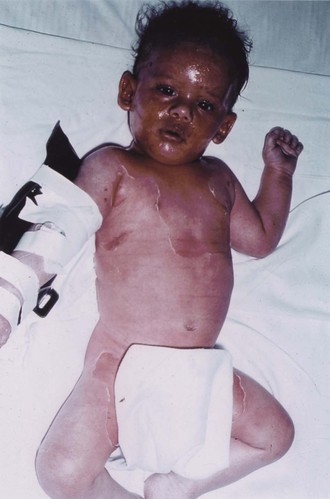
Mark S. Pasternack, Morton N. Swartz * Bacterial and mycotic infections, exclusive of those caused by the common dermatophytes, are discussed in this chapter. Classification of cutaneous infections on morphologic and clinical grounds can be very helpful in providing initial clues regarding the most likely responsible infectious agents (Table 95-1). TABLE 95-1 Classification of Bacterial and Mycotic Infections of the Skin Impetigo is an initially vesicular, later crusted, superficial infection of the skin. Most cases occur in children. The annual incidence of impetigo is 1% to 2%, with annual summer epidemics.1 There are both geographic and yearly variations in incidence related to the clonal spread of virulent or resistant pathogens.2 Previously, group A streptococcus was the principal cause of impetigo and was isolated from about 80% of cases, either alone or mixed with Staphylococcus aureus.3 In the past 20 years, group A streptococcus has been found less commonly (20% to 30%) in impetigo and has been supplanted by S. aureus,1–4 with a growing frequency of methicillin-resistant S. aureus (MRSA) isolates.5 Conceivably, the role of staphylococci may be somewhat overestimated because these organisms are common secondary invaders, and some strains produce bacteriocins that may impair the recovery of group A streptococci. Histopathologically, impetigo consists of superficial, intraepidermal, unilocular vesicopustules. In epidemiologic studies, group A streptococcal acquisition on normal skin antedates the appearance of impetigo by about 10 days.3 During that time, minor trauma (e.g., insect bite, abrasion) or primary dermatoses predispose to the development of infected lesions. Impetigo is most common during hot, humid summer weather. Two to 3 weeks after skin acquisition of streptococci, pharyngeal colonization by the same organism occurs in about 30% of children with skin lesions. (The sporadic cases of facial impetigo occurring in cooler climates probably result from contiguous spread from an initial nasopharyngeal infection, and the serotypes involved are those commonly causing pharyngeal disease.) In staphylococcal impetigo (in which S. aureus is the only pathogen), skin infection follows nasal colonization.5,6 Among returning travelers, impetigo is commonly associated with antecedent insect bites.7 Nonbullous impetigo caused by group A streptococcus (Streptococcus pyogenes) begins when the corneal layer of the epidermis is disrupted and the bacteria gain access to highly differentiated subcorneal keratinocytes. Impetigo strains, but not pharyngeal strains, of S. pyogenes preferentially bind to differentiated keratinocytes,8 mediated by S. pyogenes M protein.9 A second streptococcal surface protein (protein F, fibronectin-binding protein), mediates adherence to antigen-presenting Langerhans’ cells located along the basal layer of the epidermis. Staphylococcal infection usually develops after nasal or skin colonization. Bacteriocin production may inhibit competing commensal flora, and several virulence factors have been proposed to enhance adhesion to epithelial cells and to underlying matrix proteins.10 Surprisingly, the production of exfoliative toxins A and B has been associated with both nonbullous and bullous impetigo; Panton-Valentine leukocidin is generally absent among staphylococcal impetigo isolates.5 Impetigo is a highly communicable infection. Spread in families (particularly among preschool-aged children) is facilitated by crowding and poor hygiene. Streptococcal impetigo begins on exposed areas as small vesicles, sometimes with narrow inflammatory halos, that rapidly pustulate and readily rupture. The purulent discharge dries and forms the characteristic thick, golden-yellow, “stuck-on” crusts. Pruritus is common, and scratching of lesions can spread infection. Occasionally, large crusts are produced by coalescence of smaller pustules. The lesions remain superficial and do not ulcerate or infiltrate the dermis; mild regional lymphadenopathy is common. Healing generally occurs without scarring. The lesions are painless, and constitutional manifestations are minimal. Gram-stained smears of vesicles show gram-positive cocci. Culture of exudate beneath an unroofed crust reveals S. aureus, group A streptococci, or a mixture of streptococci and S. aureus. The anti–streptolysin O titer after streptococcal impetigo is scant, probably related to inhibition of streptolysin O by skin lipids at the infection site. In contrast, the anti–DNase B response readily occurs after streptococcal impetigo (with elevated titers in 90% of patients with nephritis complicating streptococcal skin infections).11 The group A streptococci responsible for impetigo usually belong to different M serotypes from those of strains that produce pharyngitis. M surface proteins, which are key virulence factors, are encoded in emm genes. Five major emm chromosomal patterns (groups A through E) have been described based on nucleotide sequences encoding part of the peptidoglycan-spanning M protein domain.12 Almost all group A streptococcal impetigo isolates belong to emm chromosomal patterns D and, to a lesser extent, E, whereas most isolates from patients with uncomplicated pharyngitis or acute rheumatic fever belong to emm chromosomal pattern group A, B, or C. Groups C and G streptococci may rarely cause impetigo; group B streptococci have been associated with impetigo in neonates. The rising incidence of MRSA impetigo has been associated with both hospital- and community-acquired strains.5 Although the initial vesicular lesions may resemble early varicella, the crusts of impetigo are darker brown and harder. The central clearing of a confluent cluster of lesions of impetigo may suggest tinea circinata but can be distinguished by the thick crusts, which are not formed in the fungus infection. When the vesicles of herpes simplex become turbid, they may resemble those of impetigo. Distinguishing between herpes simplex and impetigo is important because irritation from topical therapy may exacerbate primary herpes simplex lesions. Acute palmoplantar pustulosis, a sterile, idiopathic, self-limited pustular eruption on the palms and soles that sometimes occurs after pharyngitis, may initially resemble impetigo.13 Localized acute pustular psoriasis may also be mistaken for impetigo. Primary cutaneous listeriosis, an occupational disease of veterinarians and farmers involved in calving, is characterized by papulovesicular and pustular lesions on the forearms that may resemble those of impetigo.14 Atopic or contact dermatitis, discoid lupus erythematosus, and infestations such as scabies may mimic impetigo or develop secondary impetiginization.15 Penicillin was the classic drug of choice for the treatment of impetigo, because of the predominant role of group A streptococci and the subsequent risk for acute glomerulonephritis. Because mixed streptococcal-staphylococcal or staphylococcal impetigo is clinically indistinguishable from streptococcal impetigo, and S. aureus, either alone or in concert with S. pyogenes, currently is the predominant cause of impetigo, primary therapy must be revised accordingly. Penicillinase-resistant oral penicillins (e.g., dicloxacillin or amoxicillin-clavulanate) or cephalosporins (e.g., cephalexin, cefadroxil) are generally effective for treating methicillin-sensitive strains of S. aureus as well as S. pyogenes. The relative incidence of MRSA (vs. methicillin-susceptible S. aureus [MSSA]) causing impetigo is not well established. Erythromycin or newer macrolides generally are reserved for β-lactam–allergic patients. The efficacy of macrolides may be reduced in areas in which erythromycin-resistant staphylococci and streptococci are prevalent. Local care (removal of crusts by soaking with soap and water) is helpful. Patients with extensive disease should undergo wound culture and reevaluation after an initial trial of oral therapy. The presence of MRSA must be strongly considered if there is a poor initial response to therapy, even if no culture data are available, and empirical therapy must be broadened (see later). Co-trimoxazole has excellent activity against community-acquired MRSA, and in vitro data support its possible use against S. pyogenes as well, although clinical data are lacking.16 Clindamycin and macrolides have variable efficacy against many community-acquired MRSA isolates. Topical antibiotic therapy may be considered when treating limited impetigo, including that caused by MRSA. Topical mupirocin ointment in a polyethylene glycol base is as effective as oral erythromycin for the treatment of impetigo17 and is more effective when treating erythromycin-resistant S. aureus.18 In the past decade, the incidence of mupirocin resistance among S. aureus isolates has been rising slowly but steadily. A second topical antibiotic, retapamulin ointment, is approved for the treatment of impetigo. This is the first of a novel class of bacterial protein synthesis inhibitors (pleuromutilins) and is effective against S. aureus and Streptococcus pyogenes independent of other antibiotic susceptibilities.19 Fusidic acid cream is available in Europe and is effective in treating childhood impetigo (primarily S. aureus), but rising resistance rates2 and policies to restrict its applicability for systemic infection have limited its use. Gentle application of topical agents is important to minimize tissue maceration and spread of infection. Systemic therapy is preferred when treating widespread impetigo. Close follow-up of patients is important because of rising rates of resistance to these topical and systemic agents. Mupirocin has also been used topically to eradicate MRSA from secondarily infected skin lesions and from colonized patients. However, because resistance in S. aureus strains has emerged sooner than anticipated after the introduction of mupirocin,18 particularly when long-term therapy was used, prolonged administration should probably be avoided. The bullous form of impetigo is caused by S. aureus of phage group II (usually type 71); it occurs principally in neonates and young children and accounts for about 10% of all cases of impetigo. The lesions begin as vesicles that turn into flaccid bullae initially containing clear yellow fluid. No erythematous areola is noted, and the Nikolsky sign is absent. The bullae quickly rupture, leaving a moist red surface, and then form thin, varnish-like light brown crusts. Bullous impetigo, like the staphylococcal scalded skin syndrome (SSSS) and the staphylococcal scarlatiniform syndrome, represents a cutaneous response to the two extracellular exfoliative toxins (ETA and ETB) produced by phage group II S. aureus. S. aureus isolates from patients with bullous impetigo uniformly carry these exfoliative toxins.5,20 ETA is chromosomally encoded, and the heat-labile ETB is plasmid encoded. Both are glutamate-specific serine proteases that bind to and cleave desmoglein-1, a desmosomal transmembrane glycoprotein necessary for epidermal cell adhesion.20,21 The toxins may act locally at the site of cutaneous infection to produce bullous impetigo or may spread systemically to cause generalized blistering when produced at a site of cutaneous S. aureus infection. Staphylococci are regularly isolated from the skin lesions of bullous impetigo. Streptococcal superinfection rarely complicates bullous impetigo, probably because type 71 strains of S. aureus produce a bacteriocin that inhibits streptococci. Fever and constitutional symptoms are uncommon, and healing occurs without scarring. Mild infections are often missed22 and have even been misdiagnosed as nonaccidental scalds in young children.23 Rarely, bullous impetigo has been attributed to group A streptococcal infection. Extensive bullous impetigo caused by MSSA generally responds to treatment with a penicillinase-resistant penicillin agent (e.g., dicloxacillin, 25 to 50 mg/kg daily in divided doses orally every 6 hours for a child, or amoxicillin-clavulanic acid), cephalosporin (e.g., cephalexin, 25 to 50 mg/kg daily in divided doses orally every 8 to 12 hours for a child), or erythromycin or clindamycin for a penicillin-allergic patient. When MRSA is widespread in the community, culture and initial oral therapy with co-trimoxazole, clindamycin, or linezolid may be considered for mild to moderate disease, with initial intravenously administered vancomycin reserved for patients with widespread disease. SSSS is the most severe and systemic manifestation of infection with S. aureus strains producing an exfoliative exotoxin; it is characterized by widespread bullae and exfoliation.21,24 Pemphigus neonatorum (Ritter’s disease) is SSSS in the neonate. The more general term toxic epidermal necrolysis is often used to encompass both SSSS and a morphologically similar syndrome of various causes (drug reactions, viral illnesses; see Chapter 196). SSSS has been associated with both MSSA and MRSA. SSSS usually occurs in younger children but can rarely develop in adults. Epidemics have occurred in neonatal nurseries.25 SSSS begins abruptly (sometimes a few days after a recognized staphylococcal infection) with fever, skin tenderness, and a scarlatiniform rash. The Nikolsky sign can be demonstrated. Large, flaccid, clear bullae form, promptly rupture, and result in the separation of sheets of skin. New bullae appear over a period of 2 to 3 days. Exfoliation exposes large areas of bright red skin surface (Fig. 95-1). S. aureus may be recovered from a distant site of infection or colonization, in contrast to the ready recovery of organisms from sites of localized bullous impetigo. In settings in which the diagnosis of SSSS is uncertain, a biopsy obtained from an area of desquamation will demonstrate bland intraepidermal cleavage at the granular layer, which is distinct from the subepidermal separation observed in bullous disorders and toxic epidermal necrolysis. With appropriate fluid replacement and antimicrobial therapy, the skin lesions heal within 2 weeks, in contrast to drug-induced toxic epidermal necrolysis, in which recovery is more prolonged because the entire epidermis must be replaced and scarring is more frequent. The mortality rate from SSSS in children is less than 3% but is often higher in adults, many of whom have underlying immunodeficiency, renal failure, or other significant comorbidities. Intravenous vancomycin is indicated for the initial treatment of SSSS because of the widespread staphylococcal infection and rapid progression of the skin lesions, particularly in settings such as a neonatal nursery and in communities with high rates of MRSA disease. Once MSSA is identified, intravenous nafcillin (100/mg/day for neonates, 100 to 200 mg/kg/day for older children) is appropriate. Topical treatment consists of cool saline compresses. Systemic corticosteroids alone should not be used in the treatment of SSSS, although they may be indicated in therapy for drug-induced toxic epidermal necrolysis. Staphylococcal scarlet fever is fundamentally a forme fruste of SSSS that does not progress beyond the initial stage of a generalized erythematous eruption. However, S. aureus enterotoxins (A through D) and toxic shock syndrome toxin 1 (TSST-1) are more frequently associated with staphylococcal scarlet fever than are ETA and ETB.26 The rash is indistinguishable from that of scarlet fever, and Pastia’s lines can develop. However, pharyngitis is not usually present and an enanthem does not develop. Desquamation, beginning on the face and involving most of the body, occurs 2 to 5 days after onset of the scarlatiniform rash. Antibiotic treatment (penicillinase-resistant penicillins or alternative therapy effective against MRSA) is indicated. Toxic shock syndrome is another acute febrile illness with a generalized scarlatiniform eruption associated with S. aureus infection. Other elements of the syndrome include (1) hypotension (shock), (2) functional abnormalities of three or more organ systems, and (3) desquamation in the evolution of the skin lesions24,27 associated with the elaboration of TSST-1 or one of several staphylococcal enterotoxins. These exotoxins act as superantigens that activate large numbers of T cells, triggering systemic inflammation, distinct from the direct-acting exfoliative toxins responsible for bullous impetigo and SSSS (see Chapter 196). Folliculitis is a pyoderma located within hair follicles and the apocrine regions. The lesions consist of small (2 to 5 mm), erythematous, sometimes pruritic papules often topped by a central pustule and a fine surrounding collar of desquamation.28 Sycosis barbae is a distinctive form of deep folliculitis, often chronic, that occurs on bearded areas. S. aureus is the usual cause of folliculitis. Pseudomonas aeruginosa (most often serotype O-11) has been responsible for folliculitis acquired from swimming pools and whirlpools contaminated with large numbers of these organisms29; Aeromonas folliculitis has been reported rarely in similar settings. This type of skin infection produces pruritic, sometimes tender, papulourticarial lesions (appearing within 48 hours after exposure) that eventuate in pustule formation. Lesions in different stages of development (macules, papules, papulopustules) are present simultaneously. Preferred sites include the buttocks, hips, and axillae, particularly areas in contact with bathing suits; the palms and soles are generally spared. Otitis externa is also a common manifestation. Healing occurs spontaneously within 5 days, by drainage or regression. Rarely, scarring develops when a pustule progresses to furuncle formation. If folliculitis is acquired in a whirlpool, the lesions are sharply limited to the trunk below the upper part of the chest or neck. Inadequate chlorine levels in whirlpools, hot tubs, and swimming pools have been responsible for many of the outbreaks reported. P. aeruginosa can also cause superinfection in acne and rosacea after prolonged broad-spectrum antibiotic therapy. In granulocytopenic and immunosuppressed hospitalized patients, P. aeruginosa O-11 from tap water used for washing was implicated in folliculitis that rapidly progressed to ecthyma gangrenosum.30 Immersion is not required to develop Pseudomonas folliculitis; it has been reported in a toddler exposed to a contaminated washcloth and bathmat.31 Folliculitis, often perioral and perinasal, caused by Enterobacteriaceae, can occur as a complication in patients with acne and rosacea, usually during prolonged courses of oral antibiotic therapy.32 Candida may cause folliculitis, typically producing pruritic satellite lesions surrounding areas of intertriginous candidiasis, in infants or patients receiving prolonged antibiotic or corticosteroid therapy. Malassezia furfur, a common skin saprophyte, may also produce a folliculitis with pruritic erythematous papules and papulopustules on the trunk, upper extremities, and face, particularly in the setting of diabetes mellitus, corticosteroid administration, or granulocytopenia,33,34 and may be confused with acne vulgaris.35 These lesions, particularly the early papular nodular ones, may suggest those of systemic candidiasis, a diagnosis that may seem to be supported by the presence of budding yeast forms on Gram-stained material from unroofed lesions. Unlike Candida, M. furfur requires lipid-supplemented media for primary isolation. Additional nonbacterial folliculitis syndromes have been associated with herpes simplex, particularly in the presentation of severe sycosis barbae,36 which may or may not be associated with secondary staphylococcal infection. Folliculitis, localized or disseminated, may occur after smallpox vaccination but does not represent progressive cutaneous viral infection.37 Folliculitis has been associated with Demodex mite infestation38 and rarely with parasitic disease. Eosinophilic pustular folliculitis, a rare pruritic dermatosis characterized by recurrent crops of follicular papules and pustules with eosinophilic infiltration of perifollicular dermis, occurs particularly in the setting of acquired immunodeficiency syndrome (AIDS). It resembles bacterial or mycotic folliculitis but is a sterile process confirmed by biopsy.39 Amicrobial pustulosis, another noninfectious folliculitis syndrome, may develop in normal hosts.40 Folliculitis may develop after the use of tyrosine kinase inhibitors and immunomodulatory agents, including infliximab.41 Local measures such as saline compresses and topical antibacterial agents (e.g., mupirocin) or antifungal agents (e.g., clotrimazole) are usually sufficient to control the infection.42 Severe or widespread disease (e.g. pseudomonal infection) may respond to oral fluoroquinolone therapy. Severe or refractory lesions should be cultured and considered for biopsy to assess uncommon causes of infection or noninfectious processes.
Cellulitis, Necrotizing Fasciitis, and Subcutaneous Tissue Infections
Cellulitis and Superficial Infections
TYPE OF LESION
CAUSATIVE AGENTS
Primary Pyodermas
Impetigo
Staphylococcus aureus, group A streptococci
Folliculitis
S. aureus, Candida, Pseudomonas aeruginosa, Malassezia furfur, Pityrosporum ovale
Furuncles and carbuncles
S. aureus
Paronychia
S. aureus, group A streptococci, Candida, P. aeruginosa
Ecthyma
Group A streptococci
Erysipelas
Group A streptococci
Chancriform lesions
Treponema pallidum, Haemophilus ducreyi, Sporothrix, Bacillus anthracis, Francisella tularensis, Mycobacterium ulcerans, Mycobacterium marinum
Membranous ulcers
Corynebacterium diphtheriae
Cellulitis
Group A or other streptococci, S. aureus; rarely, various other organisms
Infectious Gangrene and Gangrenous Cellulitis
Streptococcal gangrene and necrotizing fasciitis
Group A streptococci, mixed infections with Enterobacteriaceae and anaerobes
Progressive bacterial synergistic gangrene
Anaerobic streptococci plus a second organism (S. aureus, Proteus)
Gangrenous balanitis and perineal phlegmon
Group A streptococci, mixed infections with enteric bacteria (e.g., Escherichia coli, Klebsiella) and anaerobes
Gas gangrene, crepitant cellulitis
Clostridium perfringens and other clostridial species; Bacteroides, peptostreptococci, Klebsiella, E. coli
Gangrenous cellulitis in immunosuppressed patients
Pseudomonas, Aspergillus, agents of mucormycosis
Secondary Bacterial Infections Complicating Preexisting Skin Lesions
Burns
P. aeruginosa, Enterobacter, various other gram-negative bacilli, various streptococci, S. aureus, Candida, Aspergillus
Eczematous dermatitis and exfoliative erythrodermas
S. aureus, group A streptococci
Chronic ulcers (varicose, decubitus)
S. aureus, streptococci, coliform bacteria, P. aeruginosa, peptostreptococci, enterococci, Bacteroides, C. perfringens
Dermatophytosis
S. aureus, group A streptococci
Traumatic lesions (e.g., abrasions, animal bites, insect bites)
Pasteurella multocida, C. diphtheriae, S. aureus, group A streptococci
Vesicular or bullous eruptions (varicella, pemphigus)
S. aureus, group A streptococci
Acne conglobata
Propionibacterium acnes
Hidradenitis suppurativa
S. aureus, Proteus and other coliforms, streptococci, peptostreptococci, P. aeruginosa, Bacteroides
Intertrigo
S. aureus, coliforms, Candida
Pilonidal and sebaceous cysts
Peptostreptococci, Bacteroides, coliforms, S. aureus
Pyoderma gangrenosa
S. aureus, peptostreptococci, Proteus and other coliforms, P. aeruginosa
Cutaneous Involvement in Systemic Bacterial and Mycotic Infections
Bacteremias
S. aureus, group A streptococci (also other groups such as D), Neisseria meningitidis, Neisseria gonorrhoeae, P. aeruginosa, Salmonella typhi, Haemophilus influenzae
Infective endocarditis
Viridans streptococci, S. aureus, group D streptococci, and others
Fungemias
Candida, Cryptococcus, Blastomyces dermatitidis, Fusarium
Listeriosis
Listeria monocytogenes
Leptospirosis (Weil’s disease and pretibial fever)
Leptospira interrogans serotypes
Rat-bite fever
Streptobacillus moniliformis, Spirillum minus
Melioidosis
Burkholderia pseudomallei
Glanders
Burkholderia mallei
Carrión’s disease (verruga peruana)
Bartonella bacilliformis
Scarlet Fever Syndromes
Scarlet fever
Group A streptococci, rarely S. aureus
Scalded skin syndrome
S. aureus (phage group II)
Toxic shock syndrome
S. aureus (pyogenic toxin-producing strains)
Parainfectious and Postinfectious Nonsuppurative Complications
Purpura fulminans (manifestation of disseminated intravascular coagulation)
Group A streptococci, N. meningitidis, S. aureus, pneumococcus
Erythema nodosum
Group A streptococci, Mycobacterium tuberculosis, Mycobacterium leprae, Coccidioides immitis, Leptospira autumnalis, Yersinia enterocolitica, Legionella pneumophila
Erythema multiforme–like lesions (rarely), guttate psoriasis
Group A streptococci
Other Lesions
Erythrasma
Corynebacterium minutissimum
Nodular lesions
Candida, Sporothrix, S. aureus (botryomycosis), M. marinum, Nocardia brasiliensis, Leishmania brasiliensis
Hyperplastic (pseudoepitheliomatous) and proliferative lesions (e.g., mycetomas)
Nocardia, Pseudallescheria boydii, Blastomyces dermatitidis, Paracoccidioides brasiliensis, Phialophora, Cladosporium
Vascular papules/nodules (bacillary angiomatosis, epithelioid angiomatosis)
Bartonella henselae, Bartonella quintana
Annular erythema (erythema chronicum migrans)
Borrelia burgdorferi
Primary Pyodermas
Impetigo
Pathologic Characteristics and Pathogenesis.
Clinical Manifestations.
Laboratory Findings.
Etiologic Agents.
Differential Diagnosis.
Presumptive Therapy.
Bullous Impetigo
Clinical Manifestations.
Presumptive Therapy.
Staphylococcal Scalded Skin Syndrome
Clinical Manifestations.
Presumptive Therapy.
Staphylococcal Scarlet Fever
Toxic Shock Syndrome
Folliculitis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cellulitis, Necrotizing Fasciitis, and Subcutaneous Tissue Infections
95