14.1
Introduction
Bone apposition and resorption are constantly occurring processes that enable bones to adapt structurally by optimizing their shape and size to meet the physical requirements of their external environment . For a seemingly inert tissue, bone has the ability to remodel, reorganize, and regenerate in response to mechanical loading and injury. Mechanical force is an important regulator of biology and plays a critical role in tissue development and maintenance . Mechanisms by which mechanical loading regulates bone remodeling and adaptation involve first the sensing of an extracellular physical signal, second the conversion of this mechanical signal into a biochemical signal, a process known as mechanotransduction, and third the response of cells to the biochemical signals. An impressive range of tissues and cells is regulated by mechanical loading, and this regulation is central to disease processes such as osteoporosis, atherosclerosis, and osteoarthritis.
Frost proposed in 1987 the idea of the “mechanostat” as a mechanism to control bone growth, modeling, and remodeling activities to explain the observation that in a healthy skeleton, bone mass is optimized for the mechanical usage . The mechanostat theory proposes that a certain range of strains promotes bone apposition (thought to be 1500–3000 microstrain in healthy skeletons based on in vivo studies), while a lower range of strains (100–300 microstrain) promotes resorption. However, these mechanical set points are thought to be regulated by other factors and can change in response to decreases in estrogen associated with menopause or are influenced by genetics, for example. Raising the set points to higher strain levels would then promote bone resorption, which can result in osteoporosis.
The mechanostat theory also proposes that reduced mechanical loading (disuse) leads to a decrease in bone density and strength, which has been observed in those who experience spaceflight, bed rest, aging, and injury. For example, prolonged microgravity associated with extended spaceflight can lead to an increase in bone turnover markers and bone loss . In addition, several studies have shown that individuals disabled by spinal cord injury have a profound loss in bone mineral density below the site of injury .
In contrast, physical activity can increase the bone size and density. Professional tennis players have greater bone density and bone diameter in their dominant (playing) arm compared to their nondominant arm . In addition, professional soccer players have significantly higher bone mineral content and density in their lower limbs compared to age-matched controls , and professional baseball players exhibit adaptive changes in bone mass and structural properties in the proximal humerus of their dominant versus nondominant playing arm, with some changes persisting long after their playing career is over . Taken together, these studies confirm that mechanical loading is important in maintaining bone structure. A lack of a mechanistic understanding of these processes is one of the primary drivers of the field of mechanobiology, which, as a consequence, has enormous potential to make critical new insights into both physiological function and etiology of disease .
Physical activity applies loads to bone on the organ level, producing tissue-level stress and strain. These, in turn, result in a complex pericellular mechanical environment that presents physical signaling to bone and osteoprogenitor cells. Intracellular molecular signaling as a response to physical signals leads to changes in cell metabolism, cell–cell signaling, and an integrated tissue-level response. In this view the fundamental transduction event, whereby a mechanical signal is converted to a biochemical signal, occurs at the molecular level. The mechanism by which cells may sense these forces applied at the organ level and create a biochemical response by activating signaling pathways remains unclear.
The purpose of this chapter is to review the state of knowledge systematically with respect to skeletal mechanobiology, and material is borrowed heavily from previous reviews by the authors . This revised chapter contains updates on the nature of the physical signals experienced by progenitor and bone cells, potential molecular mechanisms responsible for cellular mechanosensing, and the resulting biochemical response. In addition, a new section covers the effects of aging on bone cell mechanobiology, as this concept is central to understanding the etiology of osteoporosis.
14.2
Bone mechanotransduction
Mechanotransduction, the process by which cells convert external mechanical forces into biochemical responses, is important in maintaining adult bone health and homeostasis. Application of mechanical loading has been shown to accelerate bone formation rates in animal studies ( Fig. 14.1 ) . The use of animal models has not only provided insights into the effects of various loading parameters such as magnitude of load, frequency, and rest insertion but has also enabled evaluation of the roles of biochemical and biological components in bone mechanotransduction through the use of genetically modified animals.
When mechanical loading is applied to bone at the organ level, strains and fluid movement may result at the tissue level. However, because bone apposition and resorption occur through the coupled activity of osteoblasts and osteoclasts, understanding how tissue-level mechanical changes are transduced at the cellular level is important.
Mechanical loading has been proposed to result in physical signals such as electric/streaming potentials, substrate strain, and flow-induced shear stress that can be sensed by bone and osteoprogenitor cells . Due to its ability to elicit cellular responses at physiological loading levels in in vitro studies, the current paradigm is that the combination of fluid flow–induced shear stress along the osteocyte body and cell processes is a mechanism by which mechanical loading from daily activity elicits changes in bone cell metabolism .
When bone is loaded, fluid is displaced from regions of compression to regions of relative tension. Mechanical loading from daily activities such as walking and running produces higher pressures in areas of relative compression compared to tension. This differential in pressure causes fluid to move through the lacunocanalicular network (LCN) in bone. Additional evidence for this theory comes from an in vivo study by Qin et al. in which oscillatory fluid flow induced bone formation in turkey ulnae in the absence of other mechanical stimulation .
Osteocytes are located within fluid-filled voids (lacunae) within the bone matrix. They form an interconnected network with one another and with osteoblasts at the bone surface via their cell processes that extend through channels called canaliculi. Osteocytes make up a vast majority of the bone’s cell population and are distributed throughout the bone matrix . Osteocyte ablation in a mouse model was shown to result in increased intracortical porosity and decreased sensitivity to unloading, supporting the role of osteocytes in mechanotransduction . Interestingly, these mice exhibited new bone formation in response to mechanical loading suggesting that osteogenic cells at bone surfaces may be able to directly sense and respond to mechanical stimulation.
Osteoblasts originate from various populations of progenitor cells. While adherent bone marrow stromal cells have been most widely studied, populations of nonadherent bone marrow cells, cells in peripheral blood, and pericytes also have characteristics of osteoprogenitor cells . Various in vitro studies have shown that mechanical stimulation of osteoblasts and stem cells results in an osteogenic response . Each of these cell types contributes to bone remodeling and adaptation, experiencing different mechanical environments based on their location within bone. For example, stem cells in the bone marrow may experience fluid flow–induced shear stress, tensile and compressive strains, and intramedullary pressure .
Mechanical loading activates multiple signaling pathways, resulting in osteogenic and antiresorptive responses. Within seconds to minutes after exposure to fluid flow, osteocytes respond with the release of nitric oxide (NO), adenosine triphosphate (ATP), calcium (Ca 2+ ), and prostaglandin E2 (PGE2) . These initial responses lead to downstream effects that mediate bone modeling and remodeling, including cytochrome oxidase subunit 2 (COX-2), which converts arachidonic acid to PGE2 . In relaying mechanical cues to effector cells, osteocytes communicate with osteoblasts via gap junctions formed at the osteocyte cell process . Osteocytes also regulate osteoclast differentiation and activation . Receptor activator of nuclear factor kappa-B (NF-κB) ligand (RANKL) stimulates osteoclast formation by binding to its receptor on the surface of osteoclast precursors, while osteoprotegerin (OPG), a decoy receptor for RANKL, inhibits osteoclastogenesis by competitively binding RANKL. The ratio of OPG/RANKL expression is used as an indicator of osteoclastogenesis, with higher ratios indicating lower levels of osteoclastogenesis. Dynamic fluid flow has been found to increase the OPG/RANKL ratio in osteocytes, demonstrating that physical stimulation can influence osteocyte-mediated inhibition of osteoclastogenesis .
With age, various factors such as a decrease in physical activity, decrease in number of osteoblasts, and a diminished cellular response to mechanical stimulation may combine to contribute to the development of osteoporosis . For example, osteoblasts isolated from old rats have been observed to display less fluid flow–induced calcium oscillations than cells from mature rats . Aging can also impair the agonist-stimulated activity of second messengers in osteoblasts .
An important aspect of the mechanotransduction process, which is less well understood, is the molecular mechanism(s) by which bone and progenitor cells sense mechanical loads and initiate these intracellular signaling cascades. Several proteins or assemblies of proteins have been proposed to regulate local bone modeling or remodeling in response to physical stimuli and include integrins , connexin (Cx) hemichannels , and ion channels . The primary cilium, a microtubule structure that protrudes from the cell membrane like a cellular antenna, is another candidate for an extracellular sensor of mechanical loading. It was first implicated in mechanosensing in the kidney and liver . Recent studies have suggested that the primary cilium is also a mechanosensor in bone .
14.3
Forms of mechanical stimulation—tissue mechanics
Daily activities apply loads to bones at the organ level, producing tissue-level deformations or strains. In turn, these tissue-level mechanics are transduced into biophysical effects, which are sensed at the cellular level. In this section the mechanical behavior of bone as a tissue is presented.
14.4
Electromagnetic fields
When bone tissue is dynamically loaded, it produces well-characterized dynamic electric fields and magnetic fields. Initially described by Friedenberg and Brighton , these potentials have been ascribed to both the piezoelectric effect and streaming potentials due to convective ion transport . It has been established that the application of exogenous electromagnetic fields to bone is osteogenic , particularly in healing fractures, and it has become an accepted therapy in several contexts. In addition, the endogenous electric fields that occur with loading have been proposed to be a physical signal important to bone mechanoresponsiveness.
14.5
Deformation or strain
Owing to the mineralized nature of bone tissue, the habitual strains in this tissue are quite small relative to other tissues. Early estimates of in vivo strains are on the order of 500 microstrain (0.05%) during habitual activity and could double or triple with strenuous activity. More recent results confirm these early studies with a maximum reported strain during vigorous activity of approximately 2000 microstrain (0.2%) . Interestingly, habitual strains do not appear to be consistent between subjects or anatomical sites, nor do high-impact activities designed to protect against bone loss generate more strain than activities that are less protective. An upper limit to bone tissue strains can be assumed to be the failure strain, which is roughly 3%. How tissue-level strains scale-down to cellular-level strains is an open question. Recently, Verbruggen et al. showed that an applied peak tissue strain of 3000 με resulted in an approximate 10× amplification of strain at the cell membrane in osteocytes and osteoblasts, levels that exceed the experimentally determined strain thresholds (>1050 με) for initiating new bone formation at the tissue level and activating mechanoresponsive signaling pathways in bone cells (>10,000 με) .
14.6
Fluid flow due to loading
Bone is fundamentally a hydrated tissue, and mechanical loading has the potential to affect the distribution and flow of its fluid, which is contained in three fundamental spaces: the vascular space, the fluid component of the mineralized tissue, and the network of osteocyte lacunae and interconnecting canaliculi.
The vascular component includes primary nutrient vessels, which branch into intermediate bundled arterioles and venules that ultimately bifurcate to form capillaries where gas, nutrient, and metabolite exchange occurs. In cortical bone, the vasculature is associated with nerve bundles and housed in longitudinal channels in the center of osteons known as Haversian canals. The Haversian canals are interconnected via perpendicular Volkmann’s canals. Fluid flow in the bone vascular space is primarily due to arterial pressure, which is also the primary driving force for fluid exudation from this space. However, owing to the large diameter of vessels and extremely high permeability, tissue loading has little direct influence on vascular pressures and flow .
The converse situation applies to fluid in the bone matrix. The porosity of this fluid compartment is quite small. Some evidence collected in the 1990s suggested that loading can result in mobilization of this fluid. However, this hypothesis was explored primarily in the context of stress-generated potentials. Currently, the fluid component of bone matrix is believed to be too tightly bound to undergo significant flow with loading . Thus the primary loading-induced fluid motion appears to be through the LCN. These voids in the bone matrix are occupied by the cell body in the case of lacunae, and long cellular processes in the case of canaliculi. However, there is a well-defined extracellular fluid compartment between the osteocyte and the bone tissue matrix ( Fig. 14.2 ). In 1977 Piekarski and Munro first advanced the idea that periodic skeletal loading pumps fluid through this network as the mechanism by which osteocytes remain viable despite their distance from the vascular supply . Lacunocanalicular fluid flow is the sum of a steady wash-through flow due to circulatory-induced intramedullary pressure and load-induced flow . When bone is loaded, fluid is displaced from regions of compression to regions of relative tension. Osteocytes are ideally situated to sense fluid flow as they are dispersed throughout the bone matrix and can communicate with one another and with cells at the bone surface through gap junctions .
Experimental evidence for this phenomenon comes primarily from tracking the motion of injected tracers. Using in vivo and ex vivo tracers in rodents, Tate et al. have shown that the LCN can accommodate molecules up to 40–70 kDa or ~7 nm in size by diffusion alone . Larger molecules were not able to penetrate the cortical bone in mature animals suggesting that mechanical loading is necessary for the diffusion of molecules larger than 10 nm . Indeed, they later showed that mechanical loading enhanced intracortical fluid flow in a site-specific manner . In a separate study, Dillaman et al. showed that unloading reduced the perfusion of horseradish peroxidase (4–5 nm) . Qin et al. showed that short daily bouts of a sinusoidal fluid pressure signal (60 mmHg, 20 Hz), in the absence of strain, prevented disuse bone loss and led to new bone formation in isolated turkey ulnae , thereby providing compelling evidence that fluid flow was a key regulator of load-induced adaptation as mentioned previously. Flow through the lacunocanalicular system is also influenced by the morphology and connectivity of the network , suggesting that changes in the network due to disease or aging may affect the flow experienced by osteocytes.
Despite the lack of direct effect of bone tissue loading on vascular pressure, mechanisms whereby artery-driven flow is regulated by loading or activity have been proposed. Direct observation of neovascularization within a bone ingrowth chamber has led to the proposal that enhanced venous return due to muscle contractions with physical activity increased net (or steady) flow and was an important anabolic cellular signal. Another alternative is that pressure in the intramedullary canal drives an outward radial flow and induces an anabolic effect. Indeed, intramedullary pressurization occurs with loading, pressurizing the intramedullary canal directly , or venous ligation . The converse is also true: disuse leads to loss of intramedullary pressure and bone loss . However, it has been suggested that the flow through the LCN due to intramedullary pressure is much lower than that due to loading in normal conditions. In this case, periosteal cells rather than osteocytes may be responding, a concept that is supported by work showing that mice in which osteocytes have been ablated retain the ability to make new bone in response to applied mechanical loading . Nonetheless, dynamic (oscillatory) flow through the osteocyte network without applied loading clearly results in bone formation .
Less is understood about the flow of fluid in marrow-filled spaces and within in-filling osteons, both of which house osteoprogenitors and preosteoclasts. Intramedullary pressure and induced fluid flow are possible mechanical signals that regulate the activities of cells found in the marrow. Dickerson et al. showed that whole-body vibration, a known anabolic stimulus for vertebral trabecular bone , resulted in fluid flow shear stress (1–20 dyn/cm 2 ) at trabecular surfaces in the marrow similar in magnitude to previously reported estimates of what occurs in vivo . More recently, Metzger et al. predicted that volume averaged fluid flow shear stress in the marrow cavity of whole porcine femurs was 1–24 dyn/cm 2 in response to whole bone loading and found that mechanical stimulation of trabecular implants resulted in new bone formation . In some cases, shear stress may reach up to 5 Pa when the marrow is modeled as a highly viscous material . It is also interesting that models predict that high-frequency mechanical loads induce higher levels of fluid flow than the same level of mechanical loading applied at a low frequency . This result, combined with experimental in vivo strain gauge studies showing that high-frequency (up to 20 Hz) loading does occur physiologically , suggests the possibility that high-frequency oscillatory fluid flow is an important physical signal, both in the marrow and canalicular space. Furthermore, due to the enclosed space within in-filling osteons, fluid shear stress acting over these cells is potentially larger than in the intramedullary space. In fact, low-amplitude high-frequency signals elicit an osteogenic response in bone cells and are anabolic for trabecular, but not cortical, bone . In conclusion, load-induced fluid flow in the cortical bone and in the marrow space certainly occurs and cannot be dismissed as a potent regulator of bone metabolism in vivo.
14.7
Vibration
It was first suggested that dynamic mechanical loads were dramatically more potent than static loads in regulating bone metabolism in the 1980s. In surgically isolated turkey ulnae, high-frequency vibration prevents disuse bone loss. This result was also observed in sheep for which vibration affects cancellous, but not cortical, bone. However, vibration-induced cortical formation has been shown in other animal models. For example, vibration increases both cortical and cancellous bone formation in rodent models . Studies also suggest that vibration enhances bone repair . Most recently, the anabolic and anticatabolic effects of vibration have begun to be demonstrated in human clinical trials . The most rigorous trial to date showed no effect of vibration on bone or muscle in older adults , though vibration has been reported to reduce adiposity in marrow and muscle . The existence of these high-frequency low-magnitude vibrations with normal activities has also been documented.
14.8
Damage
The potential for loading to produce damage, or the accumulation of microscopic cracks, in bone tissue was initially described in 1960 by penetration of stain prior to sectioning. In addition to its deleterious effect on mechanical competence, damage induces bone turnover and remodeling and bone remodeling tends to be increased in regions of cracks . Interestingly, an increased number of microcracks have been reported with age and appear to change their morphology. Microcracks have been reported in trabecular bone as well, observed through the use of both staining and synchrotron micro–computed tomography. Finally, inhibition of bone turnover with bisphosphonates is associated with higher levels of damage.
Although load-induced bone formation has been thought to require dynamic loading, a study by Lynch and Silva found that static creep loading could also lead to bone formation in vivo. They found that applying a static load stimulates a damage-dependent dose–response in periosteal woven bone formation. Furthermore, after 14 days, functional repair of the damage resulted in loaded bones with mechanical properties equal or superior to control bones .
14.9
Cellular and pericellular mechanics
We next consider how tissue-level behavior induces changes in the biophysical environment at the cellular and pericellular level ( Fig. 14.3 ). Bone cells are tightly coupled to their extracellular environment in a complex dynamic fashion that has only recently begun to be understood and described in detail. This new area of biomechanical research has great potential to lead to important new insights into skeletal mechanobiological mechanisms.
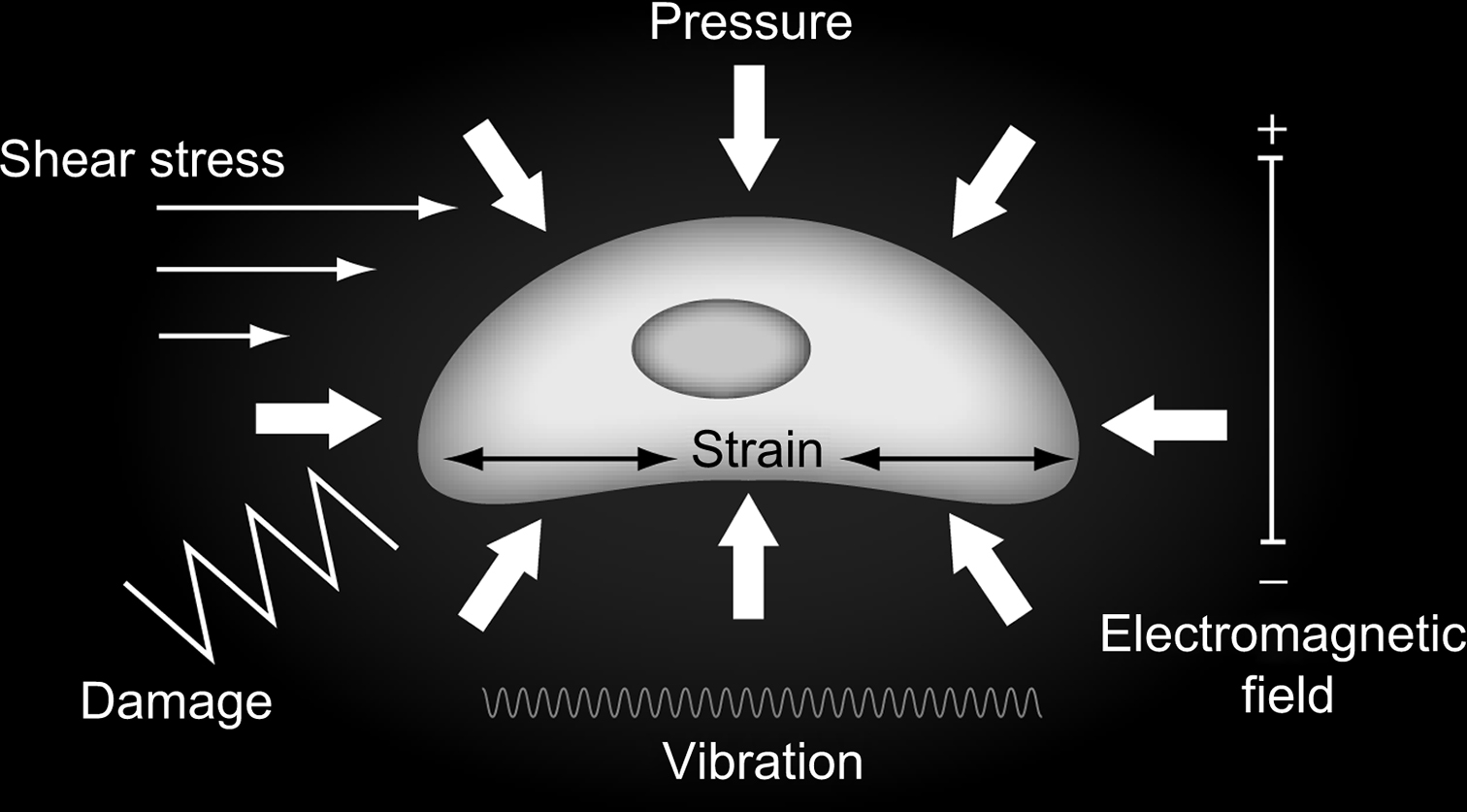
14.10
Electromagnetic fields
Electromagnetic fields regulate bone cell behavior in vivo and in vitro. For example, they alter cellular morphology, increase alkaline phosphatase activity, induce detachment and apoptosis, suppress and enhance osteoclastogenesis, induce proliferation, and regulate differentiation and mineralization depending on the parameters of stimulation. In terms of signaling, electromagnetic fields activate intracellular calcium, insulin-like growth factor, transforming growth factor β (TGF-β), PGE2, and the RANKL-OPG system. The electrosensitivity of ensembles of bone cells linked by gap junctions is greater than uncoupled cells . However, critical evaluation of both in vitro and in vivo experimental evidence has suggested that although exogenous electromagnetic fields can stimulate bone cellular activity, the endogenous fields produced by habitual loading may not elicit a significant cellular effect. This has led many investigators to consider alternative loading-induced cell-level physical signals as mediators of bone mechanobiology.
14.11
Direct cellular deformation
With the apparent lack of an effect of loading-induced electric fields on bone cells in culture, the focus turned to direct cellular deformation. Specifically, research focused on whether the strains that bone cells experience through direct coupling to the matrix can elicit a change in their metabolism. Several experiments have been conducted to address this question by examining the sensitivity of bone cells to substrate strain. Owan et al. identified a fundamental concern with bending, the commonly used method for inducing substrate strain. They decoupled strain from slide displacement by using different slide thicknesses and found that cellular responsiveness was less sensitive to strain than to displacement. They concluded that the cells were responding to the induced flow from the movement of the slide through fluid media. Furthermore, direct comparisons of strain to flow have been conducted, and in each case, no cellular response was observed until roughly 5%–10% substrate strain, which exceeds the approximate 3% failure strain level of bone tissue .
Substrate strain continues to be studied in investigations of bone cell mechanosensing with displacement-based systems that are known to induce both small and large amounts of secondary fluid shear stress. This may be due, in part, to the ease of use of commercially available stretching systems. Some effort to control for the secondary flow has been made; however, the complexity of the fluid dynamics involved is substantial and has been calculated for only a few systems . In addition, the downstream cellular responses to stretch and flow appear to have substantial overlap .
However, there is potential for strain amplification because the osteocyte lacuna acts as a void or hole in the matrix. The magnitude of this amplification has been quantified . Although average lacunar strain amplification was in a range of one to four, amplification of as much as 15 was observed in small regions . With habitual activities sufficient to maintain bone loss (roughly 0.05%), the amplified osteocyte strains remain short of those that regulate cellular behavior in vitro (roughly 5%), but they are certainly much closer. Thus although the significance of these small regions of high strains is currently unclear, they may prove to be an important part of the bone cell mechanosensing system. Furthermore, this emphasizes the complex nature of the cellular biophysical environment and the need for more research in this area.
14.12
Fluid flow
The failure of either direct cellular deformation or induced electric fields to regulate bone cell metabolism robustly in cell-culture models has been termed a “fundamental paradox” in bone physiology . This disconnect between how relatively small tissue–level strains regulate cells, which appear to require quite large deformations to respond , has led to a rediscovery and intense interest in loading-induced fluid flow in bone. Laminar flow over a surface, such as the cell membrane, will expose the cell to shear stress and a resulting deformation. This deformation has been quantified, and the time-dependent mechanical behavior of osteocytes may explain how they distinguish steady from oscillatory flows .
Cyclic loading due to ordinary activities, such as ambulation, forces fluid from regions of high compressive strains. The fluid then returns when loading is removed, thus exposing cells to an oscillating fluid flow . Oscillatory flow profiles activate specific biochemical signaling pathways in bone cells with important distinctions from those activated by unidirectional steady flow . For example, intracellular calcium mobilization was observed in both the response to steady as well as oscillatory flow . However, in the case of steady flow, the source of this calcium increase was found to be from both intracellular stored calcium and extracellular calcium influx through stretch-activated membrane channels. In contrast, oscillatory flow resulted in the release of calcium from the endoplasmic reticulum via inositol triphosphate (IP3)-sensitive calcium channels only with no involvement of stretch-activated channels. Similarly, medium-duration (1 hour) exposure to steady flow produces a dramatic reorganization of the actin cytoskeleton including densification and stress fiber formation , whereas oscillatory flow does not . However, longer oscillatory flow times (5 hours) do eventually lead to actin filament reorganization . The differences in the response to oscillatory versus unidirectional flow are likely due to large cellular viscoelastic deformations expected from a unidirectional steady flow that may activate additional pathways .
Beyond exerting a force, flow has the potential to interact with membrane-associated proteins that decorate the cell surface and protruding cellular structures . However, these measurements have been made in cultured cells, and a detailed understanding of cellular deformation due to flow rests primarily with models described in the following section. Another intriguing possibility is that the effect of loading-induced fluid flow is not to mechanically stimulate the osteocyte at all but to regulate the osteocyte’s biochemical environment. Indeed, the role of loading in supplementing purely diffusive nutrient supply with convection due to flow was the impetus for the initial descriptions of bone flow due to loading . This possibility has been advanced more recently and supported by tracer studies that quantify both flow and convection . However, it is difficult or impossible to separate the mechanical and biochemical effects of flow in vivo, although in vitro experiments suggest that both are important considerations .
14.13
Models of pericellular flow
Although experimental evidence demonstrates that bone tissue loading produces pericellular fluid flow, to date it has proven impossible to make detailed measurements of the cellular forces and deformation that results. Thus theoretical modeling has been utilized to provide insight beginning with numerical simulation of the original observations of Piekarski and Monroe and the suggestion that cellular sensing of this flow is important in bone metabolism as well as quantifying convective transport in detail . Weinbaum et al. introduced the first models that included the micromechanical details of the osteocyte . These detailed analytical models accounted for momentum transfer between the fluid and the proteoglycans surrounding the osteocyte process. This model was further refined to a theory that small tissue strains lead to large cellular strains through fluid-structure coupling to a stiff actin-dense osteocyte process anchored by periodic linear linker proteins. With the discovery of direct focal contacts between the cell process and the mineralized matrix , the model was further refined to calculate the transmembrane molecular forces consistent with an integrin-based attachment . Computational models have also provided important insights into osteocyte pericellular mechanics. Finite element analysis has been used to simulate flow through the lacunocanalicular system and has the potential to overcome the geometric simplifications of analytical approaches . They have also been applied to understand cell-level signaling in stem cell and tissue differentiation in bone . Other modeling approaches such as multiscale simulation and lumped parameter models have also been utilized. Most of these models have been validated with experimental bulk measurements . As such, they provide estimates of the range of shear stress cells experience in vivo with habituation loading (0.1–2 Pa) and form the basis of the in vitro mechanobiology experiments described in the following sections.
14.14
Pressure
In addition to fluid flow, loading must produce hydrostatic pressure, and in vitro studies have shown that pressure regulates changes in bone cell behavior. For example, in 1975, Rodan et al. showed that 5.9 kPa regulated cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) accumulation in primary cells . Dynamic hydrostatic pressure at 13 kPa applied to bone rudiments leads to increased mineralization . Thirteen kilopascals can upregulate TGF-β signaling, alkaline phosphatase activity, collagen, actin, osteopontin gene expression, and periosteal cell proliferation . Dynamic 4.9 kPa pressure applied to osteoblasts has been associated with interleukin-1 β, PGE2, and resorption, but 37 or 101 kPa applied to bone marrow cells was antiosteoclastogenic. A pressure of 4 MPa leads to increased cell–cell and cell–matrix adhesion and reorganization of the cytoskeleton . Cyclic pressure in the range of tens of kilopascals activates intracellular calcium signaling, increased proliferation, and osteogenic differentiation of progenitor cells. Live bone explants exposed to daily 3 MPa hydrostatic pressure exhibited enhanced cell viability, but not when cultured for shorter periods on titanium mesh. Interestingly, it has been recently suggested that pressure arising from loading decreases with age due to changes in osteon morphology . In addition, the effect of pressure on RANKL/OPG signaling may change with age .
However, similar to strain, whether the pressures that occur in vivo are sufficient to induce cellular responses remains unclear. Experimentally validated models show that significant pressures in the LCN develop only at supramegahertz frequencies and that, physiologically, pressures are only “a few pascals” . This is at least one order of magnitude lower than the pressures that regulate bone cell behavior. Also, a direct comparison of gene expression changes due to applied pressure showed less of an effect compared with other physical stimuli .
14.15
Vibration
Vibration experienced at the organism or organ level has the potential to generate cell-level physical signals such as fluid flow, pressure, or electric fields. Often, these induced signals exhibit a dependence on frequency . Other than the dampening effects of articular cartilage and other soft tissue, vibration—particularly high-frequency vibration—has the potential to be experienced by bone cells directly. It has been shown to increase NO but inhibit PGE2 signaling in osteocytes and to affect gene expression in osteoblasts, perhaps through oscillation of the relatively higher density nucleus . However, there has been a suggestion in the literature that the effect of vibration may be primarily on hemodynamics and fluid flow rather than directly on bone cells .
14.16
Damage
As described previously, tissue damage is a potent activator of bone remodeling and repair, both in the bulk and in targeting turnover to regions of damage. For example, microcracks are associated with osteocyte apoptosis . This finding has led to the suggestion that cracks may cause cellular damage, likely to the osteocyte process, that induces apoptosis and signals remodeling . A complication is that disruption of the osteocyte process in vivo occurs simultaneously with blockage of canalicular fluid flow . Thus some have concluded that osteocytes perceive the lack of flow as reduced mechanical loading. In 2010 it was reported that fatigue damage in bone kept in disuse does not induce remodeling . More recently, Kennedy et al. blocked osteocyte apoptosis and showed that the remodeling response to damage was abrogated , suggesting that the microdamage repair system involves targeted remodeling of apoptotic osteocytes.
14.17
Mechanosensing mechanisms
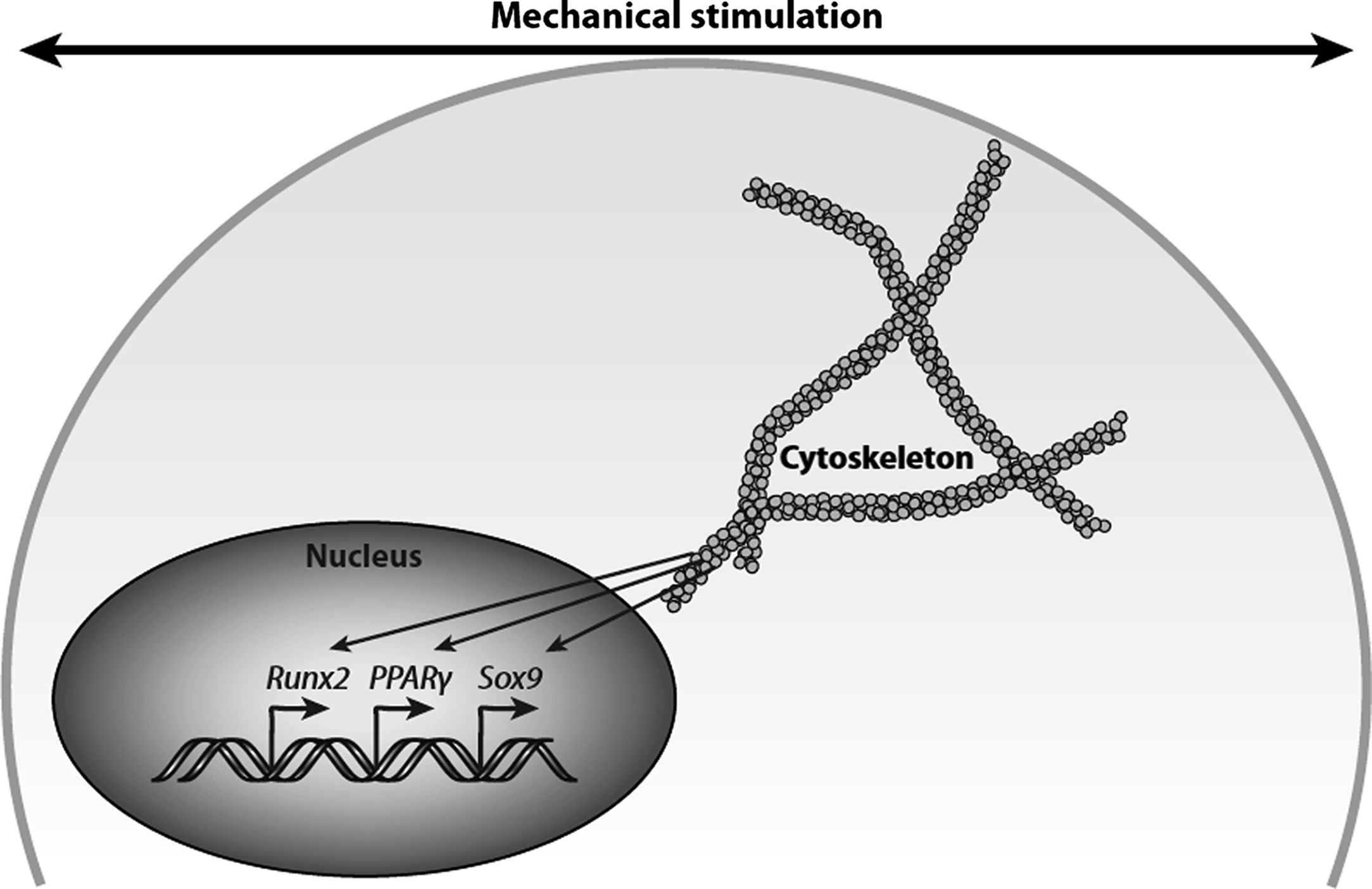
In the previous section, we describe the state of knowledge in cellular and pericellular mechanics in terms of how habitual loading of bone tissue produces a diverse range of biophysical signals at the cellular level. However, in order for a biological response to occur in response to loading, these mechanical signals must induce cellular biochemical signaling. Although the mechanism by which this occurs is currently poorly understood, almost all potential mechanisms involve a change in the conformation of a protein due to applied physical forces . In this section, we consider some likely candidates for these molecular-level mechanisms ( Fig. 14.4 ).
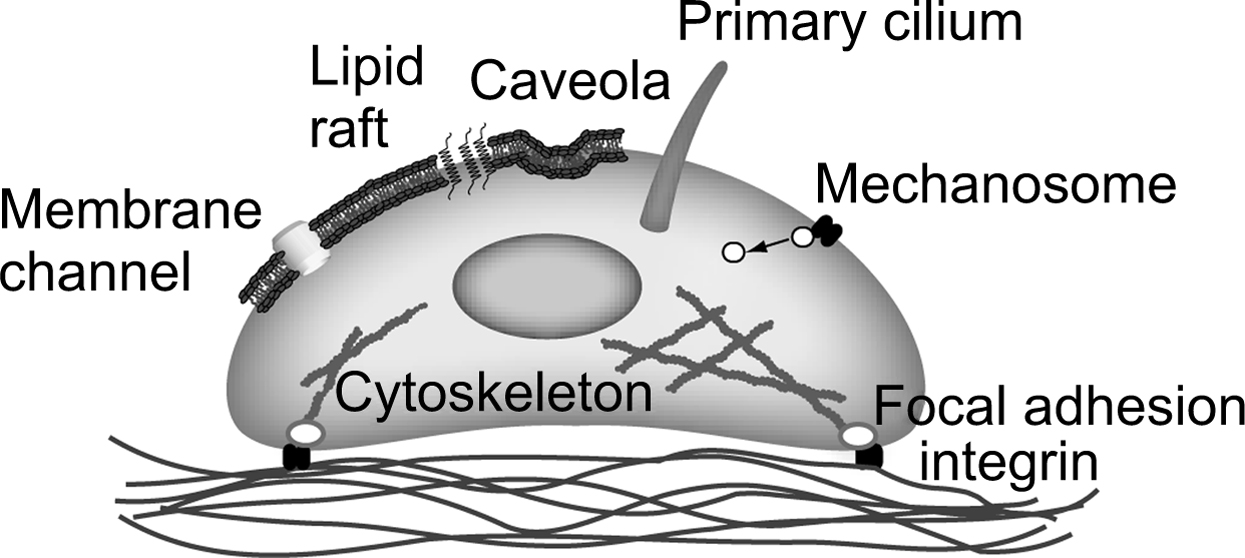
14.18
Molecular dynamics
One of the most powerful techniques available to predict changes in conformation of proteins is molecular dynamics. In molecular dynamics, the behavior of large systems of particles, atoms, or molecules is simulated, subject to assumptions about the interparticle forces. A typical system is a protein or small collection of proteins. In terms of understanding mechanotransduction, molecular dynamics can be used to predict changes in protein conformation as a function of applied molecular forces. For example, Mofrad et al. found that the ability of focal adhesion kinase (FAK) to bind paxillin was altered as a function of force on the FAK molecule . Talin–vinculin binding and actin cross-linker activity have also been studied . In addition to cytoskeletal proteins, the gating of mechanosensitive ion channels and the mechanosensitive primary cilium-associated protein polycystin (PC)1 has been simulated . However, despite its great potential, molecular dynamics has important limitations stemming largely from computational complexity. Although covalent interactions act over short distances, noncovalent forces extend to large numbers of particles, causing the computational requirements to scale as the square of the number of particles. Current simulations are limited to the nanosecond timescale, and explicitly modeling the solvent (water) is problematic. However, multiscale, coarse-graining, and other strategies to improve computational efficiency hold promise. Indeed, there is recent experimental evidence suggesting that force-induced protein unfolding as predicted by molecular dynamics does occur in several contexts .
14.19
Cytoskeleton
The proteins of the cytoskeleton are particularly strong mechanosensitive candidates because one of their primary functions is structural ( Fig. 14.5 ). The cytoskeleton is composed primarily of three cytoskeletal filaments (actin microfilaments, intermediate filaments, and microtubules). The dynamic interplay between these filaments and cell–cell and cell–matrix adhesions regulates cell shape and structure. Tensegrity (tensional integrity) describes how the structural organization of the cell is modified by its physical environment . The tensegrity model predicts that the cytoskeleton serves to connect the extracellular matrix (ECM) to the cell nucleus via focal adhesions making the cell “hard-wired” to respond to mechanical stress. That is, the cytoskeleton actively generates tensile forces via contractile microfilaments that are balanced by other structural elements, namely, integrins and focal adhesions, to stabilize cell shape mechanically. Mechanical stress results in a perturbation of the entire prestressed network and triggers a series of signaling events that lead to further integrin activation and recruitment of additional focal adhesions.
Indeed, disruption of the actin cytoskeleton interferes with the ability of bone cells to respond to fluid shear stress , and enhancing actin polymerization increases osteogenic differentiation . Studies in osteoblasts showed that the upregulation of COX-2 and c-fos in response to 1 hour of steady flow involves the rearrangement of the actin cytoskeleton . Conversely, oscillatory fluid flow in the same cell type did not lead to actin filament reorganization, and disruption of actin filaments did not affect flow-induced intracellular calcium and PGE2 release . Finally, the disarrangement of the actin cytoskeleton in a multipotent cell line abrogates the flow-induced increase in runt-related transcription factor 2 (Runx2) expression suggesting that the cytoskeleton plays a key role in flow-induced osteogenic differentiation ( Fig. 14.6 ) .
There is also evidence that microtubules may be critical and disruption of the primary cilia microtubules interferes with bone cell mechanosensing . Microtubule disruption attenuated flow-induced proliferation , collagen I, and matrix metalloproteinases (MMP) 1 and 3 , whereas disruption of microfilaments had no effect. The pharmacological disruption of actin microfilaments, microtubules, or intermediate filaments did not affect laminar flow-induced increases in PGE2 release, and of the three, only disruption of intermediate filaments reduced flow-induced COX-2 expression . However, cytoskeletal disruption experiments are difficult to interpret because agents typically compromise the mechanical integrity of the cell as well as intracellular transport and trafficking. There is also evidence for transduction independent of the cytoskeleton. For example, nuclear translocation of nuclear factor kappa-light chain enhancer of activated B cells (NF-κB) due to flow has been reported to be independent of the actin and microtubule cytoskeletons. This has been extended to PGE2 release , and there is evidence for different roles of the cytoskeleton in osteocytes versus osteoblasts. Cytoskeletal changes have also been proposed as a pressure sensing mechanism.
14.20
Integrins and adhesion-associated proteins
As described previously, osteocyte processes appear to be anchored to the extracellular mineralized matrix by structures reminiscent of focal adhesions or focal contacts. Transmembrane integrins and the collection of associated adhesion complex proteins hold great potential as mechanosensors. This is because they both experience high levels of loading since they act as stress concentrations and because many have well-characterized signaling potential. Indeed, interfering with the integrin/actin connection has been shown to interrupt mechanotransduction in bone cells .
Integrins are transmembrane cell adhesion heterodimers comprised α and β subunits that bind directly to the ECM at anchoring hubs known as focal adhesions. The cytoplasmic tail binds talin and paxillin, both of which link directly to the actin cytoskeleton through various signaling molecules, including FAK. Integrin signaling is bidirectional in that extracellular domain-ECM binding activates intracellular signaling molecules (outside-in signaling), while conformational changes in the cytoplasmic tail activate the integrin head for ligand binding .
Osteoblasts express α2–α6, α8, αv, β1, β3, and β5 integrin subunits . Of these, the β1 subunit appears to play an important functional role in osteoblasts . Mice expressing an osteoblast-specific dominant negative form of β1 integrin exhibited reduced bone mass and increased cortical porosity due to a defect in bone formation and increased osteoclast resorption.
Expression of β1, β3, and CD44 has been reported in osteocytes . Specifically, β1 is predominantly expressed in the cell body, whereas β3 is expressed along the osteocyte processes and appears to tether processes to the canalicular wall at focal adhesions , which would be situated directly in the path of the canalicular fluid flow . Mechanical stimulation activates integrins and downstream signaling including mitogen-activated protein kinase (MAPK) , tyrosine phosphorylation, intracellular calcium release , c-fos gene expression , and translocation of FAK from the cytosol to the cytoskeleton during the creation of focal adhesions , all of which are important osteogenic signals. In addition, β1 expression is upregulated in osteoblasts in response to fluid flow suggesting that β1 binding may be a primary response to physical forces. Indeed, mice with osteocyte-specific gene ablation of β1 integrin exhibited significantly lower load-induced bone formation than wild-type littermates .
As integrins do not possess intrinsic catalytic activity, ECM-intracellular signal transduction is carried out by activation of downstream signaling molecules comprising focal adhesions. FAK, a nonreceptor tyrosine kinase (Tyk) , is one of the first molecules recruited to focal adhesions upon integrin binding and has been shown to be important in cell migration, proliferation, and survival. FAK has also been implicated in cellular mechanotransduction . FAK has been implicated specifically in bone cell mechanotransduction and has been shown to play a role in orthodontic tooth movement . The activation of FAK results in the autophosphorylation of tyrosine 397 creating a high-affinity binding site for the Src-homology 2 (SH2) domain of the Src family protein Tyks. FAK and SH2 binding activate MAPK signaling through interaction with c-Src, growth factor receptor–bound protein 2 (Grb2), and the small GTPase Ras . Fluid flow leads to FAK phosphorylation and MAPK activation in bone and endothelial cells . FAK phosphorylation has also been linked to NF-κB activation as well as calcium release via large-conductance calcium channels . Disruption of FAK in osteoblasts leads to decreases in the fluid flow–induced extracellular-signal-regulated kinase (ERK) phosphorylation, expression of c-fos and COX-2, as well as PGE2 release , all of which are important signaling events in osteoblast function.
Interestingly, osteocyte and osteoblast-specific gene ablation of FAK does not affect load-induced cortical bone formation in vivo . Thus FAK appears to play a lesser role in the sensing of, and response to, fluid flow in osteocytes and osteoblasts in vivo. This result may be due to a compensatory mechanism. However, FAK may play a more prominent role in downstream bone formation events, such as osteoblast differentiation and recruitment. Indeed, the disruption of FAK in osteoblasts abolishes the response of bone marrow cells to mechanical stimuli in a tibial injury model indicating a potential role for FAK in osteoprogenitor recruitment and homing. Data suggest that proline-rich Tyk 2 (Pyk2) may compensate for the loss of FAK in various cell types including endothelial cells and fibroblasts . However, Pyk2 expression was not enhanced in FAK−/− osteoblasts , and it is unclear whether Pyk2 has a compensatory effect on load-induced bone formation in vivo. In fact, Pyk2-null mice exhibit increased bone mass and bone formation suggesting that Pyk2 normally represses osteoblast differentiation . Recent studies show that the formation of a Src/Pyk2/MBD2 complex in osteocytes in response to mechanical stimulation suppresses anabolic gene expression supporting an inhibitory role for Pyk2 in osteogenesis .
Additional adhesion-associated proteins that may regulate mechanosensing in bone are talin and paxillin. Talin binds the cytoplasmic domain of the integrin β subunit and alters integrin conformation, thereby enhancing the integrin-binding affinity and receptor activation . Talin also binds FAK and actin and is a key linking protein between the ECM and the actin cytoskeleton . Recent studies show that talin unfolding is required for force transduction at focal adhesion sites , suggesting that changes in protein conformation are a key mechanism in mechanotransduction. Paxillin binds the cytoplasmic tail of α4 and contains several signaling motifs including phosphorylation sites, phosphatases, and regulators of the Rho family of small GTPases . Paxillin has been shown to align with the direction of fluid flow in osteocytes , suggesting that it is responsive to mechanical stimuli, but additional data are needed to better understand the role of these adhesion molecules in mechanosensing.
14.21
Membrane channels
Alterations in gating properties of membrane channels are one of the most powerful mechanisms regulating cell behavior. These gating changes occur as a result of changes in protein conformation that may represent the initial molecular mechanotransduction event. In bone cells, membrane potential, ion concentration, and hormonal regulation are all known modulators of channel gating . Mechanically gated ion channels are most heavily studied in bacteria but have been widely described in mammalian cells. They are involved in hearing and touch as well as maintaining osmotic homeostasis and regulating cell volume. In these contexts, they are thought to be sensitive to membrane tension. Three classes of pressure-sensitive ion channels in human osteoblast-like cells were described by Davidson et al. . The channel exhibiting the highest conductance was found to be a voltage-gated and calcium-dependent potassium channel, which later was shown to be sensitive to membrane stretch . Cyclic stretch has the ability to alter ion channel characteristics in osteoblast-like cells as evidenced by increased numbers of open channels and a reduced strain threshold compared to nonstrained controls . Ion channels are also responsive to fluid flow. Fluid flow induced a rapid increase in intercellular calcium signaling, which was inhibited by pertussis toxin , an inhibitor of G-proteins known to regulate calcium flux. l -Type voltage-sensitive calcium channels were shown to mediate load-induced bone formation in vivo . In a study of bone cells, patch-clamp electrophysiology was used to identify currents associated with calcium, potassium, and sodium ions that are mechanically activated. Voltage-activated channels are involved in bone mechanosensing in vitro . In vivo, it is presumed they are involved in sensing changes in membrane potential that occur through another mechanism.
Ion channels are also activated by treatment with parathyroid hormone (PTH) , a potent regular of serum calcium. In fact, PTH treatment enhances fluid flow–induced calcium signaling through activation of mechanosensitive and voltage-sensitive calcium channels . It has been shown that membrane strains of 800% were needed to open 50% of the channels in primary rat osteoblasts , although the precise mechanism by which mechanical stimuli activate ion channels is unclear.
Attention has focused on the role of the transient receptor potential (TRP) cation channel superfamily members in bone cell signaling. TRPV4, 5, and 6 are highly selective for calcium , and TRPV5-null mice exhibit hypercalciuria and decreased cortical and trabecular thickness in long bones . TRPV4 has been shown to localize to primary cilia , which are known mechanosensors in kidney cells . Data show that TRPV4 can be activated by mechanical stress , and gene ablation of TRPV4 in mice resulted in increased trabecular bone mass due to reductions in osteoclast number and activity . The observation that TRPV4 is also expressed in human and murine osteoblast-like cells makes it a possible candidate for fluid flow–induced calcium signaling in osteoblasts.
Another area of intense study is the role of PC1 and PC2 in skeletal mechanosensation. PC1 is an 11-pass transmembrane protein with a large extracellular domain and is a guanine nucleotide-binding (G) protein–coupled receptor (GPCR), whereas PC2 is a calcium channel that belongs to the TRP ion channel superfamily . PCs are expressed in osteoblasts and osteocytes, and, like TRPV4, have been shown to localize to primary cilia . Gene ablation of Pkd1, the gene encoding PC1, resulted in the abnormal development of the axial skeleton and long bones suggesting a role for PCs in skeletal maintenance. More recently, interference with PC1 signaling in primary human osteoblasts that were exposed to mechanical stimulation resulted in decreased levels of NFATc1 and Runx2 expression , suggesting that PC1 is potentially another important mechanoreceptor in bone.
Annexin V is a calcium channel that binds extracellular collagen and cytoskeletal actin and is involved in the intracellular calcium response to flow in osteoblasts . This suggests the possibility that mechanical force in the actin cytoskeleton may open the channel. Similarly, a large-conductance calcium- and voltage-dependent potassium channel interacts with FAK . Interestingly, annexin V has been shown to interact with the primary cilium protein PC1 .
Cxs are typically observed in gap junctions mediating cell–cell signaling, and gap junctions in bone cells are formed mainly by Cx43, a 43 kDa Cx protein . There is evidence that they can also form a hemichannel and open in response to mechanical and chemical stimulation . Hemichannels have been implicated in mechanically stimulated ATP and PGE2 release via mechanically mediated autocrine and paracrine signals, which are discussed in detail later. Given that osteocytes communicate with one another and with progenitors and osteoblasts on bone surfaces via Cx43 gap junctions , Cx43 channels have been proposed as a predominant mechanism of mechanotransduction in bone. In vitro studies have shown that mechanical stimulation enhances Cx43 expression, phosphorylation and localization to the cell membrane in osteocytes and osteoblasts , suggesting that mechanical signals increase the number of Cx43 gap junctions available for signaling and improve the ability of cells to communicate with one another. Additional studies show that PGE2 release from osteoblastic cells in response to mechanical stimulation depends on Cx43 hemichannels . Interestingly, disruption of Cx43 in osteochondral progenitor cells, preosteoblasts, osteoblasts, and osteocytes results in enhanced, rather than abrogated, mechanically induced osteogenesis in vivo . Mechanistically, Cx43 has been shown to bind β-catenin and prevent its translocation to the nucleus in response to Wnt signaling , suggesting that Cx43 normally acts to suppress osteogenesis via cross-talk with the Wnt signaling pathway. That Cx43 appears to be involved in both release of osteogenic factors in response to loading and interference in the Wnt signaling pathway suggests a complex role in bone mechanotransduction.
14.22
Plasma membrane dynamics and mechanotransduction
The plasma membrane is far from simply a two-dimensional hydrophobic domain that functions as a barrier separating the intracellular and extracellular spaces. It has the potential to change its fluidity and to segregate itself into smaller microdomains (caveolae or rafts, for example) and to have barriers (known as pickets or fences). In these ways, it can regulate signaling by changing the reaction kinetics of membrane-bound proteins. A number of biophysical approaches have been applied to measure changes directly in membrane dynamics in the context of mechanotransduction including time-correlated single-photon counting, laser scattering, and molecules whose fluorescent properties change with membrane viscosity. Membrane fluidity increases with fluid shear stress exposure .
Rubin et al. showed that the antiosteoclastic effect of mechanical stimulation required the small GTPase H-Ras, which resides in lipid raft microdomains, and that cholesterol depletion of these microdomains blocked mechanical activation of H-Ras . The caveolar microdomain has also been implicated, as caveolin-1 plays a significant role in bone mechanosensing . Interestingly, estrogen receptors (ERs) are required to localize to the membrane and interact with caveolin-1, but not bind estrogen, for bone cells to sense strain. Also, mechanical stress has been shown to alter the configuration of the PTH receptor in a manner reminiscent of membrane fluidization.
14.23
Mechanosome
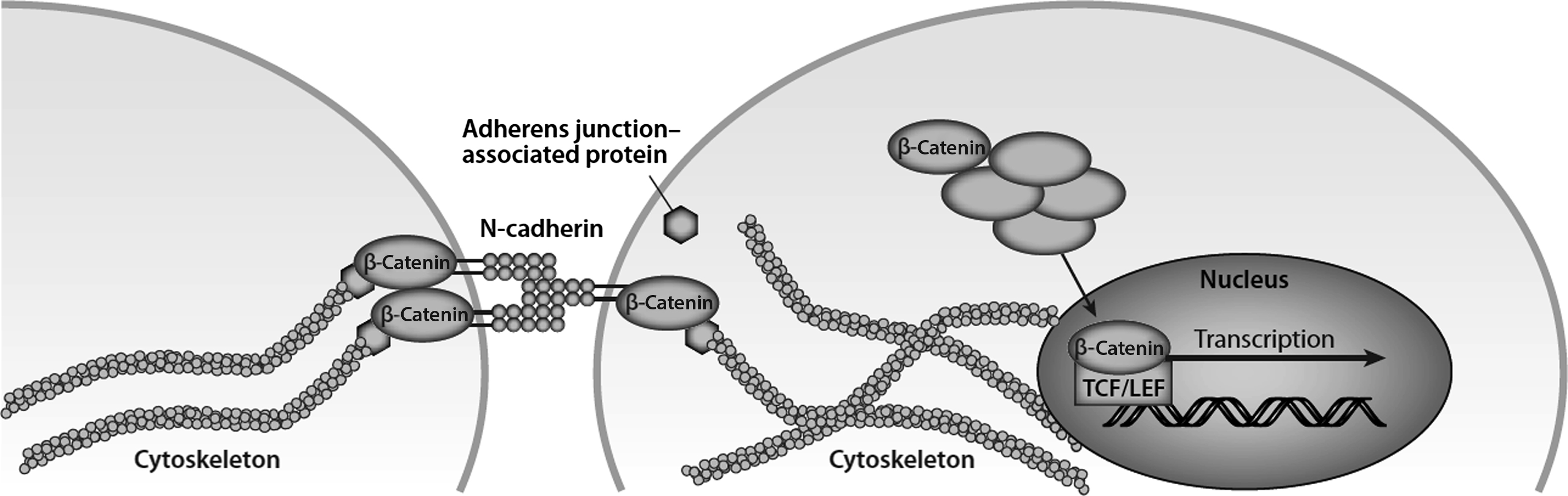
Often, signals at the cell membrane are carried intracellularly via protein complexes that are liberated from the membrane and/or focal adhesions when activated . In the context of mechanical signals, such a protein complex has been termed a mechanosome . One candidate for this role is β-catenin, because it is a well-described transcription factor and it is also a structural protein found in adherens junctions. There is no direct evidence that the two β-catenin pools overlap (i.e., nuclear translocation of junctional β-catenin); however, one report noted that the osteogenic effect of fluid shear stress involves nuclear β-catenin activity that coincided with liberation of β-catenin from its association with N-cadherin ( Fig. 14.7 ) . Nuclear translocation of β-catenin occurs with mechanical stimulation and leads to expression of Wnt targets such as Wnt1-inducible signaling pathway protein 1 (Wisp-1) and COX-2 , osteogenic differentiation , and altered gene expression.
14.24
Primary cilia
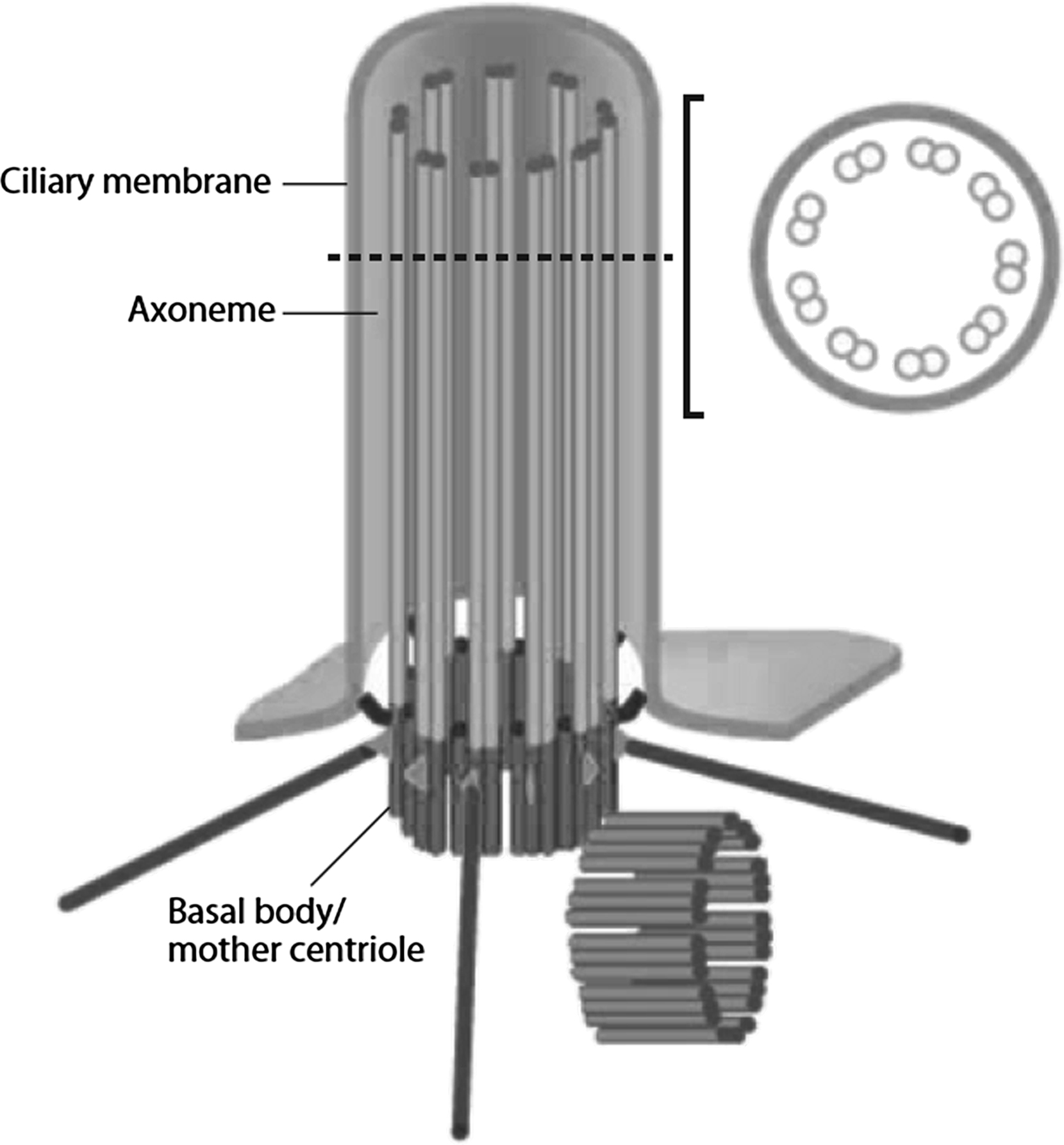
The primary cilium is a solitary, nonmotile organelle that projects from the surface of almost all nondividing eukaryotic cells and was first described in 1898. They are virtually ubiquitous in mammalian cells and have been found in an astonishing diversity of cells in vitro and in vivo. Despite this, for much of their known existence their biological function remained undiscovered, leading some scientists to propose that they may be vestigial . However, a dedicated few individuals were persistent in their conviction that such a common organelle must be critical to cellular function .
Owing to the link between primary cilia, polycystic kidney disease, and mechanotransduction , the role of primary cilia has become a hot topic in cell and molecular biology. Their function is starting to emerge as a nexus of extracellular sensing and integration of both biochemical and biomechanical signals . Primary cilia are made up of a microtubule-based axoneme surrounded by a specialized ciliary membrane. They originate from the basal body, which is a modified version of the mother centriole ( Fig. 14.8 ). Centrioles are more commonly known for their role as microtubule-organizing centers for the mitotic spindles formed during cell division. However, outside of mitosis, the mother centriole (the centriole that was inherited during cell division as opposed to the one that is formed after division is complete) becomes the basal body of the primary cilium. As such, it functions as both an anchor for the primary cilium and a template for cilium extension.