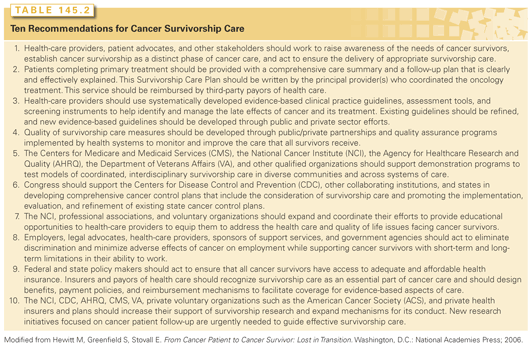
■ Raise awareness of the medical and psychosocial problems faced by cancer survivors and establish cancer survivorship as a distinct phase of the cancer trajectory during which specific clinical interventions are needed.
■ Define quality health care for cancer survivors and identify strategies to achieve it.
■ Improve quality of life through policies to ensure cancer survivors’ access to psychosocial services, fair employment practices, and health insurance.
There are many unanswered questions about surveillance strategies, models of health-care delivery, research strategies, and education of both patients and health-care providers. This chapter is designed to serve as an introduction to understanding the scope of the survivorship problem.
DEFINITION OF SURVIVORSHIP AND SCOPE OF THE PROBLEM
Cancer survivorship is an evolving concept with multiple definitions. Prior to the recent evolution of curative cancer therapy, cancer survivors were defined as family members left behind after a loved one had succumbed to the disease.2 In the latter half of the 20th century, as survival rates increased, the dramatic impact of life-saving but potentially toxic therapies began to emerge. Izsak and Medalie3 are credited with first describing the “costs” of cancer survivorship: “Survival rates . . . do not relate to how the patient survives, at what cost to his physical functioning, how he adapted to his condition from a psychological point of view, and how he is fulfilling his roles in his family, at work, among friends, and in the wider society.” Three decades ago, Fitzhugh Mullan,4 a young physician and cancer survivor, challenged the binary concept of illness versus cure and suggested that survivorship was a process with predictable stages, ranging from the acute diagnosis and treatment phase, through the post-therapy phase of watchful waiting, and finally to the phase of permanent survival, when the focus shifts from concerns about risk of recurrence to those impacting long-term quality of survival. Soon after, the National Coalition for Cancer Survivorship (NCCS) was founded, raising awareness of the importance of the survivorship experience and setting the stage for recognition of survivorship as a distinct phase along the cancer control continuum.5 In the mid 1990s, the National Cancer Institute established the Office of Cancer Survivorship, which was charged with directing and supporting research, training, and education regarding issues relating to cancer survivorship. Over the past decade, many influential groups, including the IOM,1 the President’s Cancer Panel,6 and the American Society of Clinical Oncology (ASCO),7 have released reports highlighting issues relating to cancer survivorship. For the purposes of this chapter, the IOM’s definition of cancer survivorship will be used, which focuses on the phases of cancer care following the completion of primary treatment and lasting until cancer recurrence or the end of life.1
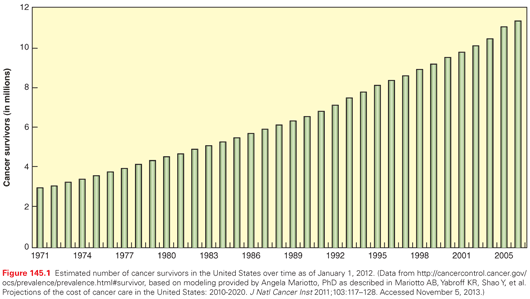
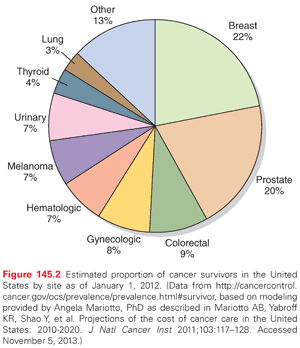
The number of cancer survivors has increased more than threefold since 1975 as a result of improvements in early detection and therapeutic successes (Fig. 145.1).8 There are currently nearly 14 million cancer survivors in the United States8 and more than 25 million worldwide.9 The 5-year relative survival rate has also continued to increase steadily, and has now reached 68% for adults and 83% for children.10 The number of cancer survivors is expected to increase in the United States by 31% to almost 18 million over the next decade,8 because of the general population growth and the increasing proportion of older adults in the population for whom cancer prevalence rates are the highest. More than 8 million cancer survivors in the United States are age 65 or older, representing 59% of all cancer survivors.11 Among survivors, the most common diagnoses are breast cancer (22%) and prostate cancer (20%); survivors of colorectal cancer, hematologic malignancies, gynecologic, urinary, melanoma, lung, and other cancers each represent less than 10% of the population of cancer survivors (Fig. 145.2).11 Dramatic improvements in childhood cancer survival has resulted in a growing population that now exceeds 379,000 in the United States alone,12 and multiple studies regarding the impact of cancer therapy on health-related outcomes in this population have been published.13 In a retrospective cohort of 10,397 childhood cancer survivors diagnosed between 1970 and 1986 (the Childhood Cancer Survivor Study), Oeffinger et al.14 reported that 62.3% experienced at least one treatment-related late effect, 37.6% developed multiple late effects, and 27.5% developed a late effect that was severe or life-threatening. The impact of cancer therapy on health-related outcomes in the rapidly growing population of adult cancer survivors is largely unknown, but the need for ongoing follow-up care and late effects surveillance for all cancer survivors is clear.1
GOALS OF SURVIVORSHIP HEALTH CARE
Identifying Late Effects of Cancer Therapy
The modern era of cancer therapy is predicated on the safe intensification of radiation, chemotherapy, and biologic therapies. Malignancies resistant to therapy have demanded an aggressive treatment approach that often resides on the edge of normal tissue tolerance, or even exceeds tolerance to some “acceptable” degree. The potential to ameliorate or prevent such normal tissue damage, or to manage and rehabilitate affected patients, requires an understanding of these persistent long-term or late-onset chronic effects. Because late effects can manifest months or years after the cessation of treatment, therapeutic decisions intended to obviate such effects can be based only on the probability, not the certainty, that such effects will develop.
Determining the frequency and pathogenesis of late effects is difficult for several reasons: (1) Patients must survive long enough for tissue injury to develop, (2) the number of patients both affected and unaffected by therapy must be known, and (3) the latent period to the manifestation of damage compromises discernment of the responsible component of multimodality therapy. Further complicating our understanding of organ tolerance to therapy is that cancer and host factors interact with therapy in the causation of late effects. Cancer factors include direct tissue effects (e.g., from organ invasion such as the lung), systemic effects of cancer-induced organ damage or loss (e.g., hepatic dysfunction or loss of a kidney), and indirect mechanical effects (e.g., renal or airway obstruction). Host factors include genetic (e.g., ataxia-telangiectasia) and comorbid (e.g., vascular disease, diabetes) conditions, the developmental age at treatment, and underlying structural abnormalities (e.g., cardiac). The previously mentioned report by Oeffinger et al.14 documents the high frequency of chronic health conditions in adult survivors of childhood cancer, many of which are severe or life-threatening. At 30 years, almost three-fourths of survivors had a chronic health condition, more than 40% had a serious health problem, and one-third had multiple conditions. Additionally, Yeh et al.15 have recently developed a model based on Childhood Cancer Survivor Study data to estimate cumulative excess mortality in a simulated cohort of 5-year survivors of childhood cancer. These investigators predict a substantial decrement in life expectancy of 10.4 years (range: 4 to 17.8 years) for the childhood cancer survivors, compared with the general population.15,16 Moreover, the model estimates that the reduction in life expectancy is up to 28%, and that approximately one in four survivors will die of either a late recurrence or late effects related to secondary cancer and cardiopulmonary conditions. These data underscore the importance of understanding the toxic effects of our therapy. Several chapters in this text provide detailed information on the long-term physical effects of cancer therapy for each organ system.
Surveillance/Guidelines for Late Effects
Treatment-related sequelae can manifest at any time during or after therapy, and may be clinically silent at times when a diagnosis might lead to effective interventions. Some chronic or late effects can evolve during normal development or aging. Health-care providers need to anticipate these effects as well as evaluate those that are overt in order to optimize measures that can enhance quality of life. The need for standardized guidelines to direct follow-up care after cancer treatment and surveillance for late effects of therapy has been recognized as an important component of cancer care for over a decade.1,5,17,18 However, the development of evidence-based long-term follow-up guidelines has proven to be a challenging endeavor. The Children’s Oncology Group (COG) developed the Children’s Oncology Group Long-term Follow-up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers, a set of risk-based, exposure-related screening recommendations to guide the long-term follow-up care of pediatric cancer survivors with the goals of improving quality of life and decreasing complication-related health-care costs.19
The COG guidelines (available at www.survivorshipguidelines.org)20 exemplify a hybrid of evidence-based and consensus-driven approaches to guideline development. The strength of the evidence linking specific therapeutic exposures with adverse outcomes is considered for the inclusion of a therapeutic agent or modality. Screening recommendations in these guidelines were initially determined by consensus from a panel of experts in the late effects of pediatric cancer treatment; evidence is now emerging regarding the yield and utility of these recommendations, and further guideline refinement is in process.21,22 The screening recommendations outlined in the COG guidelines are organized by therapeutic exposure and are appropriate for asymptomatic survivors presenting for routine exposure-based medical follow-up 2 or more years following the completion of therapy for a pediatric malignancy, and can be customized for individual patients based on age, gender, and treatment history. Multidisciplinary task forces continue to monitor the literature to ensure that guideline recommendations reflect emerging knowledge about late effects; version 1.0 of the COG guidelines was published in 2003, and version 4.0 was released in 2014.
Guidelines for follow-up of pediatric cancer survivors have also been developed by the Scottish Intercollegiate Guidelines Network (SIGN),23 the United Kingdom Children’s Cancer and Leukemia Group (CCLG),24 and the Dutch Childhood Oncology Group (DCOG).25 An international effort is currently underway to harmonize these guidelines.26 Joint European–American guidelines for follow-up after hematopoietic cell transplantation were released in 200627,28 and updated in 2012.29–31
The lack of standardized guidelines to direct the care of adult cancer survivors with regard to health promotion and the early detection of treatment-related complications is an ongoing focus of concern, particularly given the IOM’s recommendation for the development of such guidelines in their 2006 report.1 In 2011, ASCO formed a Cancer Survivorship Committee, which has recently partnered with ASCO’s Clinical Practice Guidelines Committee to develop consensus-based guidance documents, informed by evidence, to address long-term effects in cancer survivors.7 Two ASCO guidance documents have now been released.32,33
Intervention to Prevent Potential Late Effects
In the past decade, a growing number of studies have been designed and conducted with the intent of treating health conditions that are identified during or after the completion of therapy as well as preventing later occurring conditions. Randomized clinical trials have tested the effectiveness of a wide range of interventions, including ones aimed at increasing levels of physical activity, promoting healthy diets and smoking cessation, and reducing adverse psychosocial outcomes.
To illustrate the evolution of survivor-focused intervention studies, one can look at studies of breast cancer survivors. In the general population, it has been well documented that physical inactivity and obesity are associated with an increased risk of all-cause mortality, several cancers, cardiovascular disease, insulin resistance, hypertension, osteoporosis, and diminished quality of life. Women with pre- or postmenopausal breast cancer who are obese and physically inactive usually experience lower 5-year survival rates, increased rates of recurrence, and increased all-cause and cancer-related mortalities. Several randomized clinical trials conducted in breast cancer survivors have demonstrated that increasing levels of physical activity are associated with improved cardiorespiratory fitness, weight management, decreased fatigue, and quality of life.34 A prospective trial now in progress is designed to assess the feasibility of a cognitive–behavioral intervention on achieving sustained weight loss in overweight/obese women with early stage breast cancer and to examine the impact of weight loss on comorbidities and quality of life.35 Similar interventions designed to promote physical activity and weight management are also being tested in other populations of cancer survivors.36,37
Although many interventions have been found to be effective at reducing psychosocial morbidities such as depression and anxiety in patients undergoing therapy for cancer, Stanton38 notes that relatively few studies have focused on long-term survivors. Kazak et al.,39 Pai and Kazak,40 and Alderfer et al.41 have published an elegant series of studies describing post-traumatic stress symptoms experienced by childhood and adolescent cancer survivors and testing interventions to reduce these symptoms.
With this experience in survivor intervention studies, a growing survivor-based data set and increased sophistication in study design, it is becoming more feasible to compare outcomes across trials. Important challenges remain, including the ability to test specific interventions in survivor populations that are relatively small in number, and to recruit and retain long-term survivors in these studies.42 Several potentially helpful interventions have yet to be studied, such as the use of statins to reduce the progression of radiation-associated atherosclerosis or chemoprevention to reduce the incidence of breast cancer in women who were treated with chest radiation.
Promotion of Adjustment and Healthy Lifestyles
The importance of providing appropriate support to facilitate positive psychosocial adjustment following treatment for cancer has been a central facet of the survivorship movement since its inception.18 The rates of depression and other types of psychosocial distress in cancer survivors (such as anxiety, anger, and feelings of isolation) have been found to exceed those in the general population, and unmet psychosocial needs often persist following the completion of cancer treatment.38 The oncology health-care provider should play a key role in screening for psychosocial distress in cancer survivors, and appropriate referrals to psychosocial professionals or other resources should be provided. Numerous print and online materials are available to address patient concerns, including: Facing Forward: Life after Cancer Treatment, from the National Cancer Institute (www.cancer.gov/cancertopics/life-after-treatment.pdf); the Cancer Survivor Toolbox, from the NCCS (www.canceradvocacy.org/resources/cancer-survival-toolbox/); and online resources from the Office of Cancer Survivorship (http://cancercontrol.cancer.gov/ocs/) and the American Cancer Society’s (ACS) Cancer Survivors Network (http://csn.cancer.org/).
The potential for positive modification of lifestyle behaviors following a cancer diagnosis and treatment in adulthood has been recognized as a window of opportunity during which cancer survivors may be open to making significant changes in their health habits oriented toward decreasing the likelihood of cancer recurrence and enhancing their overall health status.43 In a study of more than 1.2 million records from the Surveillance, Epidemiology, and End Results Program, cancer-specific death rates were found to underestimate the mortality associated with a cancer diagnosis; death rates from non–cancer-related causes were noted to be higher among persons with a history of cancer than among the general population.44 Taking advantage of the teachable moment, which occurs when patients transit from active cancer therapy to follow-up care, is an important role for oncology health-care professionals who are poised to serve as powerful catalysts in promoting behavioral change and modifying adverse health risks.45 Although a substantial proportion of cancer survivors may be willing to initiate positive lifestyle changes at this juncture, there are known groups of survivors who are less likely to adopt healthy lifestyle changes, including males, those with lower educational levels, and those who live in urban areas.46 Additional support and intervention may be required in order to achieve positive lifestyle modification in these subgroups.
Survivors of childhood cancer often lack a distinct teachable moment for lifestyle modification such as that experienced by survivors of adult-onset cancers, given the wide range of ages and developmental stages of patients treated for pediatric malignancies. They are known to be at increased risk for multiple chronic health conditions such as obesity and cardiovascular disease.14 Several suboptimal health behaviors, including smoking and unhealthful dietary and exercise habits, have been observed in subsets of these survivors.46 The importance of health promotion in this population should be emphasized, and attention should be given to providing targeted health counseling appropriate to each patient’s cancer history, therapeutic exposures, age, gender, and developmental stage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








