Cancer screening and early detection
Otis W. Brawley, MD, MACP
Overview
Cancer screening is an intervention to find cancer at an early stage. The primary goal of screening is to reduce the mortality from the disease being screened for. Secondary is to successfully treat the disease with the least morbidity. In order for screening to be effective, the disease must have a phase in its natural history in which the disease is localized and therapeutic interventions can successfully stop disease progression. The natural history of some cancers is such that screening is not effective. One can best assess a disease as screenable and a screening intervention as useful through a prospective randomized in which people at risk for the cancer are enrolled and randomized to the screening intervention, which is administered on a regular basis or not to get the screening intervention. People who are diagnosed with the disease on both arms should have access to adequate treatment. Over time, the death rate from those who were to be screened is compared to those who were not to be screened. Of note, assessment should be by intention to screen. A successful screening test reduces the death rate. Assessment through a prospective randomized trial is important as some screening tests have been associated with an increase in overall and 5–year survival rate without a decrease in mortality or risk of death. Indeed, the benefit and risk of a screening test is important in determining whether a screening test should be recommended or used.
Cancer screening is a means of early detection of malignancy in an asymptomatic individual at risk for a disease. A positive test indicates that disease may be present and additional “diagnostic” testing is necessary to confirm the presence and extent of disease. In a symptomatic person, the same test is often considered “diagnostic” rather than screening.1
The purpose of screening is not simply to find disease, but to reduce the incidence of advanced disease, and find disease at a point in its natural history where treatment will prevent death. In clinical study, prevention of death is demonstrated by reduction in cancer mortality rates.2 Some screening tests decrease morbidity associated with treatment and/or improve quality of life. Some screening efforts now focus on identifying and treating precursor lesions to prevent malignancy.
Key criteria for screening
Cancer screening is most effective and efficient if performed for diseases with high prevalence and significant population impact. The sojourn time is the period in which an occult tumor can be detected by screening before metastasis or the onset of symptoms.2 For successful screening, the sojourn time should be sufficiently long to allow periodic screening to detect cancer in the target population before disease has spread; treatment of disease should have greater benefit when given before compared to after symptom onset; and the screening test should meet acceptable levels of accuracy and cost.1
Evaluation of early detection programs
Evaluation of a screening test should assess whether the test finds cancer, truly increases survival time, and whether subjects have a lower risk of dying from the disease as a result of screening. Evaluations of screening tests outside the context of a rigorous research design are subject to many biases that can invalidate the conclusions drawn.
Potential biases in the evaluation of screening
Lead-time bias: The time from an occult condition being detected by screening and the moment that condition would have become known through development of symptoms is known as the lead time. There is always a bias toward better survival in a screened group because screening moves the point of diagnosis earlier. True lead time bias occurs when earlier detection only advances the time of a patient’s diagnosis, without prolonging life (Figure 1). Because of the effect of lead-time bias, an increase in survival or improved 5-year survival rates cannot be used alone to assess a screening test.
Length bias is the bias toward detection of less-threatening cancers (Figure 2). Cancers that grow more slowly and have a long sojourn time are more likely to be detected in screening. Faster growing, more aggressive, cancers with a short sojourn time are more apt to escape detection and be diagnosed due to symptoms in between scheduled screens. A cohort of cancer patients with screen-detected tumors will have a higher proportion of slow growing tumors compared to a population diagnosed without screening, and thus will appear to have better survival.
Overdiagnosis is the concept that some tumors fulfill all histologic criteria of malignancy, but are not destined to cause death and would not have become known to the patient without screening (Figure 3).3 Overdiagnosis is an extreme example of length bias. There are two kinds of overdiagnosis. First is the disease that histologically is indistinguishable from precancer or cancer but has no biological propensity to progress.4 This is common among screen-detected premalignant lesions of the uterine cervix and cancers of the prostate and thyroid.5 Second are tumors that can be lethal, but not in the specific patient because she/he would have died from another cause during the sojourn time.4 A persistently higher incidence rate within a population can be due to lead-time or the introduction of unscreened cohorts into a screening program and overdiagnosis.
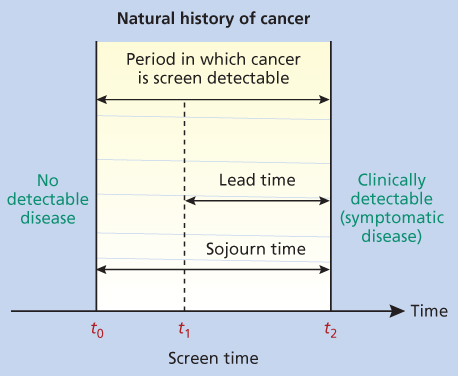
Figure 1 The natural history of cancer. In this figure, t2–t0 is the duration of the preclinical screen-detectable period, known as the “sojourn time”; t2–t1 is the amount of time by which the diagnosis is advanced by screening, known as the “lead time.” Under the assumption of an exponential distribution of sojourn time, the expected lead time of an individual screen-detected case is equal to the mean of the distribution of the sojourn time.
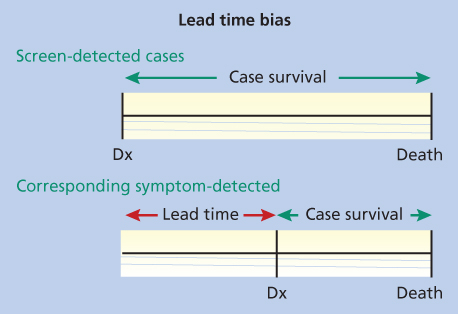
Figure 2 Lead-time bias. In this figure, Dx is the time of diagnosis. Note that the screen-detected cases and the cases diagnosed with symptoms each die at the same time, but the survival time looks greater in the screen-detected case because of lead-time bias.
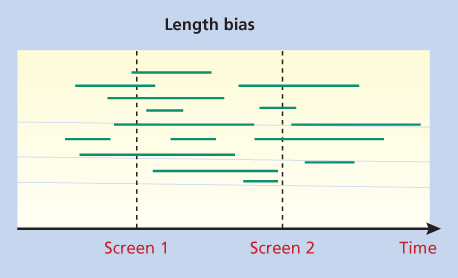
Figure 3 Length bias. In this figure, the horizontal lines represent the sojourn times of individual tumors detected in a screening program. The two screening examinations detect six out of eight long sojourn time tumors, but only two out of six short sojourn time tumors.
Selection bias is the concept that individuals who participate in screening may differ from those who do not, that is, they may be more health conscious, more likely to control risk factors, more disease aware, more adherent to treatment, and generally live healthier lives.6 Selection bias can give the appearance of better screening outcomes than expected and may even limit the generalizability of results of studies designed to overcome the influence of selection bias. For example, current and former smokers who entered the National Lung Screening Trial (NLST) had substantially less emphysema, cardiac disease, and diabetes compared to a representative sample of Americans.6
A unique form of selection bias can occur in a randomized trial in which subjects in the intervention arm are consented, while those in the control arm are not aware they are being followed in the study. Less-healthy individuals in the intervention arm may refuse participation, while those in the control arm cannot.7
Characteristics of a screening test
Sensitivity and Specificity: Sensitivity or the positive rate is the probability of a positive result when applied to a person who truly has the disease. Specificity or the true negative rate is the probability of a negative result in a person who does not have the disease (Table 1). Both high sensitivity and high specificity are desirable in a screening test. Unfortunately, they tend to be negatively correlated. While tests with high sensitivity succeed at detecting most occult disease, a test with low specificity will lead to many false positives and additional workup.
Table 1 Measures of screening performance
| Disease | Status | |
| Screening test results | Yes | No |
| Positive | a | b |
| Negative | c | d |
Sensitivity = a/(a + c); specificity = d/(b + d); positive predictive value (PPV) = a/(a + b); negative predictive value (NPV) = c/(c + d).
Positive and negative predictive values: The positive predictive value (PPV) is the probability that a subject with a positive screening result actually has the disease (Table 1). The negative predictive value (NPV) is the probability that a subject who screens negative is truly free of disease and provides some quantification of the reassurance value of a negative test. It is arguably more important to have a high NPV, as this will mean that few disease subjects are missed and potentially have delayed diagnosis and treatment.
Efficacy versus effectiveness: Screening studies usually assess the efficacy of an intervention, that is, whether the test saves lives among the population enrolled in the trial. An additional question is the effectiveness of the intervention when it is widely used. A few large clinical trials come close to assessing effectiveness, although these studies may be influenced by healthy volunteer effects due to the consent process.1 Ultimately, the effectiveness of a screening intervention should be assessed through program evaluation in “real-world” situations rather than experimental studies, in order to consider factors such as provider experience, improvements in screening technology over time, acceptability to the population, and cost effectiveness.
Research designs to evaluate a screening intervention
Descriptive studies are the easiest screening studies to perform and often provide the first evidence that screening may contribute to disease control. These are uncontrolled observations based on the experience of physicians, clinics, or cancer registries. Descriptive studies can yield useful information, but provide the weakest evidence owing to inherent biases and cannot establish efficacy because of the absence of a control group.
Case–control studies are typically retrospective studies comparing a group of patients (cases) who have an outcome of interest (disease, death, interval cancer, etc.) with a group of patients (controls) who do not, in order to examine factors that may have contributed to the outcome.8 Cases and controls are matched for key characteristics, such as age, gender, and socioeconomic status. Case–control studies are generally inexpensive and provide evidence more quickly than prospective studies.8 These studies are challenging owing to the need to avoid biases, and results can be easily confounded by uncontrolled factors.
Prospective randomized clinical trials (RCTs) are the most rigorous assessment of screening, in that they typically compare disease-specific mortality in a group randomized to receive a screening invitation with a group that receives usual care. The distorting effects of self-selection and other biases are minimized through randomization. The mortality endpoint is not subject to the effects of lead-time, length bias, or overdiagnosis. RCTs should be analyzed by intention-to-treat (ITT), meaning end results are based on comparisons between invited and uninvited groups rather than screened and unscreened groups. Analysis that includes only those who are screened interjects bias.
The large sample size required, the expense, and the long duration has limited the number of prospective RCTs conducted. Some have argued that all-cause mortality is preferred to disease-specific mortality as the primary endpoint, as it avoids potential biases in allocation of cause of death and avoids failure to measure other causes of death that may be an outcome of diagnostic and treatment interventions. The greater size and cost of an RCT measuring all-cause mortality makes them highly impractical.9
A number of medical and professional organizations review the scientific literature and develop screening recommendations or guidelines.5 The US Preventive Services Task Force (USPSTF) is known for using a very conservative process designed to minimize the influence of conflicts of interest, financial, intellectual, and emotional.10 These recommendations are available at www.uspreventiveservicestaskforce.org. The American Cancer Society (ACS) also publishes guidelines (Table 2). A number of medical specialty societies publish guidelines or screening recommendations for specific their disease.
Table 2 American Cancer Society screening guidelines for the early detection of cancer in average-risk asymptomatic people
| Cancer site population test or procedure frequency | |||
| Breast | Women, ages 40–44 | Mammography | All women should become familiar with the potential benefits, limitations, and harms associated with breast cancer screening. Women should have the opportunity to begin annual mammographic screening between the ages of 40 and 44 years |
| Women, ages 45–54 | Mammography | Women with an average risk of breast cancer should undergo annual screening mammography starting at age 45 years | |
| Women, ages 55+ | Mammography | Women who are 55 years and older should be screened every 2 years but may choose to continue annual screening | |
| Mammography | |||
| Cervix | Women, ages 21–65 | Pap test & HPV DNA test | Cervical cancer screening should begin at age 21. For women ages 21–29, screening should be done every 3 years with conventional or liquid-based Pap tests. For women ages 30–65, screening should be done every 5 years with both the HPV test and the Pap test (preferred), or every 3 years with the Pap test alone (acceptable). Women ages 65+ who have had ≥3 consecutive negative Pap tests or ≥2 consecutive negative HPV and Pap tests within the past 10 years, with the most recent test occurring within 5 years, and women who have had a total hysterectomy should stop cervical cancer screening. Women should not be screened annually by any method at any age |
| Colorectal | Men and women, ages 50+ | Fecal occult blood test (FOBT) with at least 50% test sensitivity for cancer, or fecal immunochemical test (FIT) with at least 50% test sensitivity for cancer, or | Annual, starting at age 50. Testing at home with adherence to manufacturer’s recommendation for collection techniques and number of samples is recommended. FOBT with the single stool sample collected on the clinician’s fingertip during a digital rectal examination is not recommended. Guaiac-based toilet bowl FOBT tests also are not recommended. In comparison with guaiac-based tests for the detection of occult blood, immunochemical tests are more patient-friendly, and are likely to be equal or better in sensitivity and specificity. There is no justification for repeating FOBT in response to an initial positive finding |
| Stool DNA test, or | Every 3 years, starting at age 50 | ||
| Flexible sigmoidoscopy (FSIG), or | Every 5 years, starting at age 50. FSIG can be performed alone, or consideration can be given to combining FSIG performed every 5 years with a highly sensitive gFOBT or FIT performed annually | ||
| Double-contrast barium enema (DCBE), or | Every 5 years, starting at age 50 | ||
| Colonoscopy | Every 10 years, starting at age 50 | ||
| CT colonography | Every 5 years, starting at age 50 | ||
| Endometrial | Women, at menopause | At the time of menopause, women at average risk should be informed about risks and symptoms of endometrial cancer and strongly encouraged to report any unexpected bleeding or spotting to their physicians | |
| Lung | Current or former smokers aged 55–74 in good health with at least a 30 pack-year history | Low-dose helical CT (LDCT) | Clinicians with access to high-volume, high-quality lung cancer screening and treatment centers should initiate a discussion about lung cancer screening with apparently healthy patients aged 55–74 who have at least a 30 pack-year smoking history, and who currently smoke or have quit within the past 15 years. A process of informed and shared decision making with a clinician related to the potential benefits, limitations, and harms associated with screening for lung cancer with LDCT should occur before any decision is made to initiate lung cancer screening. Smoking cessation counseling remains a high priority for clinical attention in discussions with current smokers, who should be informed of their continuing risk of lung cancer. Screening should not be viewed as an alternative to smoking cessation |
| Prostate | Men, ages 50+ | Digital rectal examination (DRE) and prostate-specific antigen test (PSA) | Men who have at least a 10-year life expectancy should have an opportunity to make an informed decision with their health care provider about whether to be screened for prostate cancer, after receiving information about the potential benefits, risks, and uncertainties associated with prostate cancer screening. Prostate cancer screening should not occur without an informed decision-making process |
| Cancer-related checkup | Men and women, ages 20+ | On the occasion of a periodic health examination, the cancer-related checkup should include examination for cancers of the thyroid, testicles, ovaries, lymph nodes, oral cavity, and skin, as well as health counseling about tobacco, sun exposure, diet and nutrition, risk factors, sexual practices, and environmental and occupational exposures | |
Organized versus opportunistic screening: Screening can be offered in a regimented program in which subjects are tracked for compliance with screening recommendations and quality of screening is measured through systematic audit. This is how screening is done in many European countries. In the United States, most screening is opportunistic, with limited tracking of compliance or quality. Some American mammography facilities do meet high world-class standards. In the United States and Europe, there are very few broad standards for screening in other diseases.
Breast cancer screening
Among women worldwide, breast cancer is the most commonly diagnosed cancer and the leading cause of cancer death. In the United States, the median age at diagnosis is 61, and the median age of death is 68.11
Screening methods for breast cancer
Mammography is an X-ray examination of the breasts that produces high-quality craniocaudal (CC) and mediolateral oblique (MLO) images of each breast with minimum X-ray dose. Evidence suggests that digital imaging allows for more accurate interpretation, improved diagnosis in women with dense breasts, and equivalence to traditional film mammography for postmenopausal women.12
The rate of abnormal interpretations is higher for first screening (5–10%) than for later mammograms. Most abnormalities are resolved through additional imaging with other mammographic views or by ultrasonography. Abnormalities that cannot be resolved are biopsied with ultrasonographic or radiographically directed fine needle aspiration, core needle biopsy, or surgical excision.
Nine RCTs of breast cancer screening have been published to date (Table 3). The trials vary in the age range of women enrolled, the screening tests used [mammography alone or with clinical breast examination (CBE)], the screening intervals, and even the years of follow-up. All RCTs were started and most completed before the modern era of adjuvant chemotherapy, and several before the advent of hormonal therapy. Screening and diagnostic equipment have also improved.13 Experts try to take these limitations and flaws into account by considering nonrandomized studies and mathematical models.14, 15 There is consensus that a well-run screening program has the potential to reduce risk of breast cancer-specific death.8
Table 3 Breast cancer screening randomized trials
| Study | Randomization | Sample size | Intervention | Follow up | Finding |
| Health Insurance Plan, United States 1963a | Individual | 60,565–60,857 | MMG and CBE for 3 years | 18 years | RR 0.77 (0.61–0.97) |
| Malmo, Sweden 1976b,c | Individual | 42,283 | Two-view MMG every 18–24 months × 5 | 12 years | RR 0.81 (95% CI, 0.62–1.07) |
| Ostergotland (County E of Two-County Trial) Sweden 1977d,e | Geographic cluster | 38,405–39,034 study 37,145–37,936 control | Three single-view MMG Every 2 years women-50 Every 33 months women 50+ | 12 years | RR 0.82 (95% CI, 0.64–1.05) Ostergotland |
| Kopparberg (County W of Two-County Trial) Sweden 1977d,e | Geographic cluster | 38,562–39,051 intervention 18,478–18,846 control | Three single-view MMG Every 2 years women-50 Every 33 months women 50+ | 12 years | RR 0.68 (95% CI, 0.52–0.89) |
| Edinburgh, United Kingdomf | Cluster by physician practice | 23,266 study 21,904 control | Initially, two-view MMG and CBE Then annual CBE with single-view MMG years 3, 5, and 7 | 10 years | RR 0.84 (95% CI, 0.63–1.12) |
| NBSS-1, Canada 1980g | Individual | 25,214 study (100% screened after entry CBE) 25,216 control | Annual two-view MMG and CBE for 4–5 years | 13 years | RR 0.97 (95% CI, 0.74–1.27) |
| NBSS-2, Canada 1980h | Individual | 19,711 study ( 100% screened after entry CBE) 19,694 control | Annual two-view MMG and CBE | 11–16 years (mean 13 years) | RR 1.02 (95% CI, 0.78–1.33) |
| Stockholm, Sweden 1981i | Cluster by birth date | Declined from 40,318 to 38,525 intervention group Rose from 19,943 to 20,978 control group | Single view MMG every 28 months × 2 | 8 years | RR 0.80 (95% CI, 0.53–1.22) |
| Gothenberg, Sweden 1982j | Complex | 21,650 invited 29,961 control | Initial two-view MMG. Then single-view MMG every 18 months × 4. Single read first three rounds, then double-read | 12–14 years | RR 0.79 (95% CI, 0.58–1.08) |
| AGE Trialh | Individuals | 160,921 (53,884 invited; 106,956 not invited) | Invited group aged 48 and younger offered annual screening by MMG (double-view first screen, then single mediolateral oblique view thereafter); 68% accepted screening on the first screen an 69% and 70% were reinvited (81% attended at least one screen). | 10.7 years | RR 0.83 (95% CI, 0.66–1.04) |
a Shapiro S, Venet W, Strax P, Venet L, Roeser R. Ten- to fourteen-year effect of screening on breast cancer mortality. J Natl Cancer Inst 1982;69:349–55.
b Andersson I, Aspegren K, Janzon L, et al. Mammographic screening and mortality from breast cancer: the Malmo mammographic screening trial. Bmj 1988;297:943–8.
c Nystrom L, Andersson I, Bjurstam N, Frisell J, Nordenskjold B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet 2002;359:909–19.
d Tabar L, Fagerberg G, Duffy SW, Day NE, Gad A, Grontoft O. Update of the Swedish two-county program of mammographic screening for breast cancer. Radiologic clinics of North America 1992;30:187–210.
e Tabar L, Fagerberg G, Duffy SW, Day NE. The Swedish two county trial of mammographic screening for breast cancer: recent results and calculation of benefit. Journal of epidemiology and community health 1989;43:107–14.
f Roberts MM, Alexander FE, Anderson TJ, et al. Edinburgh trial of screening for breast cancer: mortality at seven years. Lancet 1990;335:241–6.
g Miller AB, To T, Baines CJ, Wall C. The Canadian National Breast Screening Study-1: breast cancer mortality after 11 to 16 years of follow-up. A randomized screening trial of mammography in women age 40 to 49 years. Ann Intern Med 2002;137:305–12.
h Miller AB, Baines CJ, To T, Wall C. Canadian National Breast Screening Study: 2. Breast cancer detection and death rates among women aged 50 to 59 years. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne 1992;147:1477–88.
i Frisell J, Eklund G, Hellstrom L, Lidbrink E, Rutqvist LE, Somell A. Randomized study of mammography screening—preliminary report on mortality in the Stockholm trial. Breast Cancer Res Treat 1991;18:49–56.
j Bjurstam N, Bjorneld L, Warwick J, et al. The Gothenburg Breast Screening Trial. Cancer 2003;97:2387–96.
k Moss SM, Cuckle H, Evans A, et al. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years’ follow-up: a randomised controlled trial. Lancet 2006;368:2053–60.
There has been tremendous debate about whether screening of normal risk women should begin at age 40 or 50.13 The dispute is based largely on the lack of clear evidence from RCTs that mammography screening for women aged 40–49 years is effective. Some studies show a statistically significant advantage to screening women in their forties and others do not.16 The most recent RCT is the AGE Study, in which some patients may have received modern adjuvant therapy.17 In all, 53,884 women aged 39–41 years were invited to screening. The control group was unaware of their participation and received “usual medical care.” With 10.7 years of follow-up, there was a statistically nonsignificant 17% lower relative risk of breast cancer death in the screening versus control group (RR = 0.83, 0.66–1.04).17
The USPSTF meta-analysis of large RCTs (Table 4), including AGE, concluded a 15% relative risk reduction in mortality (RR = 0.85, 95% CI, 0.75–0.96) for women aged 40–49 invited to 2–9 rounds of screening after 11–20 years of follow-up.18 This translates into 1904 women being invited to screening to prevent or delay one breast cancer death.19 More than half of these women are estimated to have a false-positive scan during the 10 years of screening. Changing the screening interval to every 2 years marginally decreases the number of lives saved while nearly halving the false positive rate.20
Table 4 Pooled RRs for breast cancer mortality from mammography screening trials for all ages
| Age (years) | Trials included, n | RR for breast cancer mortality (95% CrI) | NNI to prevent 1 breast cancer death (95% CrI) |
| 39–49 | 8a | 0.85 (0.75–0.96) | 1904 (929–6378) |
| 50–59 | 6b | 0.86 (0.75–0.99) | 1339 (322–7455) |
| 60–69 | 2c | 0.68 (0.54–0.87) | 377 (230–1050) |
| 70–74 | 1d | 1.12 (0.73–1.72) | Not available |
CrI, credible interval; NNI, number needed to invite to screening; RR, relative risk.
[a Health Insurance Plan of Greater New York,f Canadian National Breast Screening Study-1,g Stockholm,e Malmö,e Swedish Two-County trial (two trials),e,j Gothenburg trial,i and Age trial.h][b Canadian National Breast Screening Study-1,g Stockholm,e Malmö,e Swedish Two-County trial (2 trials),1,31 and Gothenburg trial.i][c Malmöe and Swedish Two-County trial (Östergötland).e][d Swedish Two-County trial (Östergötland).e]
1 Nyström L, Andersson I, Bjurstam N, Frisell J, Nordenskjöld B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet 2002;359:909–19.
2 Habbema JD, van Oortmarssen GJ, van Putten DJ, Lubbe JT, van der Maas PJ. Age-specific reduction in breast cancer mortality by screening: an analysis of the results of the Health Insurance Plan of Greater New York study. J Natl Cancer Inst. 1986;77:317–20.
3 Miller AB, To T, Baines CJ, Wall C. The Canadian National Breast Screening Study-1: breast cancer mortality after 11 to 16 years of follow-up. A randomized screening trial of mammography in women age 40 to 49 years. Ann Intern Med 2002;137:305–12.
4 Moss SM, Cuckle H, Evans A, Johns L, Waller M, Bobrow L; Trial Management Group. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years’ follow-up: a randomised controlled trial. Lancet 2006;368:2053–60.
5 Bjurstam N, Björneld L, Warwick J, Sala E, Duffy SW, Nyström L, et al. The Gothenburg Breast Screening Trial. Cancer 2003;97:2387–96.
6 Tabar L, Fagerberg G, Chen HH, Duffy SW, Smart CR, Gad A, et al. Efficacy of breast cancer screening by age. New results from the Swedish Two-County Trial. Cancer. 1995;75:2507–17.
Source: Taken from The Breast Cancer Evidence Review: An Update for the U.S. Preventive Services Task Force. Heidi D. Nelson, MD, MPH; Kari Tyne, MD; Arpana Naik, MD; Christina Bougatsos, BS; Benjamin K. Chan, MS; and Linda Humphrey, MD, MPH. www.uspreventiveservicestaskforce.org/Page/SupportingDoc/breast-cancer-screening/final-evidence-summary9.
The higher prevalence of mammographic density among women in their forties compared to their fifties complicates interpretation of mammogram in the younger group. Studies show that the accuracy, sensitivity, and specificity of a given mammogram vary by radiologist, but in general improve with increasing patient age.21, 22 Among women screened regularly, interval cancers are more prevalent in women aged 40–49 years.23
CBE and breast self-examination (BSE): A competent CBE involves physical palpation of the breast by a trained healthcare provider in small segments, from the nipple to the periphery of the breast, including the axilla.24 In some settings (especially in developing countries), this may be the only method of breast cancer screening available.18
No RCT has adequately evaluated CBE as a single screening modality. A small proportion of palpable masses are not seen on mammography, leading to screening guidelines that include routine CBE, ideally just before mammography. Some data suggest that CBE contributes very little in a setting of high adherence with regularly scheduled high-quality mammography.18
BSE has appeal as a screening test because it is simple, convenient, and noninvasive. The “monthly BSE” was once widely advocated.25 However, two prospective RCTs have reported a lack of evidence of efficacy and even some evidence of harm, although both studies have some methodological issues.26, 27 Monthly BSE promotion distracts from the importance of mammography, can provide false reassurance, can heighten anxiety about breast cancer, and create false positives. Today, no professional organization in the United States or Europe encourages monthly BSE, instead advocating “breast awareness” and consulting a healthcare provider if a possible abnormality is found.25, 28
Evaluation of the effectiveness of screening
While the RCTs of breast cancer screening suggest a benefit, estimating the effectiveness of routine screening programs in the community is not straightforward. The United States has had a 35% decline in age-adjusted mortality from 1989 to 2011. Mathematical modeling estimates that screening and improvements in breast cancer therapy, especially hormonal therapy, are each responsible for about half of the mortality decline.29
The harms of breast cancer screening
Every screening test has some associated harms. Slightly more than half of women screened in a 10-year period will have a false positive requiring at least additional imaging.30, 31 Surveys show about a quarter of these women still suffered distress and anxiety 3 months after cancer was ruled out.32 False negatives lead to a false sense of security. Overdiagnosis estimates in breast cancer screening range from 0% to 54% of cancers diagnosed with the aid of mammography, with most estimates suggesting overdiagnosis is 10–30%.14, 33
Mammography screening has also caused a dramatic rise in the diagnosis of ductal carcinoma in situ (DCIS). Previously a rare, incidental finding after mastectomy, there are now >70,000 cases diagnosed annually in the US Study which has yet to show that early detection and treatment of DCIS reduces mortality. It is believed that most DCIS lesions do not progress to invasive cancer,15, 34 and there is increasing interest in its overtreatment.35 Renaming DCIS has been suggested to remove the word carcinoma.36 In the future, genomic medicine may allow us to distinguish the tumors (in situ and true cancer) that need aggressive therapy from those that should be observed.
New technologies
Digital breast tomosynthesis (DBT) and 3D mammography are newer technologies that appear to offer increased sensitivity and a reduction in recall rates.37
Magnetic resonance imaging (MRI) is more sensitive but less specific than mammography in detecting breast cancer.38 Initial MRI is associated with a very high number of false positive results, which is reduced with subsequent studies. MRI has found a niche in screening women with significant breast density and women who may be at elevated risk due to mutations in breast cancer susceptibility genes.
Ultrasound imaging has been used for many years as a diagnostic. The limitations of mammography in screening the dense breast have led to ongoing interest in using ultrasound for primary screening or as an adjunct to mammography.39, 40
In 2003, the American College of Radiology Imaging Network (ACRIN) initiated a multicenter trial in women at increased risk for breast cancer due to family history and significant breast density.41, 42 The breast cancer detection rate was increased by 4.2 cancers per 1000 women screened with mammography and ultrasound versus mammography alone (11.8 vs 7.6 per 1000). However, the false-positive and negative biopsy rates were also very high. While a significant improvement was seen in the diagnostic yield of small, node-negative cancers in this higher-risk population, it is unclear that it will save additional lives and questions remain about the potential for screening ultrasound.40 Routine ultrasound screening is not advocated at this time.
An interesting technology that is gaining use is 3D automated breast ultrasound. It offers more standardized imaging compared to conventional ultrasound and can be performed by a technologist instead of a radiologist. It is still used as a diagnostic, but there is interest in using it as an initial screening test with mammography and CBE.
Screening recommendations
As of 2015, the ACS guideline recommends that all women become familiar with the potential benefits, limitations, and harms associated with breast cancer screening. Women with an average risk of breast cancer should undergo regular screening mammography starting at age 45 years. Women who are aged 45–54 years should be screened annually. Women who are 55 years and older should be screened every 2 years but may choose to continue annual screening. Women should have the opportunity to begin annual screening between the ages of 40 and 44 years. Women should continue screening as long as their overall health is good and they have a life expectancy of 10 years or more. Beginning in 2015, the ACS does not recommend clinical breast examination for breast cancer screening among women at any age with an average risk of breast cancer. The ACS guideline applies to women who have access to mammographic screening.
The 2009 USPSTF recommendation is women aged 50–75 years have a mammogram every 1–2 years.43 USPTF did not recommend CBE, citing the lack of study to show it beneficial. The Task Force recommends against “routine” mammographic screening for women aged 40–49 years, instead advising this decision be made following physician–patient discussion including individual risks and concerns.
As of December 2014, the USPSTF, the American College of Physicians, and the Canadian Task Force on the Periodic Health Examination recommend routine screening beginning at age 50.44 An advisory committee on cancer prevention in the European Union recommends that screening be offered to women aged 50–69 years in an organized screening program.45
On the basis of the accumulation of data, but no RCTs, the ACS now recommends that women at very high risk of breast cancer begin annual mammography and MRI at age 30, or perhaps earlier if she and her physician believe it is prudent.38 It is impossible to perform a prospective RCT in this population. High risk is defined as having a known mutation of BRCA1 or BRCA2, 20–25% or greater lifetime risk based on family history, a high-risk genetic syndrome, such as Li-Fraumeni syndrome or Cowden disease, or having received high-dose mantle radiation to the chest.22
Colorectal cancer
Worldwide, colorectal cancer is the third most commonly diagnosed cancer in men and the second in women and accounts for 8% of all deaths. In the United States, colorectal cancer is the third most commonly diagnosed cancer and the third leading cause of cancer death among men and women. While most cases are diagnosed among individuals aged 60–80 years, there is increasing concern about colorectal cancer in those aged 40–50 years.11
The goal of colorectal screening is both the detection of early-stage adenocarcinomas and the detection and removal of adenomatous polyps, given the significant evidence that adenomatous polyps are precancerous lesions. Colorectal polyps are common in adults over age 50. One-half to two-thirds of all colorectal polyps are adenomatous, which can progress to cancer, although the majority never will. It is estimated that it takes 10 years for an adenomatous polyp <1 cm to become an invasive lesion.46 Other polyps, including incidental hyperplastic polyps and mucosal tags, are not significant in the development of colorectal cancer.47
Screening methods for colorectal cancer
Evidence suggests that a number of tests, applied in a program of regular surveillance, have the potential to reduce deaths from colorectal cancer. They include:
- 1.
Fecal occult blood tests (FOBTs) aim to discover occult blood in stool, which is often caused by polyps (especially >2 cm). Common FOBTs include guaiac-based tests (gFOBTs) and fecal immunochemical tests (FITs).
The gFOBT detects blood in the stool through the pseudoperoxidase activity of heme. This test requires diet modification, as it can react positively with red meat, cruciferous vegetables, and some fruits. Rehydration of gFOBT not only improves sensitivity but also increases the false-positive rate.48 The gFOBT can be done in the physician office but it is preferred that patients collect the specimen at home for processing in a laboratory. Any positive stool blood test requires follow-up diagnostic colonoscopy.
Limitations of gFOBT have led to a decrease in usage but some high-sensitivity tests are still available. The gFOBT performed with stool collected during a digital rectal examination (DRE) is not recommended.
The FIT reacts to human globin. FIT is not reactive to diet as gFOBT is, and will not react to digested human blood from upper gastrointestinal bleeding. The fecal hemoglobin threshold can be set to balance sensitivity and specificity based on individual risk or programmatic requirements. FITs are usually processed in a laboratory. Sensitivity declines with delay in sample processing. Even a 5-day delay is significant.49
In 1000 ambulatory patients (with and without symptoms of colorectal cancer), the sensitivity for cancer with three FIT samples with a hemoglobin threshold of 75 ng/mL was 94.1%, and specificity was 87.5%. In a screening study with one FIT evaluation of stool followed by colonoscopy, FIT had higher sensitivity for detection of advanced neoplasms in patients taking low dose aspirin compared to those not taking aspirin. There was a minor decrease in specificity.50
In a systematic review of gFOBT and FIT, there was no clear evidence for superiority of either test.51 Bleeding from cancers or large polyps may be intermittent. Generally, FIT requires two tests over a week or so and gFOBT requires three. In practice, FIT is replacing gFOBT.
- 2. Stool DNA (sDNA) testing detects relatively well-defined DNA markers associated with colorectal neoplasia in cells exfoliated by colorectal polyps and malignancies into the colonic lumen. Available sDNA tests focus on >21 separate point mutations in the K-RAS oncogene, a probe for BAT-26 (a marker of microsatellite instability), and a marker of DNA integrity analysis (DIA) to achieve high sensitivity.52 As with stool blood testing, any positive stool blood or DNA test must be followed up with colonoscopy to rule out the presence of polyps or cancer.
- 3. Flexible sigmoidoscopy (FSIG) with a 60 cm scope is a relatively simple procedure, requires minimal preparation, and allows for examination of about half of the average colon.53 Generally, the test is performed without sedation. The presence of polyps in the distal bowel signals an elevated risk for polyps or cancer in the proximal bowel. If FSIG is positive, the patient is referred for colonoscopy.
Quality indicators for FSIG have been published and emphasize appropriate training, satisfactory examination rates to >40 cm, expected adenoma detection rates based on age and gender, and ability to biopsy suspected adenomas.
- 4.
Barium enema is an X-ray examination of the bowel that derives contrast from barium (single-contrast study) or the combination of barium and instilled air (double-contrast barium enema study, DCBE). DCBE is more sensitive than single-contrast study for both malignancies and polyps. If the patient has a positive test, the next step is a colonoscopy.54
Evidence for the efficacy of DCBE is largely indirect, based on its performance in detecting small malignant lesions and polyps and on the known benefits of early detection and polypectomy for reducing mortality. The proportion of examinations in which adenomatous polyps identified by colonoscopy were also detected by DCBE was significantly influenced by lesion size. Among all currently recommended screening tests, barium enema is the least utilized.
- 5.
Colonoscopy has a unique advantage among colorectal cancer screening tests in that direct visualization of the entire bowel is possible, with >90% of examinations terminating at the cecum, and clinically significant adenomas can be identified and removed during the examination.24, 55 Colonoscopy is done in an outpatient setting predominantly. While conscious sedation is standard, some patients receive general anesthesia. Proper bowel preparation is critical to ensure that the bowel is clean.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








