Carcinoid tumors appear as hyperintense luminal masses due to increased vascularity. The tumors typically have intense desmoplastic reaction in response to biochemical products produced by the tumor that results in puckering of the adjacent mesentery, giving a characteristic stellate pattern on the CT scan not necessarily reflecting mesenteric invasion (Fig. 54.3).

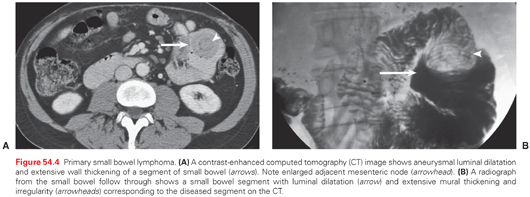
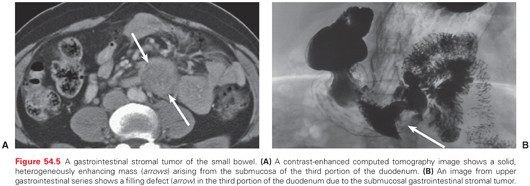
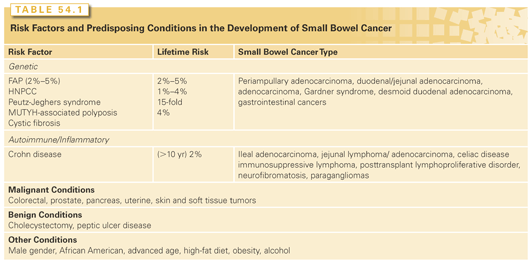
Small bowel lymphomas present as circumferential mural thickening with low homogeneous attenuation and a characteristic aneurysmal dilatation in which the involved segment shows cavitary dilatation with a nodular, irregular luminal contour and peripheral bowel wall thickening (Fig. 54.4).62 Sarcomas appear as homogeneous, well-circumscribed, hypervascular masses on CT (Fig. 54.5).63–65
The MRI has a defined niche in the assessment of early small bowel lesions given its improved soft tissue delineation and it may be pivotal in detecting early small bowel pathology (Table 54.1).66–70 Somatostatin receptor scans (octreoscan) and metaiodobenzylguanidine (MIBG) scans may be helpful in localizing and diagnosing of small bowel carcinoid tumors. Octreoscan employs a 111In-diethylenetriaminepentaacetic acid analog that targets somatostatin receptors. Over 90% of carcinoid tumors express somatostatin receptors, and this test has a sensitivity of 80% to 100%. Octreoscans may also provide a functional map of the tumor for anticipated radiolabelled immunotherapy-targeted therapy depending on the stage of the disease.71
Positron emission tomography (PET)/CT scans are useful in the initial diagnosis and in disease staging for small bowel adenocarcinomas (SBA), lymphomas, and gastrointestinal stromal tumor (GIST).72,74 Fludeoxyglucose (18FDG)-PET has a limited role in the diagnosis and staging of carcinoid tumors because they are not 18FDG avid; however, it may play important role in ascertaining treatment response and detecting disease recurrence.72–74
Two notable advances are double-balloon enteroscopy, or push enteroscopy, and video capsule endoscopy (VCE), which were both introduced in 2001. Double-balloon enteroscopy is a technically challenging procedure that permits the evaluation of the entire small bowel and tissue sampling, which is not possible with VCE. Double-balloon enteroscopy most commonly detects symptomatic lesions, including areas of ulceration, stenosis, and GI bleeds, with a sensitivity of 74% to 81%.75–83 VCE is effective at identifying 50% of new SBC lesions and 87% of all lesions, with a relatively low miss rate of 10%.84–93
Adenocarcinoma of the small bowel is the second most common histologic SBC subtype, comprising 36.9% of cases.94 The annual incidence is estimated at 7.3 case per million worldwide. In the United States, 3,050 new cases of SBA are anticipated in 2013.94,95 Patients with SBA usually present between the 6th and 8th decade of life, with earlier presentations in patients with genetic, autoimmune, or inflammatory conditions (Table 54.2).
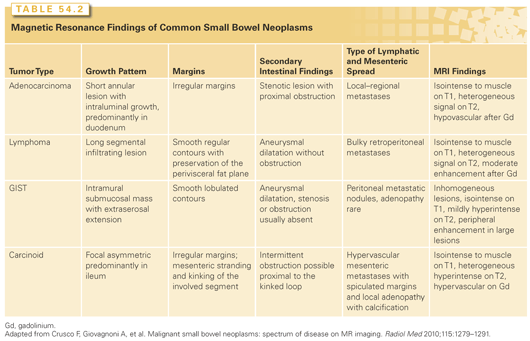
SBAs display a slight predominance for men, with age-adjusted incidence rates highest among African Americans (14.1 per 106) followed by Caucasians (7.7 per 106), Hispanics (6.2 per 106), and Asian/Pacific Islanders (5.5 per 106). Adenocarcinoma incidence decreases distally in the small bowel, with the highest incidence in the duodenum (50% to 5%), followed by the jejunum (16%) and ileum (13%). Most SBA patients present at an advanced stage (American Joint Committee on Cancer [AJCC] stage III or greater) resulting in a poor overall 5-year disease-free survival (of about 30%) and mean survival (20 months).50 Outcomes are worse for duodenal tumors (28% 5-year survival) compared to jejunal and ileal lesions (38% 5-year survival).
Etiology
SBA shares several etiologic features with colorectal cancers (CRC), leading to the adoption of similar treatment strategies, although they are biologically distinct diseases. The most obvious similarity between SBA and CRC is a common etiopathogenic pathways because both cancers are more common in patients with FAP, HNPCC, and inflammatory bowel disease. Available data imply a similar adenoma to carcinoma sequence in both SBA and CRC. As with CRC, the risk of progression is associated with the size of the adenoma (8.3% for lesions <1 cm and 30% for lesions >1 cm) and histology (14.3 % for tubular, 23.1% for tubulovillous, and 36% for villous).96–99 A number of molecular similarities and dissimilarities between these two cancers have also been reported. In a recent genomic hybridization study looking at GI cancers, SBA was more similar to CRC than to stomach cancer.100 In addition, HER2 oncogene amplification is low in SBA similar to CRC in contrast to gastric cancer.97,98,101,102 Microsatellite instability (MSI) and loss of mismatch repair proteins is present in 18% to 35% of SBAs versus 15% of CRCs.77–79 Approximately 50% of the SBA cases reflect sporadic methylation of the MLH1 gene with a relatively high incidence of MSI and MLH1 methylation in CD associated SBA (67% to 73%).106–117
Staging and Prognosis
The AJCC staging system for SBA is depicted in Table 54.3. Given the nonspecific initial presentation of SBAs, 32% of patients present with stage IV disease, 27% present with stage III, 30% present with stage II, and 10% present with stage I.102 Less than 50% of SBA patients are curative surgical candidates. Survival by stage is 63% for stage I, 48% for stage II, 32% for stage III, and about 4% for stage IV.102 Multivariate regression analyses identified age over 55 years, males, African Americans, T4 tumor, lymph node involvement and ratio, duodenal followed by ileal primary, poor differentiation, metastatic disease, and positive margins as associated with a poor prognosis.94,102,120–122 Recent investigations into molecular determinants have also identified the CpG island methylator phenotype (CIMP) status, E-cadherin loss, and aberrant β-catenin expression as also associated with a worse prognosis.106,118

Management
The site of the disease, the stage at presentation, the available expertise, patient comorbidities, and patient performance status are all important considerations in determining the optimal management for individual SBA patients.
Surgery for Local–Regional Disease
Surgery is the treatment for local–regional confined SBAs.50 The 5-year survival of resected versus unresected patients is 54% versus 0%.122 The optimal surgical treatment depends on the location of the primary tumor, with duodenal adenocarcinomas managed by either a pancreaticoduodenectomy or segmental resection. Both procedures are equivalent with regard to long-term survival, with segmental resection associated with less postoperative morbidity and length of hospitalization.122–125 Jejunal and ileal adenocarcinomas are treated with oncologic-appropriate segmental resection. A right hemicolectomy is the appropriate oncologic approach to tumors near the ileocecal valve.
Adjuvant Therapy
Recurrence in SBAs is predominantly distant and rarely local–regional (86% versus 18%, respectively).50 Although duodenal SBAs have a higher incidence of local failure, distant recurrence still predominates (59% versus 19%, respectively, and combined, 22%).94,126,127 Most treatment strategies are based on fluorouracil (5-FU) or, more recently, 5-FU/oxaliplatin–based regimens. Overman et al.129 reported adjuvant therapy improved disease-free survival (hazard ration [HR], 0.23, 95% CI, 0.06 to 0.89, p = 0.03), but not overall survival (HR, 0.48, 95% CI, 0.13 to 1.74, p = 0.26) in patients receiving adjuvant chemotherapy (5-FU or platinum based).129 Multiple retrospective studies have demonstrated a mixed response for adjuvant chemotherapy in SBAs.49,50,128–130
The role of hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of SBCs has been investigated in four small trials involving a total of 30 patients with peritoneal carcinomatosis. These patients underwent HIPEC followed by adjuvant chemotherapy, with a reported mean overall survival of 22.2 months compared to 12 months on conventional treatment strategies. The results are encouraging, but the numbers are too small to draw any robust conclusions.140
Treatment for Metastatic Disease
Limited randomized controlled trials defining a role for chemotherapy versus best supportive care (BSC) in patients with advanced SBAs have been performed. Multiple retrospective studies have demonstrated a small survival advantage for palliative chemotherapy approaches compared to BSC alone (Table 54.4).49,50,131–136
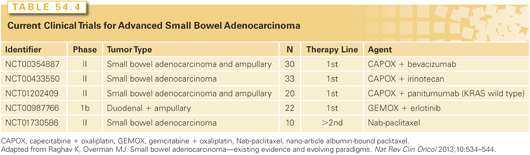
Although there are no randomized trials comparing different regimens, a number of SBA clinical trials are currently accruing in an effort to more precisely define the role of chemotherapy in advanced SBA. The role of surgery for all advanced/metastatic SBC is purely palliative. Specific patients might benefit from an intestinal bypass to maintain an enteral means of nutrition and an improved quality of life.120,137–139
Carcinoid tumors are slow growing neoplasms that arise from the neuroendocrine cells of Kulchitsky. Of these cells, 75% occur in the gastrointestinal tract (44.7% in the small bowel and 19.6% in the rectum), followed by the lung, bronchus, and rarely the liver, pancreas, or gonads. Given the fourfold increase in the incidence of carcinoids that has occurred over the past 2 decades, carcinoids are now the most common SBC (36.9%) according to the National Cancer Database.167,168
Clinical Presentation and Prognosis
Of small bowel carcinoids, 44.7% occur in the small bowel, and over 50% of these are found in the ileum.168 Carcinoid tumors are most common in the 7th decade of life (mean age, 66 years), in males (52.4%), and in Caucasians (80.4%). Most carcinoids present in an advanced stage (75%) as a result of a significant delay and difficulty in diagnosis. Despite the advanced stage, most carcinoids follow an indolent course, with an overall 5-year survival ranging from 52% to 77%.170–174 Tumors over 10 mm and those with a transmural depth of invasion are the primary risk factors for local–regional progression and metastasis.175
Carcinoid Syndrome
Carcinoid syndrome is an array of symptoms that occurs in patients with carcinoid tumors of the bronchus or metastatic to the liver. Symptoms of carcinoid syndrome include diarrhea, cutaneous flushing, and wheezing. These symptoms are precipitated by stress, alcohol intake, and certain physical activities, and are secondary to the systemic release of several tumor-derived factors, including serotonin, dopamine, tachykinins, histamine, and prostaglandins, all of which are metabolized by the liver. Up to 80% of patients with small bowel carcinoids develop carcinoid syndrome, and it is the presenting complaint in 10% to 17% of patients. The mainstay of treatment for carcinoid syndrome is somatostatin, which prevents the secretion of hormones by binding to a specific receptor on the tumor surface.192,193 Although octreotide is effective in most patients, interferon α (IFN-α) may benefit patients who do not respond to octreotide alone.187 The antihistamine cyproheptadine has also been used successfully for refractory carcinoid symptoms.188
Management
Surgery for Local–Regional Disease
An oncologic segmental resection of the tumor is the preferred treatment for localized small bowel carcinoids.176 Five-year survival after resection of localized disease ranges between 50% and 85%. Of small bowel carcinoids, 29% are associated with other noncarcinoid neoplasms, necessitating a thorough inspection of the entire bowel at surgery. Appendiceal carcinoids smaller than 2 cm can be treated with a simple appendectomy, whereas larger tumors require a right hemicolectomy. Pretreatment with octreotide prior to anesthetic induction is recommended because surgical intervention/manipulation can precipitate a carcinoid crisis.177–180
Surgery for Metastatic Disease
The liver is the most common site of carcinoid metastasis. Liver resection along with primary tumor resection is recommended for resectable metastatic disease. More radical surgical debulking or cytoreductive surgery has been used for patients with extensive bilobar liver disease, liver failure, or extensive metastatic disease to provide prolonged disease-free survival. Several studies have shown that liver resection can improve the 5-year survival rate from 36% to 61% compared to historic controls.181–183 Although long-term data are lacking, percutaneous or laparoscopic local ablative techniques such as radiofrequency, microwave, or cryoablation represent alternative cytoreductive modalities for the treatment of metastatic carcinoid tumors. Cytoreductive strategies not only prolong survival, but are also associated with a decrease in octreotide doses required to control carcinoid symptoms. A number of retrospective series have demonstrated that surgical resection is superior to systemic therapy with regard to both overall survival and symptom management.184
Hepatic Artery Embolization
Transcatheter hepatic arterial embolization (HAE) with or without chemotherapy has been utilized extensively for both symptom control and as a definitive treatment for unresectable carcinoid liver metastasis.185 HAE may be performed with gel foam, polyvinyl alcohol, or microspheres. The addition of chemotherapy allows for the delivery of much higher intratumoral concentrations than can be achieved with systemic therapy. Known complications include transient or fulminant liver failure, liver abscesses, and postembolization syndrome (fever, abdominal pain, leukocytosis, elevations in liver function tests).185
Systemic Chemotherapy
Several chemotherapeutics have been studied extensively in the management of carcinoid metastases, including 5-FU, streptozotocin, and doxorubicin. All have yielded modest response rates of ~20%. In addition, IFN-α has been purported to achieve tumor stabilization in 20% to 40% of cases, and octreotide has been shown to prevent the progression of metastatic carcinoids in several small case series.186–190 Tyrosine–kinase inhibitors like imatinib or sunitinib can induce a delay in tumor cell growth in preclinical studies and disease stability in 83% of patients treated over a 1-year period. In a recent phase II study, bevacizumab, a monoclonal antibody that targets vascular endothelial growth factor (VEGF), was shown to stabilize disease in 95% of patients when combined with octreotide compared to 68% stabilization when octreotide was combined with IFN-α.191,218,219
Palliative Surgery
The decision to perform palliative resection for disseminated carcinoid tumors should carefully balance the surgical risks and perceived patient benefits. Orthotopic liver transplantation for patients with unresectable liver-only disease remains investigational and is currently performed by only a small number of transplant centers.
Lymphoma is the third most common SBC, comprising 10% to 20% of cases. Of such cases, 20% to 40% are extranodal lymphomas, which arise within a solid organ, and ~50% of extranodal lymphomas are GI lymphomas.194–198 Of GI lymphomas, 75% are located in the stomach, followed by the small bowel, colon, and other organs such as the pancreas and the liver. GI lymphomas have a peak incidence in the 7th decade of life, with a male to female ratio of 1.5:1. The incidence of small bowel lymphomas has increased in the United States over the last 2 decades, correlating with an increase in lymphomas among immunocompromised patients. Increased immigration from the Middle East—where primary intestinal lymphoma constitutes the most common primary extranodal disease—may also account for some of the increase in small bowel lymphomas. Additional risk factors include Crohn disease and prior radiation exposure.
Staging and Prognosis
The most important prognostic indicator for intestinal lymphoma is tumor spread. Most GI lymphomas are of the non-Hodgkin type and are staged based on the Ann Arbor staging system: stage I disease is limited to a single site; stage II tumors are confined to below the diaphragm and are separated into two subgroups, namely those with regional (stage II 1E) and distant (stage II 2E) lymph node involvement; stage III has involvement of organs on both sides of the diaphragm; and stage IV represents widespread dissemination, including the liver and the spleen. Primary intestinal lymphomas can be differentiated from secondary lymphomas by the absence of superficial and mediastinal lymphadenopathies on work-up, if there is no evidence of disease on both peripheral blood smears and bone marrow biopsies, if the disease is localized to a specific small bowel segment and regional draining mesenteric lymph nodes only, and if there is no evidence of hepatic or splenic involvement (except via direct extension from the primary tumor).
Variants
Mucosal-Associated Lymphoid Tissue Lymphoma
Marginal zone B-cell lymphomas (MALT) are the most common primary gastrointestinal lymphomas. They occur more commonly in the stomach, followed by the small bowel (most commonly, the ileocecal region), the colon, and the esophagus. MALTs occur predominately in men and peak in the 6th decade of life. They present as unifocal, ulcerated overhanging lesions, characterized by cellular heterogeneity and bearing close resemblance to normal gut-associated lymphoid tissue (Peyer patch and mesenteric nodal tissue). Nonneoplastic reactive lymphoid follicles surrounded by centrocytes are characteristic, with the neoplastic focus occupying the marginal zone or intrafollicular region. These tumor cells express elevated levels of immunoglobulin (Ig)M and B-cell–associated antigens (including CD19, CD20, CD22, and CD79a). Most tumors are CD5, CD10, and CD23 negative and CD43 variable. MALT lymphomas are not associated with Bcl-2 or Bcl-1 rearrangements.
MALT lymphomas may be associated with chronic inflammatory conditions, including autoimmune disorders such as Sjögren syndrome and Hashimoto thyroiditis. The majority of patients present with stage I or II disease. Therapy is multimodal and includes surgical resection and/or chemoradiation therapy, with small bowel lymphomas having a better prognosis than gastric tumors. Some studies suggest that MALT tumors may be antigen driven, especially by Campylobacter jejuni and Helicobacter pylori. Regression has been reported with eradication of H. pylori infection using antibiotics.194
Diffuse Large B-Cell Lymphoma
The second most common small bowel lymphoma is diffuse large B-cell lymphoma (DLBCL), also known as large cell immunoblastic, large-cleaved follicular center cell, centroblastic D immunoblastic cell, or diffuse mixed lymphocytic and histiocytic cell. DLBCL occurs more frequently in men, with a median age of 54 to 61 years, and primarily involves the ileocecal region. DLBCLs present as unifocal ulcerated lesions, composed of diffuse large B cells with large nuclei that are twice the size of a normal lymphocyte. Tumor cells are CD19, CD20, CD22, and CD79a positive. Bcl-2 gene mutation is present in approximately 30% of the cases. Immunosuppression is an important risk factor for DLBCL. Surgery is the mainstay of treatment for localized disease, followed by adjuvant radiation or chemotherapy.195 Overall 5-year survival is between 50% and 70% with multimodality therapy.196
Burkitt Lymphoma
Burkitt lymphoma of the small bowel accounts for <5% of all small bowel lymphomas. It can occur endemically or sporadically and is highly aggressive. The endemic subtype is seen predominantly in Central Africa; it affects children with a peak incidence at 8 years of age, is associated with Epstein-Barr virus (EBV) infection, and involves the GI tract in only 20% to 30% of cases. Conversely, sporadic Burkitt lymphoma occurs more commonly in Westernized countries, affects a broader age group, is not associated with EBV infections and commonly affects the GI tract (ileocecal region). Clinically, they can mimic appendicitis by presenting as large masses. Microscopically, cells are monomorphic medium-sized cells with round nuclei and an abundant basophilic cytoplasm. It is a rapidly growing tumor with short doubling time; the high rate of proliferation gives it a starry sky pattern due to the numerous macrophages that have ingested apoptotic tumor cells. Treatment primarily consists of chemotherapy, usually vincristine, cyclophosphamide, doxorubicin, and methotrexate.197
Mantle Cell Lymphoma
Mantle cell lymphoma (MCL) is a rare primary GI lymphoma that follows either an indolent or very aggressive course. MCL commonly affects men (ratio, 4:1) in their 6th or 7th decades of life and has a predilection for the small bowel and colon. Macroscopically, MCLs appear as multiple, whitish polypoid lesions that share morphologic features with nodal lymphomas. CD5+ B cells are located within the mantle zone that surrounds the germinal centers. Four histologic subtypes have been described: nodular, diffuse, mantle zone, and blastic. The blastic type has the worst prognosis, whereas the nodular and diffuse types have the best prognosis. MCLs have been associated with t(11:14) (q13; q32) chromosomal translocation, causing overexpression of cyclin D1. The vast majority of MCLs present in stage IV disease.
T-Cell Lymphoma
T-cell lymphomas (TCL) of the small bowel are less common than their B-cell counterparts, accounting for approximately 15% of all small bowel lymphomas. TCLs affect men and women equally and most commonly arise in the jejunum or the proximal ileum. TCLs may remain localized; however, dissemination is common. They typically present as large circumferential ulcers, in the absence of large masses, with associated mesenteric lymphadenopathy. As with other types of lymphomas, obstruction and perforation are common presentations. Microscopically, transmural replacement of the intestinal wall by highly pleomorphic lymphoid cells may be seen. A large number of surrounding intraepithelial lymphocytes may also show cellular atypia. Tumor cells stain positive for CD3, CD7, CD8, and CD103 and negative for CD4. Small bowel TCLs are known as EATLs due to their association with long-standing enteropathies, primarily celiac disease. EATL is described in approximately 5% to 10% of all patients with celiac disease, and the relative risk of developing a lymphoma in this setting is 25- to 100-fold higher than normal patients. Prognosis is generally poor, with a 5-year survival rate of 10%.198
GASTROINTESTINAL STROMAL TUMORS
GIST tumors arise from the intestinal cells of Cajal and are characterized by the presence of a gain-of-function c-kit (CD117) mutation. The c-kit protein codes for a tyrosine–kinase receptor involved in cellular proliferation, apoptosis, and differentiation.
Epidemiology and Genetics
GISTS are the most common mesenchymal tumor of the small bowel, despite comprising only 0.5% to 1% of all GI tumors. There are between 4,500 to 6,000 GISTs per year in the United States, with the stomach being the most common site.199–204 Incidence rates are equal in both genders and peak between 50 and 60 years of age. Small bowel GISTs are most commonly found in the jejunum, followed by the ileum, and the duodenum and typically present with pain, intussusception, or bleeding. Approximately 80% to 90% of all GIST tumors contain a c-kit mutation (CD117), whereas the remainder have a mutation in another tyrosine–kinase receptor gene or platelet-derived growth factor (PDGF) receptor alpha. It is now recognized that a vast majority of tumors previously identified as leiomyomas and leiomyosarcomas were actually CD117+ GIST. The molecular discoveries have allowed for the development of the specific c-kit tyrosine–kinase inhibitor imatinib (Gleevec), a drug initially designed to treat chronic myelogenous leukemia.
Prognosis and Behavior
All GISTs have the potential for malignancy, and approximately 50% of resected patients will have disease recurrence within 5 years. Of GISTs, 30% to 50% are clinically malignant. Most GISTs have a c-kit gain-of-function mutation, allowing for the prediction of clinical behavior and recurrence risk stratification.205–208 Tumors larger than 2 cm have a higher risk of recurrence and metastasis. This risk increases significantly for tumors larger than 3 cm to 5 cm. The mitotic rate has also been studied, and GISTs with five or more mitoses per 50 high-powered field (HPF) have a worse prognosis, whereas mitotic rates higher than 10 per 50 HPF predicts high recurrence and metastatic rates regardless of tumor size or location, with 5-year survival rates ~25%. The tumor site also plays an important role in prognosis, with jejunal and ileal GISTs displaying more malignant behavior compared to duodenal, rectal, or gastric GISTs.
Management
Surgery
R0 surgical resection is the mainstay therapy for all GISTs. Extensive resection of the surrounding uninvolved tissue has not been shown to improve outcomes. A routine lymphadenectomy is not indicated because GISTs rarely metastasize to regional lymph nodes. Intraoperative tumor rupture and spillage has been linked to carcinomatosis, necessitating a meticulous surgical technique. The peritoneum and liver are the main sites of metastasis and recurrence, and both should be inspected during surgical exploration.209–212
Adjuvant Therapy
Adjuvant imatinib therapy improves recurrence-free survival after complete surgical resection of localized disease. The ACOSOG Z9001 trial was a randomized phase III double-blinded, placebo-controlled multicenter trial where 713 patients were assigned to receive either 400 mg of imatinib or placebo daily for 1 year after complete resection of a c-kit–positive, 3 cm (or more) GIST. One-year progression-free survival was 98% in the imatinib arm and 83% in the placebo arm.213
Similar results were found in the Z9000 trial, a phase II, intergroup trial led by the American College of Surgeons Oncology Group, in which 106 patients who had undergone complete surgical resection of GIST were prescribed imatinib 400 mg per day for 1 year followed by serial radiologic evaluation. At 7.7 years of follow-up, the patients receiving imatinib had 1-, 3-, and 5-year overall survival rates of 99%, 97%, and 83%, respectively, compared to a 5-year overall survival rate of 35% for historic controls. Progression-free survival for 1, 3, and 5 years was 96%, 60%, and 40%, respectively.214
In patients in whom the initial complete surgical resection is not possible, the use of imatinib therapy has resulted in a significant prolongation of survival, with 80% of patients achieving a 5-year overall survival versus 9 months’ survival with no therapy.215 Neoadjuvant imatinib is also effective at reducing large tumor burdens and may facilitate margin-negative, organ-sparing resections.216,217 Regorafenib is another novel multitargeted kinase inhibitor being used in GIST management that inhibits the Ras/Raf/Mek pathways as well as VEGF receptor 2 (VEGFR2) signaling.219
Leiomyomas and Leiomyosarcomas
Small bowel leiomyomas and leiomyosarcomas arise from the muscularis propria and muscularis mucosa. They have a varied presentation, primarily involving tumor ulceration and bleeding as the tumor enlarges. Because tumor growth is initially localized and primarily extraluminal, obstruction does not occur until very late in the disease course.223 Leiomyosarcomas stain positive for desmin and actin and are negative for CD117 (c-kit and CD34). Metastasis is via hematologic routes and is mainly to the liver and peritoneum. About one third of patients have metastasis at the time of diagnosis, and prognosis is very poor.228–231
Desmoid Tumors
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








