The plasma membrane of eukaryotic cells serves to insulate the cell from the outside environment, but this barrier must be breached to transmit signals of extracellular origin. This fundamental
problem of transmitting extracellular signals is solved in two ways. Signals cross the plasma membrane either by activating transmembrane receptors or by using ligands that are membrane permeable (
Table 5.2). Cells are exquisitely sensitive to most ligands. The affinity of receptors for ligands generally is in the picomolar to nanomolar range, and very few receptors need to be occupied to transmit a signal. For example, it has been estimated that activation of ten T-cell receptors is sufficient to send a maximal signal. Cytokineresponsive cells may express only a few hundred receptors on the cell surface. Given the small number of receptors that are activated, amplification of most signals is necessary for cellular responses. A requirement for signal amplification also allows opposing or complementary pathways to affect signal strength more efficiently.
2 As a result of ligand binding, receptors undergo conformational changes or oligomerization, or both, and the intrinsic activity of the receptor or of associated proteins is stimulated. Receptors may bind and respond to more than one ligand. For example, the epidermal growth factor (EGF) receptor binds to transforming growth factor-alpha (TGF-
α), EGF, heparin-binding EGF (HB-EGF), beta-cellulin, epiregulin, epigen, and amphiregulin. The stimulation of most receptors leads to the activation of several downstream pathways that either function cooperatively to activate a common target or stimulate distinct targets. Generally, some of the pathways activated are counter-regulatory and serve to attenuate the signal. Receptors may also activate other receptors. A well-studied example is the activation of the EGF receptor by G protein-coupled receptors (GPCR), which occurs as a result of protease cleavage and activation of HB-EGF.
There are a number of transmembrane receptor families. This chapter will discuss several of them to illustrate distinct signaling mechanisms.
Receptor Tyrosine Kinases
Receptor tyrosine kinases are transmembrane proteins that have an extracellular ligand-binding domain, a transmembrane domain, and a cytoplasmic tyrosine kinase domain.
3 The ligands for these receptors are proteins or peptides. Most receptor tyrosine kinases are monomeric, but members of the insulin-receptor family are heterotetramers in which the subunits are linked by disulfide bonds. Receptor tyrosine kinases have been divided into six classes, primarily on the basis of the sequence of the extracytoplasmic domain. Examples of tyrosine kinase receptors include the insulin receptor, the platelet-derived growth factor (PDGF)
receptor, the EGF receptor family, and the fibroblast growth factor (FGF) receptor family.
Activation of receptor tyrosine kinases is generally believed to require tyrosine phosphorylation of the receptor. In the case of the insulin receptor, an insulin-stimulated conformational change activates the kinase. Most of the tyrosine kinases are activated by oligomerization, which brings the kinase domains of distinct molecules into close proximity so that they cross-phosphorylate. Autotransphosphorylation of tyrosine in the activation loop of the kinase domain locks the kinase into a high-activity conformation, stimulating phosphorylation of other sites on the receptor, as well as other substrates. However, cancerderived mutants of the EGF receptor may be activated without receptor autophosphorylation.
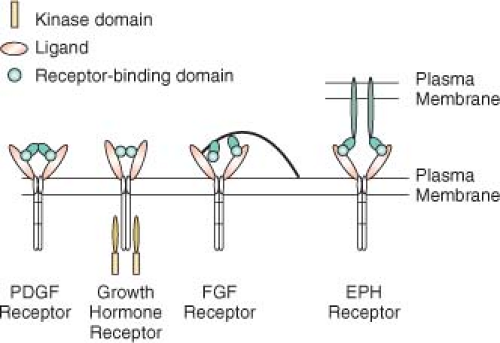
4Ligands stimulate receptor oligomerization in a variety of ways (
Fig. 5.1). Some ligands, such as PDGF, are dimeric, so that the ligand is able to bind two receptors simultaneously.
5 Other ligands, such as growth hormone, are monomeric but have two receptor-binding sites that allow them to induce receptor dimerization.
6 FGFs are also monomeric but have only a single receptor-building site. FGF molecules bind to heparin sulfate proteoglycans, which concentrates FGF and facilitates dimerization of the FGF receptor.
7 EGF is also monomeric, but binding of EGF to the receptor changes the receptor conformation and promotes interaction with a second ligand or receptor dimmer, leading to activation.
8 Some ligand-receptor interactions result in signaling by the ligand, in addition to the receptor. Ephrins are ligands for EPH tyrosine kinase activity in the target cell, but they also stimulate signaling by ephrins in the ephrin-presenting cell.
9Studies of the EGF receptor-family illustrate some important concepts. The EGF-signaling pathways involve four receptors (EGF receptor, ERB2, ERB3, and ERB4) and many ligands.
10 EGF stimulates homodimerization of the EGF receptor, but, under certain conditions, heterodimerization with other family members also occurs. Activation of EGFR proceeds via asymmetric dimerization of the receptor. Ligand causes extracellular dimerization, which then causes the kinase domains to form an intracellular head-totail dimer, which activates the receptor.
11,
12
Receptors that Activate Tyrosine Kinases
A number of receptors do not have intrinsic enzymatic activity but stimulate associated tyrosine kinases. Important examples of this type of receptor include the cytokine and interferon receptors that associate constitutively with members of the Jak family of tyrosine kinases
13 and the multichain immune recognition receptors that activate SKF and Syk family tyrosine kinases.
14,
15 The kinase appears to be inactive in the absence of ligand, but, as happens in receptors with intrinsic tyrosine kinase activity, signaling is initiated by ligand-stimulated heterodimerization and conformational changes of the receptors.
Serine-Threonine Kinase Receptors
The TGF-
β family of receptors are transmembrane proteins with intrinsic serine-threonine kinase activity.
16 TGF-
β ligands are dimmers that bind to and oligomerize type I and type II receptors. The type I and type II receptors homologous but distinctly regulated. The type II receptors seem to be constitutively active but do not normally phosphorylate substrates, whereas the type I receptors are normally inactive. Ligand-mediated dimerization of the type I and type II receptors causes the type II receptor to phosphorylate the type I receptor, converting it to an active kinase. Subsequent signal propagation is dependent on the kinase activity of the type I receptor and the phosphorylation of downstream substrates.
Receptor Phosphotyrosine Phosphatases
Receptor protein tyrosine phosphatases (RPTPs) have an extracellular domain, a single transmembrane-spanning domain, and cytoplasmic catalytic domains.
17 The extracellular domains of some receptor tyrosine phosphatases contain fibronectin and immunoglobulin repeats, suggesting that these receptors may recognize adhesion molecules as ligands. Several RPTPs are capable of homotypic interaction, but no true ligands are yet known for RPTPs. Most receptor tyrosine phosphatases have two catalytic domains, and both are active in at least some receptors. Functional and structural evidence suggests that the phosphatase activity of some of these receptors is inhibited by dimerization. Ligand-dependent dimerization could cause constitutively active tyrosine phosphatases to lose
activity, enhancing signals emanating from tyrosine kinases. RPTPs do not always function in strict opposition to tyrosine kinases, however. For example, CD45 is necessary for signaling by the B-cell receptor, which also requires tyrosine kinase activity.
18 Since some Tyr-phosphorylation events, such as phosphorylation of a Tyr near the C-terminus of src-family protein-Tyr kinases, can be inhibitory to the Tyr kinase activity, activation of certain phospho-Tyr phosphatases can paradoxically cause an increase in global tyrosine phosphorylation (discussed in more detail below).
G Protein-Coupled Receptors
GPCRs are by far the most numerous receptors.
19 Almost 700 GPCRs are present in the human genome.
20 The number of GPCRs is so high because they encode the light, smell, and taste receptors, all of which require great diversity. These receptors have seven membrane-spanning domains: The N-terminus and three of the loops are extracellular, whereas the other three loops and the C-terminus are cytoplasmic. A wide variety of ligands bind GPCRs, including proteins and peptides, lipids, amino acids, and nucleotides. No common binding domain exists for all ligands, and interactions of ligands with GPCRs are fairly distinct.
21 In the case of the thrombin receptor, thrombin cleaves the N-terminus of the receptor, freeing a new N-terminus that self-associates with the ligand pocket, leading to activation. Amines and eicosanioids bind to the transmembrane domains of their GPCRs, whereas peptide ligands bind to the transmembrane domains of their GPCRs, and peptide ligands bind to the transmembrane domains and the extracellular loops of their GPCRs. Neurotransmitters and some peptide hormones require the N-terminus for binding and activation.
Intramolecular bonds that involve residues in the transmembrane or juxtamembrane regions keep GPCRs in an inactive conformation.
22 In the inactive state, the receptor is bound to a heterotrimeric G protein, which is also inactive. Agonist binding causes a conformational change that stimulates the guanine nucleotide exchange activity of the receptor. Exchange of guanosine triphosphate (GTP) for guanosine diphosphate (GDP) on the
α-subunit of the heterotrimeric G proteins initiates signaling. Ultimately, GPCRs stimulate the same downstream pathways as other receptor types, including ion channels, cytosolic protein tyrosine and serine kinases, and enzymes that phosphorylate or hydrolyze membrane lipids.
19 Certain GPCRs also activate receptor tyrosine kinases. As mentioned earlier, GPCR-dependent cleavage of HB-EGF stimulates the EGF receptor, which is necessary for the GPCR to activate the mitogenactivated protein kinase (MAP kinase) pathway.
Notch Family of Receptors
The Notch receptor has a large extracellular domain, a single transmembrane domain, and a cytoplasmic domain.
23 Ligands for the Notch receptor are proteins expressed on the surface of adjacent cells, and activation results in two proteolytic cleavages of Notch. Initial cleavage by ADAM family proteases removes the extracellular domain and causes endocytosis. Subsequent proteolysis by the preselinin protease family releases the cytoplasmic region of Notch as a soluble signal. This fragment moves to the nucleus, where it complexes with the transcriptional repressor CBFI, relieving its inhibitory effects and stimulating transcription.