosed diabetes. Nor was there an association with internal cIMT [33].
Conversely, patients with diabetes and stroke have been found to have greater cIMT. In one study of 438 Japanese patients with T2DM, common cIMT was significantly higher in those who had stroke, even after adjustment for age, BMI and smoking status [34]. Similarly, in a Czech cohort, cIMT was increased in stroke patients with diabetes [35].
Atherosclerosis also affects the ability of endothelium to release nitric oxide (NO), a potent vasodilatator. The diabetic blood vessel has either reduced NO production or altered NO metabolism. In addition to its vasodilatating effects, NO protects against platelet aggregation and enables the blood vessel to withstand ischemic conditions. With decreased NO activity, the vessel will tend more towards vasoconstriction, with predictably poor response to ischemia.
Stroke patients have been demonstrated to have decreased NO in circulating blood, along with increased peroxynitrite (ONOO–), a reactive oxygen species. These results were particularly true in larger strokes. Because the measurements were performed for acute stroke, these levels are likely to represent the outcome of ischemia rather than the cause; however, the correlation supports a role for decreased NO in the effects of stroke [36].
The cerebral vasculature has a diminished response to inhibition of NO synthase (NOS). In a small study of men with diabetes treated with a synthetic NOS inhibitor, NG-monomethyl-L-arginine (L-NMMA), the blood flow through the internal carotid artery was significantly lower than in control subjects treated likewise [37].
As further indirect evidence of the role of NO in stroke, 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) have multiple beneficial effects beyond their most common, that of lowering plasma cholesterol. Among these effects is increasing expression of endothelial NOS as well as decreasing the activity of Rho-kinase, a proconstrictor enzyme [38]. Statins have been demonstrated to lower the risk of recurrent stroke in multiple large studies [39–41]. Whether the beneficial effect of statins in stroke is because of their cholesterol lowering effect, their ability to stabilize atherosclerotic plaques, their effects on vascular function or more likely a combination of all of the above, is very difficult to separate.
In addition to the above predisposing factors, the blood of the patient with diabetes is hypercoagulable. Studies have demonstrated increased thrombin generation [42], increased prothrombin fragments and increased thrombin–antithrombin III complexes [43]. Furthermore, the elevated prothrombotic levels were significantly associated with macroangiopathic complications.
Thrombus formation is further promoted by platelet hyperreactivity in the blood of the patient with diabetes. In patients with metabolic syndrome, platelets have been shown to have increased activity both through closure time as measured by the platelet function analyzer (PFA-100), increased fibrinogen binding after exposure to ADP, implying activation of the GPIIa/IIIb receptors, and by expression of activated ligands on the platelet surface [44]. In full-blown diabetes, platelets were hyperreactive as measured by light transmittance aggregometry and expression of surface ligands [45].
Thus, taken together, the person with diabetes has a vascular environment that is highly susceptible to thrombo-occlusive complications. With early atherosclerosis and disordered endothelial response, the conditions are predisposed to thrombophilia. The blood, in its hypercoagulable state combined with platelets that are highly active in themselves, is far more likely to form clots.
Lacunar strokes are caused by damage to smaller parenchymal vessels. The most common cause is microatheroma, as demonstrated in pathologic case series published by Fisher [46–48], the neurologist responsible for naming the lacunar syndromes. Lipohyalinosis and fibrinoid necrosis also cause microangiopathies, and both are most commonly found in the setting of chronic hypertension of severe acute BP elevations, as seen in hypertensive encephalopathy [49,50].
Primary prevention of stroke in the patient with diabetes
Primary prevention of stroke is of paramount importance as the disability from stroke and health care costs associated with the acute and chronic care of stroke are so extensive. The approach to prevention in the patient with diabetes is of necessity multifactorial.
Medical therapy aimed at achieving normoglycemia is the foundation. The Diabetes Control and Complications Trial (DCCT) investigated intensive insulin regimens including subcutaneous insulin injections and external insulin pumps in those with T1DM. The goal for treatment was HbA1c levels of less than 6.05% (42 mmol/mol). The comparison group had no such goals outside of prevention of hyperglycemia or hypoglycemia [51].
The intensive treatment group achieved a reduction in the combined endpoints of non-fatal MI or stroke, cardiac death or revascularization procedure of 57%. The majority of this improvement was associated with the decrease in HbA1c [51].
Among oral hypoglycemic agents, metformin has been shown to decrease diabetes-related endpoints including stroke by 32% and diabetes-related mortality including stroke by 42% when compared with conventional therapy. Furthermore, it was more effective in reducing these outcomes when compared with other intensive therapies such as sulfonylureas (e.g. chlorpropamide or glibenclamide) or insulin. It should be noted that the HbA1c levels were similar between the treatment groups, and so the benefits obtained were not explicable on the basis of improved glycemic control [52].
Rosiglitazone, a thiazolidinedione, has been linked with increased risk of MI and death from cardiovascular causes. Stroke was not assessed separately in the meta-analysis reporting these findings [53]. In a more recent multicenter, open-label trial directly assessing the effect of rosiglitazone on cardiovascular outcomes, the use of rosiglitazone was associated with a non-significant reduction in stroke (HR 0.72, 95% CI 0.49–1.06) [54]. Pioglitazone, another medication in the same class, was not associated with worse cardiovascular outcomes, including stroke, and, in fact, reduced a secondary outcome of all-cause mortality, non-fatal MI and non-fatal stroke by 16% [55].
The UK Prospective Diabetes Study (UKPDS) failed to show any reduction in macrovascular complications of T2DM despite an 11% reduction in HbA1c in the intensive treatment group. Microvascular complications such as retinopathy and neuropathy were significantly reduced [56]. A 10-year follow-up study of the same cohort after attempts to maintain treatment differences had desisted showed that the original treatment cohort had persistent decreases in microvascular complications as well as in MIs and deaths from any cause but not CVA [57]. Thus, glycemic control, at least in those with T2DM, has not been linked to a reduction in risk for stroke.
Given how susceptible people with diabetes are to the effects of other vascular risk factors such as hypertension and hyperlipidemia, the therapeutic regimen must also address these states. In treating hypertension in people with diabetes, the classes of medications with effects on the renin angiotensin system appear to have the greatest benefit.
The Hypertensive Old People in Edinburgh (HOPE) trial examined patients either with vascular disease (including CAD or stroke) or diabetes plus one other cardiovascular risk factor such as hypertension, tobacco use or elevated low density lipoprotein levels. In this high risk population, ramipril, an angiotensin-converting enzyme inhibitor (ACEi), decreased the risk of death from cardiovascular causes with a relative risk of 0.74 and reduced the risk of stroke with a relative risk of 0.68. As the mean reduction in BP was only 3/2, the benefits were not attributable to the BP lowering effect of the medication. The effect was similar whether or not the patients had had a stroke prior to enrollment [58].
The Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial examined patients with diabetes, hypertension and left ventricular hypertrophy. Participants were either treated with losartan, an angiotensin receptor blocker (ARB) or atenolol. Although, once again, both medications achieved the same reduction in BP, the primary endpoint of cardiovascular mortality, MI or non-fatal stroke was reduced with a relative risk of 0.76 in the Losartan group. Notably in this trial, only 40% of participants achieved a systolic BP of less than 140 mmHg, implying that further benefits would likely accrue with more intensive management [59].
A recent trial of intensive multifactorial medical management in patients with diabetes with microalbuminuria showed a reduction in all-cause mortality, cardiovascular mortality and cardiovascular events. The regimen was designed to achieve the following goals: HbA1c levels of less than 6.5% (48 mmol/mol), fasting total serum cholesterol levels of less than 175 mg/dL (4.5 mmol/L), systolic BP less than 130 mmHg and diastolic BP less than 80 mmHg. Stroke was not a pre-specified endpoint in this study; however, there were six strokes in six patients in the intensive medical group, compared with 30 strokes in 18 patients in the control group [60].
While antiplatelet treatment is recommended for the primary prevention of CAD, the Anti-Thrombotic Trialists’ Collaboration meta-analysis failed to demonstrate a significant improvement in the primary prevention of ischemic stroke in patients with diabetes. Overall, there were nearly 5000 patients treated with aspirin, with only a 7% reduction in serious vascular events. The confidence interval was wide enough to include a possible 25% risk reduction, a number that is consistent with the prevention of secondary stroke in this population. It may be in this population at high risk, that the potential benefits of prophylactic aspirin outweigh the hemorrhagic complications [61].
Most of the early trials of antithrombotic medications were performed only on men. In the Women’ s Health Study, a study performed among female health professionals with no history of coronary or cerebrovascular disease, low dose aspirin (100 mg every other day) was associated with a 17% risk reduction in stroke, a result of a 24% risk reduction in ischemic stroke and a non-significant increase in hemorrhagic stroke. These findings were especially pronounced in women older than age 65 at the time of enrollment, as well as in the subgroup with diabetes [62].
Treatment of acute stroke in the patient with diabetes
Treatment of acute stroke is limited by the vulnerability of the neuron to ischemic insult. With decreasing cerebral blood flow (CBF), the parenchyma becomes less likely to recover from even short durations of ischemia. With CBF less than 200 mL/kg of tissue, neurologic dysfunction begins to appear, but it is not until CBF falls below 100 mL/kg of tissue that irreversible ischemia occurs, in a matter of minutes [63].
Thrombolysis has been demonstrated to be effective in the treatment of acute stroke, as long as the medication is given within the first 3 hours after symptom onset, as defined by the last time the patient was seen at their neurologic functional baseline (Table 42.2). The National Institute of Neurologic Disorders (NINDS) trial of intravenous tissue plasminogen activator (t-PA) demonstrated a 30–50% increased likelihood of minimal or no disability 3 months after treatment. The thrombolysis was associated with a 6.4% chance of symptomatic intracranial hemorrhage, but mortality data at 3 months were not statistically different between the treatment and placebo groups [64].
Table 42.2 Management of acute ischemic stroke.
| 0–3 hours | Intravenous t-PA |
| 0–6 hours | Intra-arterial t-PA |
| 0–8 hours | Mechanical clot retrieval (MERCI device) |
| Permissive hypertension for the first 3–5 days | |
| Aspiration precautions | |
| DVT prophylaxis | |
| Intravenous t-PA is the only acute intervention approved by the US FDA. Both intra-arterial t-PA and mechanical clot retrieval remain experimental, although widely used. IV t-PA should remain the standard of care when it is not contraindicated, with the other interventions to be used as auxiliary therapy | |
DVT, deep venous thrombosis; FDA, Food and Drug Administration; t-PA, tissue plasminogen activator.
Based on this trial, treatment with intravenous t-PA has become standard of care in this early time period. While recent data from the ECASS-III trial suggest that it is not only safe but clinically effective to a lesser degree in a select group of patients to give intravenous t-PA in the window between 3 and 4.5 hours, this has not yet become standard practice. In addition, patients with diabetes who had a previous stroke by history or on imaging were excluded from the trial [65].
Thrombolysis in the patient with diabetes and acute stroke is not as successful as in the general population. In one series of 27 patients treated with intravenous t-PA, none of the patients with diabetes achieved recanalization of the occluded artery as measured by transcranial Doppler [66]. The series was not large enough to demonstrate a significant difference. In another study examining which factors might predict major neurologic improvement in patients treated with intravenous t-PA, there was a trend towards patients with diabetes being less likely to achieve that improvement [67].
Beyond intravenous thrombolysis, intra-arterial thrombolysis has been examined in patients up to 6 hours after the onset of stroke symptoms. The Prolyse in Acute Cerebral Thromboembolism II (PROACT II) trial found that patients treated with intra-arterial urokinase had a 58% relative risk reduction to achieve minimal or no functional disability at 90 days after treatment. Mortality rates were comparable, and recanalization rates were highly improved with the medication [68]. Current guidelines support the use of intra-arterial thrombolysis in the period of time between 3 and 6 hours, but the medication has not been approved by the US Federal Drug Administration (FDA) for this indication [69].
In one case series of 100 patients treated with intra-arterial thrombolysis with urokinase, diabetes was associated with poor functional outcome at 3 months. It was not associated with symptomatic intracranial hemorrhage [70], but because diabetes is independently associated with worse outcomes following acute ischemic stroke, it is not clear whether these data have any meaning for clinical practice.
Hyperglycemia at the time of stroke treatment is associated with worsened outcomes. In a series of 73 patients treated with intravenous t-PA, age, diabetes, admission glucose greater than 140 mg/dL (7.8 mmol/L) and early reocclusion on transcranial Doppler imaging were significantly associated with worsened functional outcome as defined by a score of greater than 3 on the modified Rankin scale, however, after logistic regression, only the hyperglycemia remained as an independent predictor of poor outcome. In particular, it was associated with larger infarct size, lesser degree of neurologic improvement and worse clinical outcome if recanalization was achieved [71].
Similarly, baseline hyperglycemia is associated with a greater likelihood of going on to symptomatic intracranial hemorrhage after intravenous thrombolysis. There appears to be a dose–response relationship between levels of serum glucose and likelihood of hemorrhage. This was especially true when levels were >11.1 mmol/L (200 mg/dL), where 25% of patients had symptomatic intracranial hemorrhage. In a repeat analysis substituting the presence of diabetes for glucose levels, diabetes was associated with an odds ratio of 3.61 for all hemorrhages and 7.46 for symptomatic hemorrhage [72].
Furthermore, both those patients who acutely worsened and those patients who showed lack of improvement at 24 hours were more likely to have elevated blood glucose at baseline. Hyperacute worsening in patients treated with either intravenous or intra-arterial thrombolysis, or both, was not surprisingly associated with intracerebral hemorrhage and lack of recanalization, but it was also associated with higher serum glucose. With every increase of 50 mg/dL glucose, the odds ratio for worsened outcome was 1.50, and the odds ratio for mortality was 1.38. Even in those patients who did achieve recanalization, higher blood glucose predicted worse outcomes [73].
Similarly, serum glucose greater than 144 mg/dL, as well as cortical involvement and time to treatment were independent predictors of lack of improvement at 24 hours after treatment with intravenous thrombolysis. The odds ratio for hyperglycemia was 2.89. Furthermore, lack of improvement at 24 hours predicted poor functional outcome at 3 months [74].
While these data are understandably disheartening, they should by no means be taken to imply that patients with diabetes and acute stroke should not receive thrombolysis, nor that these patients do not benefit from the treatment. Furthermore, it is not clear whether the hyperglycemia that is seen in patients with acute stroke and diabetes is secondary to the ischemic insult as a stress response, or instead part of the chronic diabetic state and thus purely a complicating factor.
Interestingly, one study examined how persistent hyperglycemia differed from transient hyperglycemia in functional outcomes as well as in mortality. When hyperglycemia was present at baseline and when measured 24 hours after admission, it was inversely associated with neurologic improvement in the first 7 days, 30-day functional outcome and 90-day negligible dependence. At the same time, persistent hyperglycemia was positively associated with increased mortality at 90 days, and parenchymal hemorrhage. When hyperglycemia was absent at baseline but present at 24 hours after admission, it was likewise inversely associated with 90-day negligible dependence, and positively associated with death and parenchymal hemorrhage. In this study, baseline hyperglycemia alone (without persistence at 24 hours) was not associated with poor outcomes. These data suggest that it may not be the stress response hyperglycemia that causes damage in the acute stroke setting [75].
Intensive treatment of hyperglycemia may be associated with improved outcomes, as has been demonstrated in the cardiac literature for MIs [76]. A small pilot study found that hyperglycemic patients could be treated with insulin infusions safely, but the numbers were too small to compare functional outcomes at 1 month [77]. The use of insulin drips in another study to maintain glucose levels at 5.0–7.2 mmol/L (90–130 mg/dL) in patients with acute ischemic stroke, started no later than 12 hours after the onset of symptoms, was associated with a trend towards better functional outcomes and minimal or no neurologic symptoms as measured by the Natonal Institutes of Health (NIH) stroke scale. There were hypoglycemic episodes in the group treated with the continuous infusion, but the majority of these were asymptomatic [78]. Clearly, further study is required on this subject.
Hyperglycemia, as defined by serum glucose >400 mg/dL, was a contraindication for inclusion in the NINDS trial for some of the reasons above, as well as that extreme hyperglycemia can cause focal neurologic deficits that mimic stroke [64]. Current guidelines recommend starting aggressive glycemic control if serum glucose is >200 mg/dL, while acknowledging that levels >140–185 mg/dL may still be harmful [69]. While it may be reasonable to attempt to bring down the glucose level and see if any focal symptoms improve or resolve, and then treat with thrombolysis if no improvement is seen, this approach has yet to be tested.
In terms of oral hypoglycemics in the acute stroke setting, one study looked at the role of sulfonylureas taken pre-stroke and during the acute hospitalization. Sulfonylureas have an effect on ATP-sensitive Kir6.2 potassium channels which are regulated by the sulfonylurea receptor 1 (SUR1) receptor, like pancreatic β-cells, and which are open only during ischemic episodes, causing cell death. Theoretically, then, treatment with sulfonylureas should be neuroprotective during ischemia. Although the numbers were small, and patients with more severe strokes (NIH stroke scale greater than 9) were excluded, patients on the medication were more likely to have a decrease of four points on the NIH stroke scale or a score of 0, and were more likely to achieve an excellent functional recovery at discharge. The effect was particularly noticeable in non-lacunar strokes [79].
Further care for the acute stroke patient is best handled in a certified stroke unit, with multidisciplinary care from a team consisting of vascular neurologists, stroke-trained registered nurses, physical therapists, occupational therapists, and speech and swallow specialists. This care results in around a 25% reduction in mortality [80].
BP control in acute ischemic stroke is a subject of ongoing debate. Current thinking still supports the concept of permissive hypertension in the peri-stroke period. Most vascular neurologists would allow the blood pressure to remain untreated until the systolic BP rises >220 mmHg or the diastolic BP > 120 mmHg. The period over which permissive hypertension should be allowed is also controversial. Typically, the BP is left untreated for the first 3–5 days after stroke [69].
The majority of medical complications after stroke relate to the disability associated with neurologic deficits. Thus, deep venous thrombosis (DVT) and aspiration pneumonitis are the two complications that stroke units need to prevent. Prevention of DVT is accomplished through subcutaneous anticoagulation with either heparin or low molecular weight heparin with or without external compressive devices [69]. Initiation of treatment is typically immediately on admission, regardless of the size of infarct. One unblinded study looked at heparin versus enoxaparin and found a reduction in thrombosis with the low molecular weight heparin [81].
Prevention of aspiration is more complicated. Protection of the airway is often compromised in the acute period after stroke. Frequent suctioning and positioning helps to prevent aspiration. Swallow evaluations should be undertaken before oral nutrition is started in any patient in whom dysphagia is suspected. Placement of nasogastric tubes (NGT) is often required for nutrition, and frequently patients will require percutaneous endoscopic gastrostomy (PEG) tube for long-term provision of nutrition. Hydration is necessary, either intravenously before enteral access is established, or via NGT or PEG tubes to prevent dehydration and electrolyte abnormalities. Antibiotics should be started in any patient suspected of infection, and fever should prompt aggressive search for a source. Hyperthermia itself causes neurologic deterioration, so antipyrrhetics should be administered [69].
Secondary prevention of stroke in diabetes
The management of the patient with diabetes after stroke is similar to that for primary prevention as outlined above (Table 42.3). The seventh report of the Joint National Committee on prevention and treatment of hypertension recommends that people with diabetes should be maintained at a BP <130/80 mmHg, and that it will likely take multiple antihypertensive medications to achieve this goal [82]. As suggested by the studies described above, ACEi and ARB provide greater protection against cardiovascular events including stroke.
Similarly, the National Cholesterol Education Program [83] and subsequently the Endocrine Society [84] have published guidelines on the management of cholesterol in people with diabetes. Diabetes should be considered a CHD risk equivalent and, as such, cholesterol should be lowered aggressively. This is particularly true in those with diabetes who have had a stroke. These patients are considered high risk for CHD, and their levels should be lowered at least to < 100 mg/dL, with < 70 mg/dL an ideal. In combination with the data on statins and stroke described above, all those with diabetes and stroke should be started on a statin.
Table 42.3 Prevention of ischemic stroke in the patient with diabetes.
| Tight glycemic control |
| Antihypertensives with ACEi or ARB |
| Anticholesterol treatment, especially with HMG-CoA-reductase inhibitors (statins) |
| Lifestyle modifications (e.g. tobacco cessation, weight loss) |
| Antiplatelet therapy (either aspirin, clopidogrel or dipyridamole) or |
| Anticoagulation in patients with atrial fibrillation |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; HMG-CoA, 3-hydroxy-3-methyl-glutaryl-Coenzyme A.
Antiplatelet therapy should be started in all patients who have had a non-cardioembolic ischemic stroke (Table 42.3). While the Anti-thrombotic Trialists’ Collaboration meta-analysis failed to show benefit in patients with diabetes, post hoc analysis of data from the Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) study found that people with diabetes treated with clopidogrel had a decreased rate of stroke, MI or death of 15.6% compared to 17.7% risk in those treated with aspirin [85]. Similarly, in a post hoc subgroup analysis of a study of cilostazol, those with diabetes had a decreased rate of recurrent stroke on the medication when compared with placebo, with a relative risk reduction of 41.7%. This finding was especially true in patients with lacunar stroke [86]. These findings need to be verified by further trials.
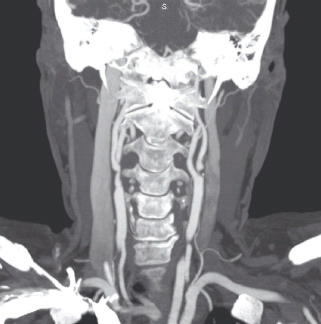
In patients who have had ischemic stroke secondary to extracranial carotid stenosis, carotid endarterectomy remains the preferred treatment of choice for carotid artery stenosis greater than 70% (Figure 42.3). For degrees of stenosis of 50–70%, the benefits of surgery are much smaller, and decisions to treat will depend on the complication rate at local institutions [19]. Carotid artery stenting for symptomatic carotid artery stenosis is still being studied. To this point, unless there is significant risk of undergoing the surgery, such as clinically significant cardiac disease or pulmonary disease, contralateral carotid artery occlusion or history of previous radical neck surgery or neck radiation therapy, carotid endarterectomy is still preferable. In these high risk situations, carotid artery stenting is not inferior to the surgery [87]. Diabetes alone is not enough to categorize a patient as high risk.
People with diabetes and atrial fibrillation, paroxysmal or otherwise, should be anticoagulated with warfarin to a goal International Normalized Ratio (INR) of 2.0–3.0. The risk reduction associated with treatment with anticoagulation is 68%, with an absolute risk reduction in the annual stroke rate from 4.5% to 1.4% [88]. This reduction in risk is so strong that one study estimated that a patient would have to fall 295 times in one year for the risk of subdural hematoma secondary to trauma to outweigh the benefits gained from anticoagulation [89]. There is no reason to assume this is any different for patients with diabetes.
Figure 42.3 Computed tomography angiography with iodinated contrast of the neck. There is mild stenosis seen at the origin of the right internal carotid artery. There is critical stenosis (“string sign”) at the origin of the left internal carotid artery, corresponding to severe atherosclerotic plaque.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree