Carbon Ions
 PRESENT STATUS AND PERSPECTIVE FOR CARBON IONS
PRESENT STATUS AND PERSPECTIVE FOR CARBON IONS
Carbon ion therapy is an innovative radiotherapy modality that is mostly dedicated to cancers considered as unresectable and radioresistant to photons. Its radiobiologic properties combine the advantages of the high-dose distribution conformity of protons for deep tumors (superior to photons and neutrons) and the higher biologic effectiveness (compared to photons and protons) of high linear energy transfer (LET) particles such as neutrons. Moreover, the combination of the biologic and ballistic properties of carbon ions greatly reduces the treatment period compared with photon or proton radiotherapy.
Lawrence Berkeley Laboratory
The use of charged particles (protons and ions) for clinical applications was first proposed in 1946 by R. R. Wilson.1 A decade later, the Lawrence Berkeley Laboratory (LBL) in California started patient irradiation. The LBL began with protons in 1954 and then with light ions. From 1957 through 1974, helium and higher particles were used in more than 2,000 patients. From 1974 through 1992, the year of closure of the center, neon ions were primarily used in 433 patients. Few patients were treated with carbon ions at the LBL. Miscellaneous cancer types were treated with neon ions, mainly base of skull tumors with several pathologic types, including chordoma, chondrosarcoma, meningioma, and adenoid cystic carcinomas (ACCs), as well as osteosarcomas, sacral chordoma, glioblastoma, cholangiocarcinoma, head and neck squamous cell carcinoma, and prostate cancer. Despite severe limitations in terms of beam application (fixed horizontal beam only), quality of imaging modalities available at that time, and limited periods for clinical applications (30% of the Bevalac running time), the LBL experience permitted first to report a high local tumor control in radioresistant tumors, especially with neon particles, and to demonstrate the feasibility and the safety of high LET particle therapy.2–6
National Institute of Radiological Sciences
The heavy ion radiotherapy project in Japan started in 1984. A unique double-synchrotron ring heavy ion accelerator system dedicated to the project was designed and constructed. It system consists of two ion sources, a radiofrequency quadrupole (RFQ) linear accelerator, an Alvarez linear accelerator, two synchrotron rings, a high-energy beam transport system, and an irradiation system. It was completed in October 1993 at the National Institute of Radiological Sciences (NIRS) in Chiba, Japan, and was named the heavy ion medical accelerator in Chiba (HIMAC). At NIRS, there are three treatment rooms with fixed vertical and horizontal beam lines. The accelerated energy of the vertical carbon ion beam is 290 MeV per nucleon or 350 MeV per nucleon, and that of the horizontal beam is 290 MeV per nucleon or 400 MeV per nucleon. The range of the 290-MeV per nucleon carbon ion beam is approximately 15 cm in water, that of the 350-MeV per nucleon beam is 20 cm, and that of the 400-MeV per nucleon carbon ion beam is 25 cm. Maximum field size is 15 cm by 15 cm.7 To produce uniform irradiation fields, a passive beam delivery system is employed with a pair of wobbler magnets and a scatterer. The range shifter is applied for adjusting the residual range of carbon ions in the patient and the ridge filter to spread out the Bragg peak in the depth-dose distribution of carbon ions. After commissioning of the system, including preclinical biologic study, in June 1994, NIRS started heavy ion radiotherapy using carbon ion beams generated by HIMAC.8
Since 1994, clinical studies to develop safe and secure irradiation technologies such as respiration gating and optimized dose fractionation for various cancers have been conducted. All carbon therapies have been performed as prospective phase I/II and II clinical trials in an attempt to identify tumor sites suitable for this treatment, including radioresistant tumors, and to determine optimal dose fractionation, especially for hypofractionation in common cancers. In the phase I/II studies to confirm the safety of carbon ion therapy and to obtain a clue to an antitumor effect, the number of fractions and treatment period were fixed for each disease, and the total dose was gradually increased by 5% to 10%. When the recommended doses were determined in the phase I/II studies, they were incorporated into the phase II studies. At NIRS, more than 6,000 patients have been treated with carbon ion beams for past 17 years, and the clinical efficacy of carbon ion therapy has been demonstrated for many malignant diseases.
In 2011, a new medical facility opened at HIMAC that uses the existing synchrotron. It comprises three rooms: two with a robotic arm–controlled patient table for fixed horizontal and vertical scanning irradiation ports and the third one equipped with a lightweight rotating gantry with the superconducting magnets. NIRS completed installation of scanning equipment and commissioning of the system at one room with fixed beam lines and started a clinical trial in May 2011.
Present Status and Perspective for Carbon Ions in Japan
At present, five carbon ion therapy facilities are operating around the world, with three of them in Japan. In 1984, the Japanese government embarked on a program of carbon ion therapy at the first facility in Chiba. Clinical studies that began in 1994 have shown promising outcomes in general and have led to the development of the other carbon ion therapy facilities in Japan.
The second center, the Hyogo Ion Beam Medical Center in Hyogo, Japan, was established in 2001—the world’s first ion beam facility providing both proton and carbon ion beams. By the end of September 2010, 2,735 patients were treated at Hyogo, including 915 patients receiving carbon ion therapy. Its lower energy (320 MeV per nucleon) and smaller field size (10 cm by 10 cm) for carbon ion beam compared with HIMAC restricted indications of carbon ion therapy for smaller and shallower tumors.
The third Japanese carbon ion therapy facility was completed in March 2010 at Gunma University Heavy Ion Medical Center. It is a concise carbon ion therapy accelerator complex almost one-third the size and construction cost of HIMAC with the same performance.
The fourth facility, SAGA HIMAT (heavy ion medical accelerator in Tosu), a concise Gunma-type system located in the southern part of Japan, is under construction and will begin treating patients in 2013. The fifth is at planning stage in Kanagawa prefecture near Tokyo and will start its operation in 2015.
Gesellschaft für Schwerionenforschung
In Germany, carbon ion research and treatment was made possible at a basically preclinical research institution. Beginning in 1997 at Gesellschaft für Schwerionenforschung (GSI) in Darmstadt, Germany, patients were treated with carbon ions by the Department of Radiation Oncology in Heidelberg, Germany. Within the research context of GSI, three beam time blocks per year were provided for patient treatment. As a collaborative effort consisting of clinicians, biologists, physicists, and engineers from the University Hospital of Heidelberg, the German Cancer Research Center (DKFZ) in Heidelberg, Germany, the Forschungszentrum Rossendorf in Dresden, and the Biophysics Group at GSI, patient treatment could be realized accompanied by extensive preclinical research.
For beam delivery provided by a synchrotron, the intensity-modulated raster scanning technique was developed, and biologic dose calculation was refined using the local effect model (LEM) developed by Kraft and Scholz.9–11 Clinical efficacy of this approach was shown and validated in more than 600 patients with special focus on radioresistant tumors such as chordomas and chondrosarcomas of the skull base, ACCs, and high-grade meningiomas.
Heidelberg Ion Therapy Center
In Europe, the only center treating patients with carbon ion radiotherapy is the Heidelberg Ion Therapy Center (HIT) in Heidelberg, Germany. The center is equipped with a synchrotron, providing different particle species for three treatment rooms. Two rooms are equipped with a horizontal beam line; in the third room, the world’s first carbon ion gantry has been realized. Since November 2009, the center has been in clinical operation offering particle treatment for approximately 1,300 patients per year. The center is directly connected to the existing Department of Radiation Oncology to allow for a streamlined work flow, and patients can be treated as both inpatients and outpatients.12,13 The center delivers particle treatments via active raster scanning; as well, treatment planning and biologic plan optimization has been adopted from the work performed previously at GSI.14
Uptake of clinical routine is based on the indications treated at GSI, focusing on skull base chordomas, chondrosarcomas, and ACCs.12 Subsequently, several clinical studies on varying indications started recruiting with different treatment concepts, including a randomized trial of carbon ion versus proton radiotherapy.15–17
Perspective for Carbon Ions in Europe, the United States, and Asia
To date, HIT is the only facility in Germany treating with carbon ions. Facilities in Marburg and Kiel, Germany, have been built but have not taken up clinical routine. In Europe, the National Centre for Oncological Treatment (CNAO) in Pavia, Italy, is a center equipped for proton and carbon ion treatments that started taking up clinical service in mid-2011. In Austria, the carbon facility (MedAUSTRON) at Wiener Neustadt is under construction and will offer proton and carbon ion treatments. The creation of a carbon ion clinical facility (the ETOILE project) in France is under discussion.
In Asia, a radiotherapy facility including photons, protons, and carbon ion beams is under construction in Shanghai.
More than 50 years after the world’s first carbon ion therapy in Berkeley, there is a growing interest within the radiotherapy community to again invest in that domain.
 TECHNICAL ASPECTS: PRESENT STATUS AND PERSPECTIVE
TECHNICAL ASPECTS: PRESENT STATUS AND PERSPECTIVE
Beam Delivery
The current passive beam delivery system used in Chiba is a quite reliable and robust system and has had proven stable performance at HIMAC since 1994. A respiratory-gated irradiation technique was also put into practical use for the treatment of moving targets with the passive beam delivery system.18 The similar systems are adopted at Hyogo and Gunma University.
A scanning irradiation method, which uses “narrow” pencil beams of carbon ions to cover a target volume by superimposing the spot beams of carbon ions slice by slice has been developed and applied at GSI and then at HIT. The new facilities at HIMAC Pavia and in Germany and Austria are (or will be) equipped with this system. Compared to passive methods, the scanning irradiation techniques permit a more conformal irradiation of the target volume, especially with a better sparing of the normal tissue located in the beam channel entrance (i.e., skin). Therefore, the technique is more appropriate for the treatment of complex-shaped lesions that cannot be adequately irradiated by the passive beams. The scanning also eliminates the need for constructing compensation filters as well as patient-specific collimators.
Gantry
To date, no gantry is available for treatment with carbon ions, whereas gantries are standard in proton therapy, primarily owing to the immense constructions required for building such a gantry to accommodate the large size and weight of the necessary magnets for beam delivery. However, to provide optimal dose distributions, especially for paraspinal tumors and tumors of the gastrointestinal tract, the use of horizontal beams is limiting treatments in these patients.
The world’s first carbon ion gantry was designed and constructed at HIT (with a total weight of more than 600 tons of steel, about 420 tons precisely turning around the patient, and a length of 25 m and diameter of 13 m). Clinical operation of the gantry is anticipated for mid-2012.
A lightweight rotating gantry using superconducting magnets has been designed and should be installed in the new HIMAC facility.
Gating and Tracking
To reduce the enlargement of irradiated volume with respiration, a respiratory-gated irradiation technique was developed for carbon ion therapy at HIMAC. In this technique, the irradiation-gate signal is generated only when the target is located at the designed position and the synchrotron can extract a beam. Thus, one of the key technologies for respiratory-gated irradiation is the beam-extraction method from the synchrotron according to the gate signal. For this purpose, the radio frequency–knockout (RF-KO) slow-extraction method, which utilizes a transverse beam heating by an RF-field tuned with a wave number of a horizontal betatron oscillation, was developed19 as well as the respiratory-gated irradiation system.18 In this system, the respiration signal is generated by observation of movement of light-emitting diode set on the surface of the patient’s body through a position-sensitive detector. The respiratory-gated irradiation system has been utilized primarily for treatment of liver and lung tumors since 1996 and has been very effective for reduction of fraction number in these tumors moving along with respiration.
Treatment of moving organs with ions applied with the raster scanning technique confront the physicist and clinician with novel challenges. Because of the interplay effect of the scanning beam and the moving target, substantial over- and underdosage within the target volume is generated; therefore, it cannot be precisely calculated and guaranteed that the planned dose will be applied homogeneously.20 Thus, effective compensation mechanisms are necessary for the treatment of moving targets.
Several approaches for moving target irradiation are under investigation: monitoring breathing motion and using the information for gating strategies (irradiating during set gating windows) can significantly reduce interaction.20 Another approach is the rescanning technique: the volume is not scanned by the beam with the whole calculated dose (i.e., particle number); the volume is scanned several times, and the particles (or dose) are divided onto the scanning runs. With this concept, a more homogeneous spread of the particles and dose over the volume can be achieved, with increasing homogeneity with the number of scanning runs.21 A sophisticated concept is monitoring organ motion and making the beam follow the movement of the target volume; this requires fast communication between the beam-scanning system and the monitoring setup to provide real-time tracking of the volume.20
The concepts presented are under evaluation; potentially, the technique used may depend on the tumor type or anatomic region treated. Additionally, target volume concepts might help to compensate for some minor organ movement. However, using only compensatory target volumes, such as internal target volumes (ITVs) used in photon radiotherapy, will not suffice.20 Variation of spot scanning size and reduction of grid size can help to compensate organ motion as well. It is most likely that a combination of several approaches—that is, specific target volume, modification of spot size, and perhaps gating—will provide optimal and clinically applicable dose distributions.
Beam Imaging
As a by-product of particle radiotherapy, b+ activity is generated and can be monitored using conventional positron emission tomography (PET) scanners to in vivo monitoring of dose delivery. Several centers offering particle therapy have established this possibility.
At GSI, an online PET imager was built into the treatment cave, allowing for online monitoring of b+ activity during each fraction and enabling later correlation with the planned and calculated treatment plan for passive beam delivery centers, such as Massachusetts General Hospital in Boston or centers in Japan.
Promising experience in PET imaging of proton and carbon ion therapy has been obtained for more than 50 patients monitored after passive proton treatments in the United States and Japan, and more than 400 patients have been monitored during scanned carbon ion beam irradiation at GSI. At the GSI pilot project, the value of PET for improving the accuracy of the semiempirical computed tomography (CT)-range calibration curve employed by the treatment planning system, as well as for detecting and quantifying deviations between planned and actual treatment delivery owing to patient misalignments or anatomic changes over the course of fractionated therapy, could be demonstrated.22 At HIT, a commercial PET/CT scanner has been installed in a dedicated room adjacent to the treatment area, and clinical evaluation is ongoing within a prospective trial.
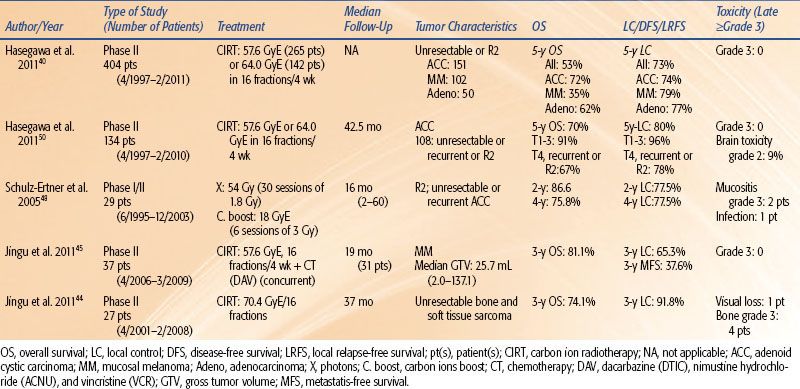
FIGURE 20.1. Bragg peak (protons versus carbon ions versus photons)

FIGURE 20.2. Spread-out Bragg peak (protons versus carbon ions versus photons).
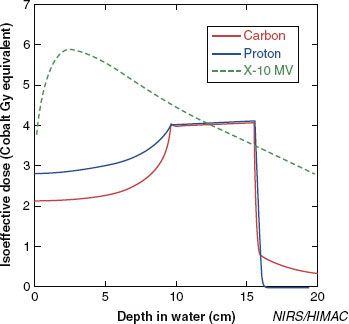
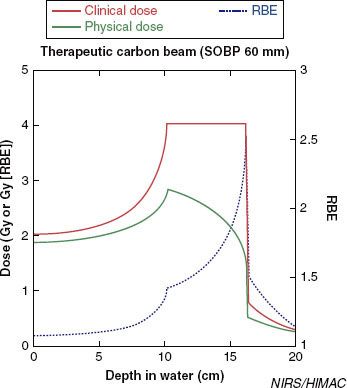
FIGURE 20.3. Design of a carbon ion spread-out Bragg peak (SOBP). The high LET region of the carbon ion beam is located in the distal part of the Bragg peak, and the SOBP becomes a weighted function of several Bragg peaks at various energies, which results in a dilution of the dose-average LET in the target volume. Therefore, the physical dose (Gray) has to be decreased as the relative biologic efficiency (RBE) value rises so as to have a flat biologically effective dose across the SOBP.
 CARBON ION RADIOBIOLOGIC PROPERTIES: RATIONALE FOR PATIENT SELECTION
CARBON ION RADIOBIOLOGIC PROPERTIES: RATIONALE FOR PATIENT SELECTION
Carbon ions for clinical applications are characterized by two main properties: (a) a depth-dose distribution with a sharp maximal energy deposition at a definite depth (the Bragg peak) related to the beam incidence energy, with almost no dose deposited beyond this peak (Fig. 20.1) and (b) a biologic efficiency increasing at the end of the beam’s range within the Bragg peak.
Although not demonstrated by large prospective randomized clinical trials, the potential clinical gains achievable by these ballistic advantages led to the definition of several “standard” indications for proton therapy (i.e., base of skull chordoma and chondrosarcoma, eye melanoma, selected pediatric brain tumors).23,24
Dose Distribution: Bragg Peak and Spread-Out Bragg Peak
Although similar, there are some notable differences between the dose-depth distribution of protons and carbon ions that may have clinical impacts (the presence of a “fragmentation tail” for carbon ions owing to nuclear interactions, allowing high-quality PET imaging) and a very narrow penumbra for carbon ions compared to protons.24
Similarly to protons, carbon ion beams are spread out to conform to the target, resulting in the spread-out Bragg peak (SOBP) after a low dose “plateau” within the entrance channel beyond the target (Figs. 20.2 and 20.3). This can be achieved using several techniques, mainly with passive scattering, but also with more advanced techniques (e.g., pencil beam with wobbling or uniform scanning) has to achieve a lower dose deposit within the normal tissue in the entrance of the beam (proximally to the tumor volume) while optimizing the distal dose distribution and therefore a higher dose distribution conformation.
Treatment Plan Intercomparisons
There have been several publications based on comparative dosimetric analyses of dose distribution outcomes with photon intensity-modulated radiation therapy (IMRT) versus particle therapy (protons and/or carbon ions) with passive or active beam delivery. The general conclusion of publications dealing with protons is that similar dose distribution can be achieved with the target volume for all techniques but that particle therapy permits lowering of the dose delivered to the surrounding critical normal tissues even for large target volumes.25–28
Few treatment planning studies with carbon ions have been published thus far. For similar and strict constraints to critical organs at risk, Schulz-Ertner et al.29 have demonstrated that carbon ions alone or a combination of carbon ions and photon IMRT was superior to photon IMRT alone for spinal chordomas and ACC with infiltration of the skull base.29,30 Amirul et al.31 have noted a dosimetric benefit both for tumor coverage and OAR preservation for selected head and neck tumors.
Integral Dose
Another advantage of particle beams is that fewer fields are required to achieve an acceptable dose distribution for difficult cases, which leads to a dramatic decrease of the integral dose (and potentially a lower incidence of radio-induced cancer, especially in pediatric indications) compared to intensity-modulated x-ray therapy (IMXT).32
Biologic Effectiveness and Equivalent Biologic Rate
As with neutrons, carbon ion interactions are characterized by a high LET that provides for a given physical dose for a higher biologic effect compared to low LET irradiation modalities (photons and protons). Therefore, the dose delivered with high LET particles is prescribed in Gray equivalents (GyE) or cobalt Gray equivalents (CGE) equal to the measured physical dose in Gray multiplied by a relative biologic efficiency (RBE) factor. The RBE is the ratio of the dose of radiation required to produce a certain biologic effect with photons relative to the dose required to produce the same effect with another form of ionizing radiation (such as protons and light ions).
Interestingly for clinical applications, this higher biologic efficiency, which may lead to a higher tumor control probability (TCP) for radioresistant tumors but may also increase the normal tissue complication probability (NTCP), is limited to the Bragg peak (thus to the SOBP). Within the “entrance” channel where the main proportion of organs at risk should be, there is no additional biologic effect.
The RBE of carbon ions is difficult to calculate; for dose-reporting purposes, a value of three is often utilized based on neutron experience. However, several other parameters should be taken into account, such as dose per fraction, fractionation, tissue type, target volume, and pO2 value at each point in the irradiated volume as well as the variation in RBE along the SOBP (higher at the distal part than at the proximal part)24 (Fig. 20.3). In NIRS, the LET dependency is taken into account in the design of clinical dose distribution by choosing the 10% survival of the human salivary gland tumor cells as end points; this was evaluated through TCP analysis for non–small cell lung cancer.33,34 The LEM, a generic model allowing for RBE-calculation in various tissue types and for various end points, has been developed and applied at GSI and is in use for biologic treatment planning at HIT.9,10
Dose Fractionation
The capacity for normal and cancer tissues to repair from sublethal radiation injury is sharply reduced with high LET radiation both for normal tissues and cancer cells. This leads to questioning the need for dose fractionation (applying a standard fractionation scheme with a low dose per fraction) justified in low LET therapy (photons and protons) by a higher kinetic of sublethal radiation injury repair for normal tissue versus radiosensitive tumors.
Experimental data with high LET particles did not find any differences in RBE values between tumor and normal cell lines in standard culture condition.35,36 However, experiments conducted with fast neutrons and carbon ions have demonstrated that increasing the dose per fraction tends to lower the RBE of both the tumor and normal tissues but with a more important decrease for the normal tissue given a higher therapeutic ratio for short-course hypofractionation schemes with carbon ion radiotherapy.35,37 At NIRS in Chiba, Japan, hypofractionated carbon ion radiotherapy has been investigated systematically for a variety of tumor entities, and it seems that a significant reduction of overall treatment time can be accomplished for many tumor entities without enhancing toxicity.8
Applications for Patient Selection and Radiotherapy Schemes
To summarize, the best indications for carbon ions based on their biologic advantages over low LET radiation (photons and protons) and poor dose-depth distribution (photons and neutron) are tumors that demonstrate low radiosensitivity when treated with photons, particularly if the tumor is surrounded by radiosensitive normal tissue.
Carbon ion biologic and physical properties also justify the use of a larger fraction dose than that used in conventional radiotherapy schemes with an impact on patient quality of life (shorter overall treatment time) as well as a major economical impact (reduction of the cost for the health insurance).
 CLINICAL DATA
CLINICAL DATA
Primarily based on the clinical experience of NIRS and GSI, various clinical data are available to assess the efficacy and tolerance of carbon ion radiotherapy in miscellaneous tumor locations and pathologies.8,23,24,38
To date, medical data on carbon ion therapy rely on prospective phase I (dose-escalation and hypofractionation assessments) and phase II trials conducted in more than 7,000 patients, mostly at NIRS and GSI. The main clinical data are summarized for each tumor site in Tables 20.1 through 20.7.
Head and Neck Cancers (Table 20.1)
Carbon ion therapy has been applied in several tumor types for primary or recurrent cancers (unresectable or R1-R2 tumors) and in some selected cases as a second irradiation in phase I and II prospective studies.39–49
The most impressive data have been obtained for ACC, malignant mucosal melanoma, and sarcomas known as highly radioresistant tumors.39,40,44–46,48,50
When compared to NIRS historical data, a dose response was demonstrated for sarcoma in the NIRS experience in terms of 3-year local control (91.8% vs. 23.6% for 70.4 GyE vs. 57.6 or 64 GyE) and 3-year overall survival (74.1% vs. 42.9% for 70.4 GyE vs. 57.6 or 64 GyE).44
The comparison of the clinical outcome for locally advanced ACC treated within the same period by the Heidelberg radiotherapy team either with a combination of photon IMRT with a carbon ion boost (39 patients) (total median dose, 72 GyE) or with photon IMRT alone (29 patients) (median dose, 66 Gy) was in favor of carbon ion boost versus photons only with a 4-year locoregional control, disease-free survival, and overall survival rates of 77.5 versus 24.6 (p = 0.08), 53% versus 23% (p = 0.19), and 75.8 versus 77.9, respectively.48
Several prospective trials have been launched at HIT.51,52
Sarcoma (Soft Tissue and Osteosarcoma) and Chordomas (Tables 20.2 and 20.3)
The initial phase I trial (64 patients) conducted at NIRS from June 1996 to February 2000 has established a reference dose and fractionation of 70.4 GyE (16 fractions, 4 weeks).53
Since then, to August 2011, 495 patients (514 tumors) were entered in a phase II trial: 405 bone sarcoma/chordoma (chordoma in 177, chondrosarcoma in 81, osteosarcoma in 81, Ewing/primitive neuroectodermal tumor [PNET] in 28, miscellaneous in 38) and 109 soft tissue sarcomas (malignant fibrous histiocytoma [MFH] in 21, malignant peripheral nerve sheath tumor [MPNST] in 15, synovial sarcoma in 11, leiomyosarcoma in 11, miscellaneous in 51). The 2- and 5-year overall survival were 79% and 59%, respectively, and 2- and 5-year local control were 85% and 69%, respectively, with 2% of grade 3/4 skin/soft tissue late toxicity (toxicity primarily related to the tumor volume and its location that may be reduced by advanced beam delivery techniques).54
Sacral Chordoma
Carbon ion radiotherapy appears effective and safe in the management of patients with sacral chordoma. It offers a promising alternative to surgery with a high local control, similar, see superior to surgical procedures alone55 or with adjuvant proton therapy56 and a higher functional outcome with few or no severe toxicities regarding urinary and anorectal function.54,57,58 The occurrence of severe neuropathy was related to the dose level (>73.6 GyE). The application of particle radiotherapy for tumors adjacent to the gastrointestinal tract may be restricted because of the low tolerance of the intestine. In that situation, a surgical spacer may be placed before particle radiotherapy.59 To note, a more than 10% of tumor volume increase at the end of the radiotherapy without further progression was described in 50% of cases.60
TABLE 20.1 HEAD AND NECK CANCERS (ADENOID CYSTIC CARCINOMA, MUCOSAL MELANOMA, ADENOCARCINOMA, SARCOMA)