Introduction
Cardiotoxic effects of chemotherapy are challenging in nature, since myocardial tissue possesses limited regenerative capacity, which renders the heart susceptible to transient and permanent side effects of chemotherapy agents. Furthermore, with increasing incidence of cardiovascular disease in the general population and improvement in survival of cancer patients as a result of marked advancement in cancer therapy, chemotherapy-induced cardiotoxicity is becoming a more significant issue for clinicians and patients. Cardiovascular toxicities of chemotherapy have a broad clinical spectrum and may manifest as hypertension, myocardial ischemia, arrhythmia, thromboembolism, systolic dysfunction, congestive heart failure (CHF), or other adverse events. Additionally, they may manifest acutely during treatment or years later after treatment has ended. Anthracyclines are perhaps the most notorious offenders and have been linked to cardiomyopathy and CHF. Failure to diagnose and treat cardiotoxic complications early can result in significant cardiac morbidity and treatment delays or suboptimal delivery. Hence, it is imperative that an oncologist has full knowledge of the cardiovascular complications of antineoplastic agents, and the appropriate management steps to be taken after their detection. In this chapter, we review the cardiovascular toxicity associated with chemotherapy by discussing cardiotoxicity profiles of individual antineoplastic agents ( Table 10.1 ) and describing the general principles for diagnosis, prevention, monitoring, and treatment of these toxicities.
| Drug | Left Ventricular Dysfunction | Arrhythmias | Myocardial Ischemia | Hypertension | Thromboembolism |
|---|---|---|---|---|---|
| Anthracyclines | |||||
| Doxorubicin | + | + | – | – | – |
| Epirubicin | + | – | – | – | – |
| Idarubicin | + | + | – | – | – |
| Liposomal doxorubicin | – | – | + | – | – |
| Alkylating Agents | |||||
| Cyclophosphamide | + | + | – | – | – |
| Ifosfamide | + | + | – | – | – |
| Cisplatin | – | + | – | – | + |
| Antimicrotubule Agents | |||||
| Vinca alkaloids | – | – | + | + | – |
| Paclitaxel | – | + | + | – | – |
| Docetaxel | + | + | + | – | – |
| Antimetabolites | |||||
| 5-Fluorouracil | – | + | + | – | – |
| Capecitabine | – | + | + | – | – |
Antineoplastic Antibiotics
ANTHRACYCLINES
Anthracyclines are a class of antibiotics isolated from pigment-producing bacillus Streptomyces and include daunorubicin, doxorubicin, epirubicin, and idarubicin. Anthracyclines are key components of many curative and palliative regimens in combination with other agents for the treatment of various malignancies, including breast cancer, sarcoma, acute leukemia, and lymphoma. They are the class of antineoplastic agents most closely associated with cardiotoxicity. A meta-analysis of published studies has concluded that patients treated with anthracycline-based chemotherapy were five times more likely to develop reduced left ventricular ejection fraction (LVEF) and CHF in comparison with patients treated with nonanthracycline regimens.
- ■
Mechanism: There are several hypotheses regarding the mechanism of anthracycline-induced cardiotoxicity, but free-radical formation after binding to iron, which leads to DNA damage, is generally the most accepted hypothesis. It is believed that the myocardium is more susceptible to free-radical damage than other tissues because it has less free-radical scavenging enzymes. Other possible mechanisms that have been postulated include mitochondrial dysfunction leading to reduced adenosine triphosphate (ATP) production in cardiac myocytes and a decrease in glutathione peroxidase concentration.
- ■
Presentation: Anthracycline-induced cardiotoxicity has been categorized into three forms: acute, early-onset chronic, and late-onset chronic. , Each form may be classified further as subclinical (without CHF) or clinical (with CHF). Acute cardiotoxicity is rare, occurring in less than 1% of patients and is observed immediately after infusion. It typically manifests as an acute but transient decrease in LVEF due to decline in myocardial contractibility. This is frequently reversible within weeks after discontinuation of therapy. Arrhythmias can also be an acute presentation, including tachyarrhythmias (supraventricular or ventricular) and bradyarrhythmias (heart block). Other findings including myocardial ischemia, dilation of the left ventricle, and in rare cases, myocarditis and pericarditis may be observed. Myocarditis typically presents as chest discomfort and shortness of breath shortly after intravenous infusion of chemotherapy. The electrophysiological abnormalities may present as an increased QT interval, ST-T changes, and decreased QRS voltage, and are generally observed in 20% to 30% of patients. Abnormalities in cardiac biomarkers, such as increased serum B-type natriuretic peptide (BNP) and cardiac troponin levels, may also occur. Usually, the manifestations of acute toxicity resolve after discontinuation of the causative agent.
- ■
Incidence: Early-onset chronic cardiotoxicity occurs in 1.6% to 2.1% of patients during treatment or within 1 year after completion of treatment, with a peak incidence at about 3 months post-treatment. The late-onset chronic form occurs in 1.6% to 5% of patients, at least 1 year after completion of treatment. In a few cases, it may not be clinically evident even 10 to 20 years after the first dose of chemotherapy. Middle-age adults make up a large proportion of cancer-survivors with clinical signs of chronic onset cardiotoxicity who have received anthracycline-based chemotherapeutics as children or young adults. Early-onset and late-onset chronic cardiotoxicity typically present as a progressive decline in the LVEF leading to a dilated cardiomyopathy or, less commonly, a restrictive cardiomyopathy. In the most severe form, they can progress to severe left ventricular (LV) systolic dysfunction and clinical heart failure which may be progressive, and is associated with a poor prognosis.
- ■
Risk factors: Many risk factors have been identified for the development of anthracycline-induced cardiotoxicity, but the most important risk factor is the lifetime cumulative dose of anthracycline. Studies that have evaluated the cumulative probability of doxorubicin-induced HF have determined that it occurs in 3% to 5% with 400 mg/m 2 , 7% to 26% at 550 mg/m 2 , and 18% to 48% at 700 mg/m 2 . Other risk factors for anthracycline toxicity include intravenous bolus administration, higher single doses, female gender, preexisting cardiac disease, and history of prior mediastinal irradiation. Also, children and older adults (age > 70 years) appear to be more susceptible to anthracycline-induced cytotoxicity. , Finally, concomitant use of other agents with known cardiotoxic effects, such as cyclophosphamide, actinomycin D, mitomycin, etoposide, trastuzumab, and paclitaxel, may have an additive effect on anthracycline-induced cardiomyopathy.
- ■
Pretreatment evaluation: Given the multitude of cardiovascular toxicities, it is generally warranted that all adult patients scheduled to receive an anthracycline-based therapy should have a comprehensive assessment of their baseline cardiac function before initiation of therapy ( Fig. 10.1 ). For patients with baseline LVEF less than 40% or patients with current CHF or history of CHF with reduced ejection fraction less than 50%, anthracycline-based chemotherapy is generally not recommended. Even for patients with no history of CHF and baseline LVEF greater than 50%, it is recommended to optimize controllable risk factors, especially hypertension, before the initiation of therapy. Routine surveillance imaging using periodic echocardiography should be offered during treatment based on the patient’s risk factors for developing cardiac dysfunction. Cardiac magnetic resonance imaging (MRI) or a multigated acquisition (MUGA) scan can be used alternatively if echocardiography is not technically feasible or available. A study by Cardinale and colleagues demonstrated the importance of monitoring cardiac function using echocardiography to detect anthracycline-related cardiotoxicity. It was observed that with repeated cardiac imaging, most cases of cardiotoxicity were detected within 1 year of chemotherapy completion. Some clinicians advocate quantification of LVEF before and after therapy and sometimes after every one to two cycles in select high-risk patient populations. A post-anthracycline LVEF measurement is critically important in breast cancer patients who are scheduled to receive trastuzumab, a monoclonal antibody associated with cardiac dysfunction and clinical CHF. It is recommended that a decrease in LVEF of more than 15% to a level less than 50% of baseline or decline to an LVEF less than 40% should result in considering cessation of anthracycline administration or switching to non–anthracycline-containing regimens. It is also recommended that in patients who require chest radiation therapy, radiation be administered at lower doses and with the use of more precise radiation fields, excluding the heart as much as possible.
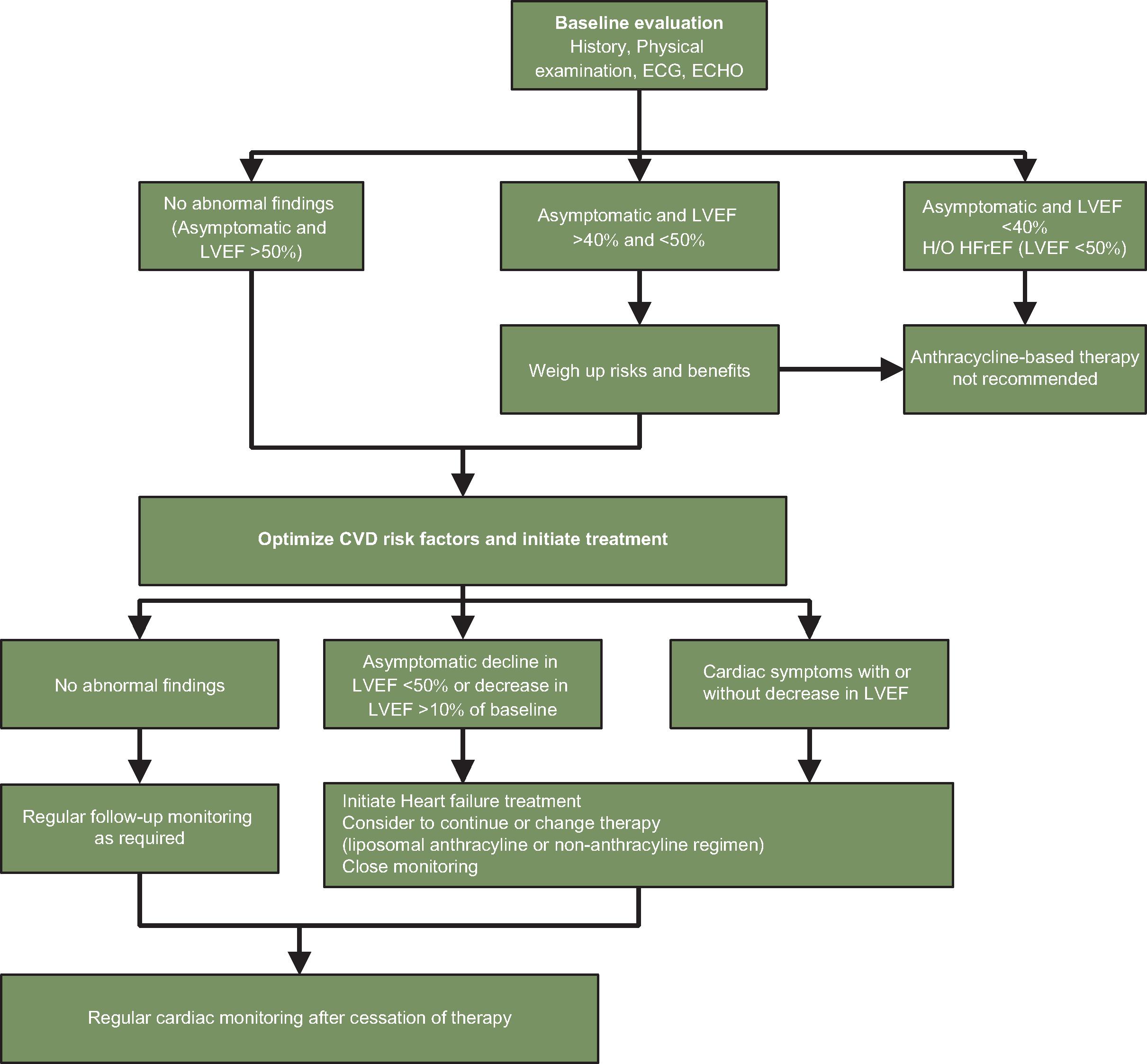
Fig. 10.1
Algorithm for monitoring patients receiving anthracycline therapy. CVD, Cardiovascular disease; ECG, electrocardiography; ECHO, echocardiography; HFrEF, heart failure with reduced ejection fraction; H/O, history of; LVEF, left ventricular ejection fraction.
- ■
Biomarkers: Blood cardiac biomarkers can also be used to identify cardiotoxicity while receiving therapy, especially plasma troponin increase. Increases in cardiac troponins T and I reflect myocardial cell death or injury, while chronic increases in BNP indicate ventricular wall stress. Troponin elevations represent an effective method for monitoring cardiac status as numerous studies have demonstrated correlations between troponin elevations and subsequent LVEF decline. Unfortunately, early rises in biomarker levels are difficult to link with final clinical endpoints because clinically apparent signs of heart failure can often arise years after initial therapy. More conclusive studies are needed to establish the utility of these biomarkers for anthracycline-induced cardiotoxicity diagnosis in clinical practice.
- ■
Treatment of heart failure: Patients developing CHF due to anthracycline-induced cardiomyopathy should cease anthracycline therapy and should be aggressively managed with current standard of care regimens for CHF using combinations of various medications including angiotensin converting enzyme (ACE) inhibitors, diuretics, β-blockers, and spironolactone. , Other procedural interventions can be considered on an individual basis and may depend on cancer status.
- ■
Treatment of asymptomatic LV dysfunction: Patients who develop asymptomatic decline in LVEF (<50%) due to cardiotoxicity can be managed with guideline-based CHF treatment using ACE inhibitors/angiotensin receptor blockers (ARB) alone or in combination with β-blockers. Studies have shown that early initiation of ACE inhibitors in isolation or in combination with β-blockers is associated with greater LVEF improvement or recovery. Also, clinicians should regularly follow up these patients and evaluate and manage cardiovascular risk factors, including hypertension, diabetes, and dyslipidemia.
- ■
Prevention: Multiple approaches have been proposed for the prevention of anthracycline-induced cardiomyopathy. Reducing the cumulative anthracycline dose limits cardiotoxicity, hence various contemporary treatment protocols that use high doses of anthracyclines (>400 mg/m 2 ) are now less frequently prescribed. It has been observed that slow continuous infusion rather than bolus administration is associated with a lower occurrence of clinical heart failure and subclinical cardiac damage. However, replacing bolus administration with a slow infusion may exacerbate exposure effects including myelotoxicity, mucositis, and alopecia, and may also lead to patient discomfort due to prolonged hospitalization. This strategy did not find merit in children with acute lymphoblastic leukemia. Liposomal encapsulation of anthracycline alters the pharmacokinetics and tissue distribution without affecting antitumor efficacy and has also been shown to reduce cardiotoxicity. , It has been proposed that due to their large size, liposomes are unable to cross the gap junctions of normal endothelium in the heart and other normal tissues, but diffuse more readily through the leaky vasculature of tumors. Liposomal infusions are generally recommended for patients who are likely to receive a lifetime cumulative dose of doxorubicin greater than 450 mg/m 2 .
- ■
Dexrazoxane: Dexrazoxane is the only US Food and Drug Administration (FDA)–approved cardioprotective agent for anthracycline-induced cardiotoxicity. In various trials, it has been shown to reduce cardiotoxicity by minimizing LVEF decline and reducing cardiac marker release. , Unfortunately, concerns about the possible compromise of antineoplastic efficacy and increase in secondary tumors, especially in childhood lymphoma and leukemia following dexrazoxane, have led to its restricted use. , Dexrazoxane has largely been evaluated in women with advanced breast cancer and adults with sarcoma, and it is currently approved for breast cancer patients undergoing treatment with extended anthracycline dosing in excess of 300 mg/m 2 .
- ■
Cardiac drugs: β-blockers, ACE inhibitors, ARBs, and statins have been evaluated in randomized controlled trials for the primary prevention of anthracycline-induced cardiotoxicity. Early trials using carvedilol and nebivolol suggested a cardioprotective role of β-blockers to prevent anthracycline-induced LV dysfunction. However, a trial including 192 women with HER2-negative breast cancer, did not demonstrate any benefit of carvedilol monotherapy over placebo with regard to LVEF decline and diastolic function. A combined role of β-blocker and ACE inhibitor in the primary prevention of anthracycline-induced cardiotoxicity was evaluated in the OVERCOME trial. The combination of carvedilol with enalapril was beneficial in preventing anthracycline-induced cardiotoxicity with treated patients demonstrating a lower incidence of CHF or death compared with placebo. A randomized trial comparing the cardioprotective effects of candesartan and metoprolol in patients with early breast cancer undergoing adjuvant chemotherapy found that candesartan, and not metoprolol, was effective in preventing anthracycline-induced decline in LVEF. Additionally, a few studies have shown that statins seem to have protective effects during chemotherapy with anthracycline. In a retrospective analysis of anthracycline-treated patients with breast cancer, incidental statin prescription was linked with less deterioration of LVEF and lower incident CHF. However, there is not enough evidence to support statin administration to the general population scheduled for anthracycline therapy and large multicenter studies are needed.
ANTHRAQUINONES
Mitoxantrone is an anthraquinone derivative used in the treatment of acute leukemias. It was developed in an attempt to produce drugs with a broad spectrum of antitumor activity and devoid of significant cardiotoxicity. However, in the initial phase studies, a few cases of dose-related cardiac dysfunction and arrhythmias were reported. , Cardiac events associated with mitoxantrone include decreased LVEF, CHF, arrhythmias, and very rarely, myocardial infarction (MI). A systemic review of the literature revealed that 0% to 6.7% of patients developed symptomatic mitoxantrone-induced cardiotoxicity and 0% to 80% of patients developed asymptomatic mitoxantrone-related cardiotoxicity. It was reported that cumulative doses greater than 160 mg/m 2 were associated with a significant increase (>5%) in the incidence of CHF. Currently, most authorities recommend that patients should not receive a cumulative mitoxantrone dose greater than 140 mg/m 2 . CHF caused by mitoxantrone should be treated with current standard of CHF medications including diuretics, ACE inhibitors, and β-blockers, as it has been reported that mitoxantrone-related CHF often responds to standard therapy for CHF.
MITOMYCIN C
Mitomycins are a family of antineoplastic antibiotics that mainly act as alkylating agents. Cardiotoxicity in the form of CHF has been reported in patients receiving multiple doses of mitomycin C. , A study of anthracycline-induced cardiotoxicity found that there was a 10% incidence of cardiotoxicity with a median cumulative dose of 60 mg/m 2 . In addition, there is evidence to indicate synergistic cardiotoxic effect of mitomycin in combination with anthracyclines. ,
BLEOMYCIN
Bleomycin is used in the treatment of lymphomas, germ cell tumors, and squamous cell tumors. Although there is a well-known association of pulmonary toxicity with bleomycin, pericarditis is an uncommon but potentially fatal cardiotoxicity associated with bleomycin. , A few episodes of acute chest pain syndrome have also been reported with bleomycin-based therapy. It is generally associated with sudden onset substernal chest pain and treatment is supportive. In addition, a few cases of coronary artery disease (CAD), myocardial ischemia, and MI have been observed in patients during and after treatment with bleomycin-based treatment regimens, although the incidence is less than 1%.
Alkylating Agents
- ■
Cyclophosphamide: Cyclophosphamide is a non–cell cycle-specific alkylating agent commonly used in combination chemotherapy regimens for the treatment of Hodgkin’s and non-Hodgkin’s lymphoma, leukemia, multiple myeloma, and breast cancer. It is a mainstay of most pretransplant preparative regimens. At low doses, cyclophosphamide rarely leads to cardiotoxicity. The administered total dose of cyclophosphamide is the main predictive factor for the development of acute cardiotoxicity. A total dose of greater than 200 mg/kg over 2 to 4 days has been reported to cause symptomatic cardiotoxicity. Also, studies have shown a lower incidence of cyclophosphamide-induced cardiotoxicity in children compared with adults.
- ■
Presentation: Common manifestations of cyclophosphamide-associated cardiotoxicity include a clinical syndrome of CHF, myocarditis, or both. , An asymptomatic decrease in LVEF that is generally transient and resolves over 3 to 4 weeks can also been seen. Electrocardiography (ECG) voltage changes, including decreased amplitude of QRS waves and non–specific ST-segment changes, can be seen 5 to 14 days after beginning cyclophosphamide therapy, even in patients without clinical signs of cardiotoxicity. Pericarditis is another complication observed in a few patients that manifests as acute-onset chest pain and pericardial friction rub. Furthermore, acute-onset fulminant CHF has been reported in patients receiving high-dose cyclophosphamide. Fatal episodes of hemorrhagic myopericarditis have also been described.
- ■
Treatment: Patients developing heart failure should be aggressively treated with current standard of care regimens for CHF. Pericardial effusions may be treated with supportive care or pericardiocentesis, or may require a pericardial window if cardiac tamponade is suspected.
- ■
Combined therapy with anthracyclines: Cyclophosphamide and anthracyclines are commonly used in combination in various antineoplastic regimens, and there is mixed evidence for the additive effects of cyclophosphamide and anthracycline-induced cardiomyopathy. , Therefore, caution should be exercised when prescribing this combination regimen and regular cardiac monitoring should be undertaken to prevent any possible development of CHF, especially in patients older than 50 years. Furthermore, the risk of cardiotoxicity may be minimized by substituting liposomal anthracyclines, and using cardioprotective agents, such as dexrazoxane.
- ■
Ifosfamide: Ifosfamide is structurally related to cyclophosphamide and has been associated with ECG changes including ST-wave abnormalities, decreased QRS complexes, and LV dysfunction. , A dose-dependent incidence of CHF has been reported in patients receiving ifosfamide in doses greater than 12.5 g/m 2 . Arrhythmias appear to be reversible after discontinuation of the drug. In addition, it has been observed that signs and symptoms of CHF resolve a few days after the initiation of supportive therapy.
- ■
Cisplatin: Cisplatin is a platinum-based alkylating agent with a wide spectrum of antineoplastic activity. Cisplatin is infamous for its nephrotoxicity and neurotoxicity but cardiotoxicity is an uncommon complication associated with cisplatin chemotherapy. Hence, cardiac monitoring is generally not recommended for its use. Cisplatin has been implicated as a cause of various conduction abnormalities, including supraventricular tachycardia, bradycardia, ST-T wave changes, and left bundle branch block. , In addition, cisplatin infusions are known to precipitate an acute clinical syndrome causing chest pain, palpitations, and occasionally, elevation of cardiac enzyme levels indicative of an MI. Various incidents of ischemic and nonischemic cardiomyopathy have also been reported with cisplatin chemotherapy. , Additionally, cisplatin has been implicated in other vascular complications, including Raynaud’s phenomenon and cerebral ischemic events. Combinations of cisplatin with other anticancer drugs such as methotrexate, 5-fluorouracil, bleomycin, and doxorubicin are associated with lethal cardiomyopathy and with occlusive thromboembolic events.
- ■
Microtubule-Directed Agents
- ■
Vinca alkaloids: The vinca alkaloids, namely: vincristine, vinblastine, and vinorelbine, are integral components of chemotherapy regimens used in the treatment of many hematologic malignancies and solid tumors. The most common cardiovascular events associated with vinca alkaloids include myocardial ischemia, hypertension, and other vaso-occlusive complications. Many cases of MI have been reported with the typical onset ranging from a few hours to 3 days after the first dose or subsequent doses of vinca alkaloids. ECG abnormalities are usually consistent with an acute MI, including ST elevation, and T wave inversion. Symptoms may last anywhere from 2 to 24 hours and are usually reversible. Cardiotoxic adverse events have been described most commonly with vinblastine, but a few cases have also been reported with vincristine and vinorelbine. Patients with coexisting myocardial ischemia are more at risk of developing these complications. If patients develop MI symptoms, immediate care with standard medical therapy for MI should be provided.
- ■
Paclitaxel: Paclitaxel is currently used in various oncology protocols for the treatment of ovarian, thyroid, lung, and breast cancers, among others. The most common cardiotoxic adverse event associated with paclitaxel administration is asymptomatic bradycardia. In one phase II study, 29% of patients treated with paclitaxel developed asymptomatic bradycardia, and two patients progressed to higher-grade heart block. In a study of 3400 patients treated with paclitaxel, there was 0.5% incidence of grades 4 and 5 cardiac adverse events (life threatening and death, respectively). Cardiac rhythm disturbances in the form of atrial flutter, atrial fibrillation, supraventricular tachycardia, and ventricular tachyarrhythmias have also been reported with paclitaxel. They may be observed occasionally during the infusion of the first cycle but are usually seen after the second or subsequent cycles. Severe conduction abnormalities can occur in patients with an underlying cardiac disease or electrolyte abnormalities. A few cases of myocardial ischemia and infarction have also been reported with paclitaxel infusion. , The exact mechanism of paclitaxel-induced myocardial ischemia is unknown, although it is appears to be linked to coronary vasospasm. Paclitaxel is frequently used in combination with anthracyclines and it is hypothesized that cardiotoxic effects of anthracyclines may be increased due to the decreased renal excretion caused by paclitaxel. Of note, CHF induced by anthracyclines may occur at a lower cumulative dose when used in combination with paclitaxel. , Hence, it has been suggested that the maximum cumulative doxorubicin dose that is administered should be decreased to less than 340 to 380 mg/m 2 , when used in combination with paclitaxel. On the other hand, sequential administration of paclitaxel and doxorubicin does not seem to result in an increased risk of cardiac toxicity.
- ■
Docetaxel: Similarly to paclitaxel, docetaxel is commonly used both alone and in combination with a number of other agents for the management of various malignant conditions, including breast, gastric, prostate, head and neck, and non–small-cell lung cancers. The most commonly encountered cardiovascular adverse events reported with docetaxel are conduction abnormalities, angina, and cardiovascular collapse. Significant elevation of BNP serum concentration has also been reported after docetaxel administration. There is evidence to suggest potentiating effect of anthracycline-induced cardiomyopathy associated with docetaxel administration.
Antimetabolites
- ■
5-Fluorouracil: 5-Fluorouracil (5-FU) is a synthetic pyrimidine antimetabolite that is commonly used in the treatment of several malignancies including colorectal, breast, gastric, esophageal, bladder, and breast cancers. The cardiotoxic effects of 5-FU range from anginal chest pain to massive MI, which can progress to cardiogenic shock and death. 5-FU is one of the most commonly known antineoplastic agents to cause myocardial ischemia, with the reported incidence ranging from 1% to 18%. , Ischemic events are more frequent when 5-FU is administered as a long continuous infusion or given in combination with cisplatin. , Also, patients with preexisting structural heart disease or CAD, or with prior chest radiation therapy, are at higher risk of developing cardiovascular events. , Hence, it is necessary for clinicians to screen patients for the presence of underlying risk factors that can predispose to the development of 5-FU–induced cardiotoxicity.
- ■
Mechanism: The most accepted mechanism for 5-FU–associated cardiac events is coronary vasospasm leading to ischemia.
- ■
Presentation: Patients exhibiting cardiotoxicity typically present with chest pain, with or without transient ECG changes. Silent ischemia has also been recorded in studies where continuous ambulatory ECG monitoring on patients undergoing 5-FU infusion was performed, and results showed that more than half of patients exhibited ECG changes. Cardiac rhythm disturbances, such as atrial fibrillation, ventricular tachycardia, and ventricular fibrillation, have been noted, which may persist for a few days following cessation of 5-FU treatment. 5-FU–mediated adverse effects can progress to symptoms of acute MI, including chest pain, diaphoresis, and shortness of breath, with myocardial injury changes suggested on ECG and LV dysfunction. Ventricular dysfunction can persist for a few days to even a few weeks following cessation of 5-FU therapy. A few cases of cardiogenic shock, cardiac arrest, and sudden death following 5-FU treatment have also been documented. , The incidence of mortality due to 5-FU–associated cardiotoxicity ranges between 2.2% and 13%. ,
- ■
Delivery of 5-FU: The development of cardiotoxicity symptoms of 5-FU varies according to the delivery mode. 5-FU is administered intravenously either as a bolus, as part of the 5-FU, folinic acid, and oxaliplatin (FLOX) regimen or as a continuous infusion (24–96 h) as part of the folinic acid, fluorouracil, and oxaliplatin (FOLFOX) regimen. The use of bolus 5-FU as a short-term infusion over a few minutes is associated with chest pain that usually occurs during the push or immediately after the first cycle. However, in the case of the absence of symptoms during the initial cycle, it is unusual to occur subsequently. The chest pain description suggests a cardiac origin and is associated with elevation of the ST segment on the ECG suggestive of acute ST-segment elevation MI ( initial bolus pattern ). Chest pain associated with continuous infusion of 5-FU may also occur with the first or sometimes second chemotherapy cycle, and typically occurs 24 to 72 hours after infusion initiation. The pain may be atypical compared with classic angina pain and may occur at rest and resolve spontaneously. These symptoms can also recur cyclically during subsequent infusions and can even persist following completion of therapy. Unlike bolus infusion, many patients can tolerate these symptoms and are able to complete the planned infusion course. Additionally, because infusions are administered on an outpatient basis, without telemetry monitoring, ECG changes are generally not captured. With subsequent cycles, symptoms can sometimes recur with more intensity and for a longer duration ( continuous exposure pattern ).
- ■
Treatment: The acute management of patients with 5-FU–associated chest pain is prompt discontinuation of chemotherapy because 5-FU–related cardiotoxicity can be potentially fatal. Also, symptoms should be treated empirically with antianginal therapy such as short-acting sublingual nitrates and/or calcium channel blockers. This approach has been shown to terminate acute symptoms in up to 69% of affected patients. The pivotal next step is an acute assessment for myocardial injury using ECG monitoring, measurement of cardiac troponin levels, and echocardiography if required. Also, it is necessary to identify if the symptoms are reasonably attributed to 5-FU. This poses a challenging question because further administration of 5-FU can potentially lead to recurrence of symptoms, whereas withholding the chemotherapy regimen can compromise cancer treatment. Unfortunately, there is no definitive test to establish diagnosis of 5-FU–induced cardiotoxicity. When it is likely that cardiotoxicity can be attributed to 5-FU infusion, the safest option is to explore alternative chemotherapy regimens with non–fluoropyrimidine-containing agents. Prophylactic treatment using nitrates and calcium antagonist has been found to be ineffective in preventing ischemia symptoms on rechallenge of the drug. , If a 5-FU–based regimen is preferable, it would be appropriate first to determine the underlying pathological process that could explain the symptoms and potentially reverse it. Patients with risk factors for cardiovascular disease can be scheduled to undergo coronary angiography and, if angiography reveals clinically significant coronary stenosis, an attempt at revascularization followed by a rechallenge of 5-FU would be reasonable. In addition, if rechallenging with 5-FU is considered, appropriate precautions, including pretreating patients with cardioprotective medication such as aspirin, calcium antagonists, or long-acting nitrates, and careful cardiac monitoring in an inpatient unit should be employed. Additionally, it has been reported that using a bolus regimen is a much safer option than a continuous infusion and should be the method of choice for administration. Further randomized clinical trials using different approaches to manage these patients are essential to determine the optimal strategy.
- ■
Capecitabine: Capecitabine is an oral prodrug of 5-FU that is known to generate 5-FU preferentially at the tumor site. Its effectiveness has been studied in cancers such as breast, gastric, and colorectal cancers. Although it has gained popularity because of its ease of administration and milder toxicity profile, multiple case reports have documented significant cardiovascular adverse events associated with capecitabine administration. It is generally suggested that the incidence of cardiotoxicity with capecitabine is 1.5% to 2% lower than that of 5-FU. However, in a retrospective study of patients undergoing chemotherapy treatment for metastatic breast and colon cancer, it was found that the incidence of capecitabine-induced cytotoxicity was comparable to that of 5-FU.
- ■
Presentation: Capecitabine-related cardiac events range from angina and reversible ST-segment deviations to MI, arrhythmias, and even cardiac arrest. , Chest pain and palpitations are the most common clinical manifestations of capecitabine-induced cardiotoxicity. It is reported that most of the symptoms of cardiotoxicity tend to occur during the first cycle, with a median time for onset of less than 4 days after initiation of therapy. , The most common ECG abnormalities noted include ST-segment deviation, sinus tachycardia, and prolonged QTc interval. , Patients developing chest pain should be immediately evaluated using ECG monitoring and measurement of cardiac enzymes. Most patients tend to respond to conservative management with antianginal agents and supportive care. The administration of capecitabine should be immediately discontinued in patients who develop pulmonary edema, MI, or arrhythmias.
- ■
Rechallenge: Once cardiotoxicity has occurred, it is uncertain whether rechallenge with a modified dose of capecitabine or together with a prophylactic antianginal agents can be done safely. A retrospective study of patients with capecitabine cardiac toxicity reported symptom recurrence in 10 of 16 patients on rechallenge and a lack of benefit of dose reduction or medical prophylaxis on rechallenging. Also, it is not advisable to administer capecitabine to patients with a history of 5-FU–induced cardiotoxicity.
- ■
Conclusion
Chemotherapy-induced cardiotoxicity is evolving as a ubiquitous complication of cancer treatment due to the ageing of the population and a disproportionate increase in the incidence of cancer among older individuals. With the discovery and approval of new therapies, clinicians should be familiar with the potential of various chemotherapeutic drugs known to cause cardiotoxicity and should promptly evaluate and closely monitor all cardiac events that occur during the course of therapy. In addition, long-term surveillance is warranted with some treatments because certain cardiovascular complications including LV dysfunction or CHF may develop more than a decade after the initial administration.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree