Like most cancers, PDA develops as a result of acquired genetic defects over many years, and therefore, most often occurs in the elderly. The median age at onset is 71 years, and nearly 74% of patients are diagnosed between the ages of 55 and 84. Only 12% of patients are diagnosed under 55, and 14% after the age of 84. The age-adjusted incidence rate in the United States is 12 out of 100,000, and the lifetime risk of developing PDA is 1.5%, or 1 in 67. African Americans have a slightly increased risk compared to Caucasians.8
Genetic Risk Factors
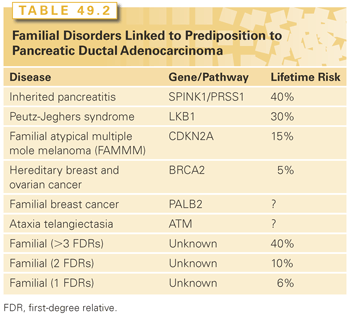
The greatest risk factor for pancreatic cancer is a strong family history. Approximately 10% of all pancreatic cancers are familial, defined as a family history involving at least two affected first-degree relatives (FDR) (e.g., parents, offspring, and siblings).9 The lifetime risk is 40% (32-fold) for patients with three or more affected FDRs, 10% for patients with two FDRs (6.4-fold), and 6% for patients with 1 FDR (4.6-fold).10 Some of the genetic defects underlying familial pancreatic cancer have been discovered, but known genetic defects only account for 10% to 15% of all familial cases.11 Specific genetic abnormalities are discussed in greater detail in the Pathology and Biology section.
Environmental Risk Factors: Tobacco, Occupational Hazards, and Alcohol Consumption
Many environmental risk factors have been formally evaluated via meta-analyses performed by the Pancreatic Cancer Case Control Consortium (PanC4, http://panc4.org/index.html). Smoking tobacco is the best characterized environmental risk factor for PDA. A large meta-analysis of 83 studies calculated a relative risk increase at 1.74 for active smokers (this is substantially less than having even a single FDR).12 Importantly, the risk decreases for former smokers, and returns to baseline after 20 years of smoking cessation.12 These results were confirmed by the PanC4 consortium, which also noted that the number of daily cigarettes is directly proportional to the risk of developing PDA.13 Not surprising, high-risk individuals with a family history who also smoke carry twice the risk, compared to similar patients who do not smoke.14,15 Cigars are also associated with an increased risk for PDA, whereas smokeless tobacco is not.16 The impact of environmental tobacco smoke (i.e., second-hand smoke) on long-term risk is unclear.17,18 Among the many carcinogens in tobacco products, N-nitrosamines and the polycyclic aromatic hydrocarbons (PAH) are suspected to be the greatest culprits.19
With regard to occupational risk hazards, chlorinated hydrocarbons and PAHs have been most consistently found to correlate with PDA, and to increase relative risk by a comparable degree as smoking.20 The former compound type is associated with dry cleaning and metal work, and, with the latter, exposure occurs with metalwork and aluminum production. According to the PanC4 consortium, mild or moderate alcohol consumption does not predispose one to PDA, whereas heavy alcohol consumption (≥9 drinks per day) is associated with an odds ratio (OR) of 1.6.21
Medical Risk Factors: Pancreatitis, Diabetes, and Obesity
Like smoking, chronic pancreatitis is a well-accepted risk factor for PDA, with an OR of 2.7 for patients with over 2 years of disease. The OR skyrockets for patients with less than 2 years of chronic pancreatitis (13.6), in large part because the pancreatitis in these patients represents a presenting symptom of PDA, as oppose to a contributing cause.22 A preponderance of the evidence from over a dozen studies suggests that obesity is a mild contributing risk factor for PDA (OR 1 to 1.5). Proposed mechanistic links include hormonal (e.g., insulin and insulin-like growth factor 1 [IGF-1]) and inflammatory influences on pancreatic cells, as well as increased carcinogen exposure related to food consumption.23 Although a link between type II diabetes mellitus (DM) and pancreatic cancer has been extensively studied, a causal association has not been clearly established. Two meta-analyses have been performed that examined more than 30 studies performed over 4 decades. Type II DM is associated with a twofold risk increase for PDA. Patients with long-standing DM (>5 years) have a mildly increased risk compared to patients without DM, suggesting that the disease may indeed contribute to tumorigenesis. Similar to obesity, increased levels of insulin and IGF-1 have been implicated. As with chronic pancreatitis, the highest risk for PDA is clearly in patients with recent onset of DM (<5 years, and particularly within 1 year), suggesting that DM is principally a manifestation of PDA, as opposed to a true risk factor in these patients.24,25
The normal pancreas contains two epithelial cell types: exocrine and endocrine cells. Most of the pancreas is comprised of exocrine cells, which line an organized ductal network. Acinar cells line the smallest ducts; they synthesize and secrete digestive enzymes. Larger ducts are lined by intercalated duct cells, and secrete bicarbonate and water. Ultimately, the ducts converge into the main pancreatic duct, which drains into the duodenum. The endocrine component makes up just 1% of the pancreas, and consists of islets of Langerhans. These hormone-producing cell clusters are primarily involved with glucose homeostasis. The principal endocrine cell types include the A (alpha), B (beta), and D (delta) cells, which synthesize glucagon, insulin, and somatostatin, respectively. The different cell types are believed to give rise to the different variants of pancreatic neoplasms.
Pancreatic cancer refers to a heterogeneous group of malignant pathologies that originate in the pancreas, and nearly all are epithelial in origin. They are categorized by their gross appearance (solid or cystic), as well as the predominant cell differentiation pattern (ductal, acinar, or endocrine). Primary pancreatic mesenchymal (e.g., sarcomas) and lymphoid neoplasms are exceptionally rare, and will not be reviewed here. Dozens of different epithelial pancreatic neoplasms have been described, but over 85% are the conventional pancreatic (tubular) ductal adenocarcinomas, and more than 98% of cancers fit into one of the following additional diagnoses: solid types, which include pancreatic endocrine neoplasms, acinar cell carcinomas, and pancreatoblastomas; and cystic types, which include mucinous cystic neoplasms, intraductal papillary mucinous neoplasms, and solid-pseudopapillary neoplasms. These diagnoses are typically made based on microscopic appearance, but a diagnosis may be confirmed with immunolabeling of specific proteins. Uncommon variants of ductal adenocarcinoma that are rarely encountered will not be reviewed. These include adenosquamous carcinoma, colloid noncystic adenocarcinoma, hepatoid carcinoma, signet ring carcinoma, medullary carcinoma, and undifferentiated carcinoma. The classification and nomenclature of pancreatic epithelial neoplasms has been reviewed by Klimstra, Pitman, and Hruban.26
Pancreatic Ductal Adenocarcinoma
Although the molecular mechanisms that underlie aggressive PDA biology remain poorly understood, there are certain features of PDA that are unique or defining, and will be highlighted in this section on pathobiology as well as in the Future Directions and Challenges section at the end of the chapter. These include molecular heterogeneity, a tendency for perineural invasion, remarkable tolerance to nutrient deprivation, and abundant stroma.
PDA Pathology
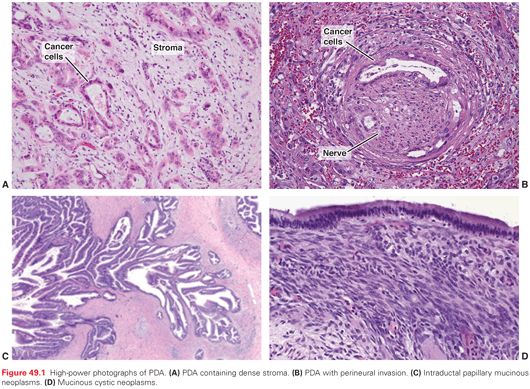
PDAs appear as ill-defined, sclerotic, yellow-white masses on gross inspection. The edges are poorly defined and infiltrative. Histologically, they are characterized by perineural invasion in almost all cases (much more frequently than in other common adenocarcinomas like colon and breast). Microscopic vessel and lymphatic invasion are common, and tumor necrosis is frequently present. Even in localized and resected cases, PDAs are rarely encountered at the T1 stage nor are they well differentiated, which drives home the fact that PDA is seldom diagnosed early in the life span of the tumor, at a curable stage.27 On light microscopy, the cells typically form infiltrative gland-forming structures, separated from each other by a tenacious desmoplastic reaction. Lymph node spread is present in the majority of resection specimens with localized disease. Immunohistochemical markers typically seen include cytokeratins (e.g., 7, 8, 13, 18, 19), CA19-9, B72.3, CA-125, and DUPAN-2. The nonneoplastic desmoplastic (stromal) component comprises more than 70% of the tumor mass (a higher proportion than other common solid tumors), and is commonly referred to as the tumor microenvironment (TME). The stroma consists of an extracellular matrix and numerous cell types, including inflammatory cells, pancreatic stellate cells, endothelial cells, nerve cells, fibroblasts, and myofibroblasts. The stroma is hypovascular with a low vessel density and high interstitial fluid pressure, resulting in a poorly perfused epithelial compartment, and the characteristic hypodense appearance on cross-sectional imaging obtained with intravenous contrast. Classic histologic features of PDA are depicted in Figure 49.1.28
The stroma creates a barrier to effective drug delivery29 and is believed to contribute to the overall virulence of the tumor. Sophisticated mathematical modeling reveals that austere conditions in the TME impose profound selection pressures on cancer cells, leading to the generation and dominance of aggressive cancer subclones.30 These subclones are chemoresistant and well adapted to survive extreme conditions present in the TME. Not surprisingly, PDA cells are more resistant to nutrient deprivation than most aggressive cancer types.31 There is now a concerted effort to develop strategies to target the tumor microenvironment in PDA (see Future Directions and Challenges).
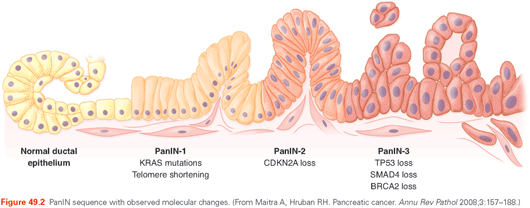
PDA development follows an adenoma to carcinoma sequence, similar to previous descriptions for colon cancer.32 Precursor lesions are referred to as PanIN lesions (pancreatic intraepithelial neoplasia), and are graded from PanIN-1 to PanIn-3. Early PanIN lesions are common (present 20% of individuals at autopsy33) and most do not progress to PDA in an individual’s lifetime. PanIN-1A lesions are tall columnar cells with abundant mucin; PanIN-1B lesions are similar but have a papillary appearance. PanIN-2 lesions have nuclear abnormalities. PanIN-3 lesions were formerly called carcinoma in situ; they exhibit true cribriforming, budding cells into lumen, loss of nuclear polarity, and mitoses. PanIN-3 lesions are found in roughly 2% of autopsy specimens from individuals who died from nonpancreatic diseases (Fig. 49.2).33 The similar lifetime risk of PDA suggests that a high proportion of PanIN-3 lesions develop into clinically significant cancer. Future effective early detection strategies would ideally discover and treat PanIN-3 lesions.28
PDA Genetics
Unlike breast and other cancers, PDA cannot be subgrouped by molecular characteristics at the present time to guide therapy or to inform prognosis. However, whole-exome sequencing of numerous PDAs were performed and provided unprecedented insight into the genetics of PDA.34,35 High throughput sequencing data emphasize the molecular complexity of PDA. Out of 24 PDA genomes, 1,327 different genes (~7% of coding genes) were found to have at least one somatic mutation, and 148 different genes had at least two mutations (0.7%).35 There were an average of 60 genetic alterations per tumor, and each PDA had a unique genetic fingerprint. Unfortunately, sequencing studies did not identify any novel high frequency mutated cancer genes as promising therapeutic targets. A more practical approach to personalized therapy is to group the genetically altered genes into 12 core signaling pathways (e.g., apoptosis, DNA damage, and 10 others), as proposed by Jones et al.35 These pathways are universally dysregulated and therefore may be more realistic therapeutic targets than single genes.35 A separate study found that axon guidance genes were also mutated at a higher rate than what was expected by chance, although the functional importance of these neuron-related genes in cancer is still unknown.34
A genetically altered gene may be categorized as an oncogene (with gain-of-function mutations), a tumor suppressor gene (loss-of-function), or a genome maintenance gene (loss-of-function). For the latter two gene types, both copies of the gene are typically inactivated; one allele is lost by somatic mutation and the other by chromosomal or allelic loss (loss of heterozygosity [LOH]). Oncogenic Kras is altered in more than 90% of PDAs, and is usually an early event in tumorigenesis.28,35,36 Somatic Kras mutations mostly occur in codon 12, and rarely occur in codons 13 and 61.37 The sequence alterations inactivate GTPase function, leaving GTP continuously engaged and the oncogene constitutively activated. Activated Kras positively regulates multiple signaling pathways, including the BRAF/mitogen-activated protein kinase (MAPK) pathway (proliferation), phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) (cell growth and survival), and Phospholipase C (PLC)/Protein kinase C (PKC)/Ca++ (calcium and second messenger signaling).38 To date, attempts to pharmacologically inhibit Kras have been unsuccessful.39 However, because of its early development in nearly all PDAs, the importance of this molecule will continue to preoccupy pancreatic researchers in hopes of finding an effective targeted therapy with a large therapeutic window (see Future Directions section).
The remaining high frequency mutated pancreatic cancer genes are tumor suppressor genes. The pattern of frequent allelic loss mutations in PDA due to chromosome instability (losses are more common than gains40), promotes a fertile environment of genetic experimentation that favors loss of function mutations. Allelotype mapping reveals that genetic loss ranges from 17% to 80% of the genome in a given PDA.41 Allelic loss is nonrandom across the PDA genome; LOH hot spots (areas where genetic loss most commonly occurs) harbor the most important tumor suppressors genes in PDA: CDKN2A (9p), TP53 (19p), and SMAD4 (18q).
CDKN2A (p16) is inactivated in roughly 95% of PDAs, either through homozygous deletion (deletion of both alleles), somatic mutation combined with LOH (deletion of one allele), or promoter hypermethylation. Inactivation of the gene abrogates the RB1-mediated G1/S checkpoint in the cell cycle (which allows unchecked inhibition of RB1 by CDK4), promoting cell cycle progression and cancer cell proliferation.42,43 TP53 mutations combined with LOH also occur in the majority of PDAs (~75%).44 TP53 is a critical component of the DNA damage response. In the face of a cytotoxic stress, TP53 induces cell cycle arrest (at G1 or G2 of the cell cycle), allowing cells to repair their DNA prior to DNA synthesis or mitosis. Thus, TP53 loss contributes to chromosome instability and aneuploidy observed in PDA.45 TP53 has been targeted experimentally by reactivating the mutant isoform46 or by targeting the G2/M checkpoint with WEE1 inhibitors (TP53-deficient tumors are particularly dependent on the G2/M checkpoint).47 SMAD4/DPC4 is inactivated in roughly half of PDAs through either homozygous deletion or mutation combined with LOH.48,49 SMAD4 is part of the transforming growth factor β (TGFβ) receptor pathway, and like the other two aforementioned tumor suppressor genes, regulates the cell cycle at the G1/S checkpoint.28 Interestingly, preservation of SMAD4 protein expression is associated with a local predominant progression pattern in PDA, which could be used to guide patient selection for radiotherapy.49
A group of genome maintenance genes (BRCA2, PALB2, FANCC, FANCG) involved in the Fanconi anemia DNA repair pathway (mutated in <10% of PDAs) are particularly intriguing because tumors deficient in this pathway are highly susceptible to DNA damaging agents and poly(ADP-ribose) polymerase (PARP) inhibitor therapy.28 Mutations in these genes are often present in the germline, and will be discussed further in the section on genetic syndromes associated with pancreatic cancer.
Analyses of laser capture microdissected pancreatic tissues have elucidated the chronologic sequence of major genetic changes in pancreatic tumorigenesis. Telomere shortening and Kras mutations are believed to be the earliest events (PanIN-1)50,51; p16 loss occurs at the PanIN-2 stage52; and TP53, SMAD4, and BRCA2 inactivation occur later during the PanIN-3 stage (see Fig. 49.2).53,54 Major chromosome instability, characterized by large-scale allelic copy number changes, is rarely observed in PanIN lesions, and is principally limited to LOH hot spots.55–57
Iacobuzio-Donahue and colleagues49 recently launched into pioneering work by extending the molecular progression model of pancreatic cancer to the most advanced stages of disease, through a rapid autopsy program in which primary tumors and paired metastases were sequenced and compared. The investigators distinguished founder mutations (those that arise early in tumorigenesis and are present throughout a tumor; about two-thirds of mutations) from progressor mutations (mutated in subclonal populations of cells, and absent in the parental clones; about one-third of mutations).58 Interestingly, progressor mutations that existed throughout a metastatic deposit were also identified in certain microdissected foci in the primary tumor, but not throughout the primary. This finding demonstrated that parent clones giving rise to specific metastases can actually be defined and mapped in the primary tumor. Although previous sequencing studies highlighted intertumoral heterogeneity, this study was the first to identify intratumoral genetic heterogeneity in PDAs, with profound implications on therapy.
Roughly 10% of PDAs are familial, and only 10% of the familial subgroup are associated with a previously defined genetic syndrome.59 Table 49.2 summarizes those familial syndromes and genetic defects that have been identified in pancreatic cancer kindreds. Hereditary breast and ovarian cancer is the most common familial syndrome, and the Peutz-Jeghers syndrome confers the highest lifetime risk. Importantly, several of the familial syndromes are associated with defects in DNA repair pathways (BRCA2, PALB2), which likely render tumors particularly susceptible to therapy with DNA damaging agents (e.g., mitomycin C, platinum agents) and PARP inhibitors.11 Other genetic predisposition alterations, such as single nucleotide polymorphisms (SNP) likely play a role in cancer risk, but remain to be fully characterized. Ongoing whole-exome sequencing efforts in familial patients will almost certainly uncover additional genetic abnormalities that predispose individuals to PDA.
There are currently no universal guidelines or proven strategies for screening high-risk individuals. The Cancer of the Pancreas Screening (CAPS) project has been the principal mechanism to study this patient population and generate recommendations. In one CAPS study, 225 asymptomatic, high-risk individuals were screened with magnetic resonance imaging (MRI), computed tomography (CT) scans, or endoscopic ultrasound (EUS) and the results were interpreted in a blinded fashion. An abnormality was identified in 92 patients (42%), and 5 were recommended to undergo a pancreatectomy (2%). High-grade dysplasia was present in three out of the five specimens.60 These data highlight that while successes are possible, the general yield of screening programs, even in this high-risk group, is relatively low.
Recently, a 49 member multidisciplinary panel made the following recommendations (with varying degrees of agreement amongst panelists): candidates for screening include FDRs of individuals with familial pancreatic cancer (at least two FDRs); patients with Peutz-Jeghers syndrome; and p16, BRCA2, and HNPCC mutation carriers with one affected FDR. There was also consensus that EUS and MRI were the preferred surveillance strategies. Routine surveillance should be performed annually, and suspicious solid masses should be further evaluated by CT scanning. Screening should begin around 50 years of age.61
Other Aspects of PDA Biology
Genetically engineered mouse models (GEMM) of PDA have provided a valuable research tool to study PDA biology.62 The workhorse of pancreatic cancer research in this area has been the conditional Kras model, where pancreas-specific promoters are exploited to target oncogenic Kras expression exclusively to the pancreas. Kras mutant mice develop pancreatic precursor lesions (e.g., PanIN lesions). Compound mutant mice with an additional genetic abnormality (e.g., mutant TP53 or CDKN2A) develop invasive and metastatic PDA, simulating human PDA. Similarly, Kras mutant mice with induced pancreatitis also develop invasive PDA. Genetic variations have also been generated that recapitulate pancreatic cystic tumors (SMAD4 loss or TGFα overexpression). The contributions of these models have been immeasurable. For instance, cell lineage tracing experiments in GEMMs reveal that PDA likely originates from acinar or centroacinar cells, as opposed to ductal cells (i.e., acinar-to-ductal metaplasia). GEMMs are enabling the development of molecular and bioimaging assays to detect late PanIN and early invasive lesions. Because GEMMs develop tumors with a robust stroma compartment, therapeutic strategies targeting the stroma can be tested. Early results suggest that this strategy enhances therapeutic efficacy of standard cytotoxic therapy. Finally, these models can be used to test and refine chemoprevention strategies before moving to humans.
Cancer genetics (e.g., mutations) have been prioritized over the last few decades in PDA cancer research, in large part because of the reproducibility of the findings and because the genetic model of tumorigenesis is straightforward and accepted. However, it is now clear that other molecular changes are also paramount for PDA development and maintenance. These include epigenetic abnormalities (methylation and histone modification), transcriptional regulation, and posttranscriptional regulation (microRNAs and RNA binding proteins).28
Less Common Pancreatic Cancers
Acinar Cell Carcinoma
Acinar cell carcinomas may have a slightly better prognosis (median survival of 33 months) than conventional PDA. The presentation is similar, except that patients may occasionally develop a paraneoplastic syndrome related to lipase hypersecretion, leading to subcutaneous fat necrosis and polyarthralgia. Microscopically, tumors grow in a trabecular pattern with minimal intervening stroma. Immunohistochemical confirmation is made with positive labeling for pancreatic enzymes (e.g., lipase, trypsin, chymotrypsin, amylase).63 Cystic variants have been reported as well, and described as acinar cystadenoma or cystadenocarcinoma.26 Twice the number of somatic mutations per tumor is present (~130 per tumor) compared to PDA, and allelic loss is comparable (27% fractional loss). Whole-exome sequencing did not reveal a consistent genetic pattern; no gene was mutated in more than 30% of cancers, and mutant genes previously identified in diverse pancreatic tumor types (e.g., TP53, SMAD4, RNF43, MEN1, GNAS) and nonpancreatic tumors (e.g., BRAF, PTEN, RB1, APC) were identified. Additionally, some mutant familial genes were identified (e.g., ATM, BRCA2, PALB2), suggesting that this tumor may arise in a familial pattern.64 Interestingly, no Kras mutations were identified.
Pancreatoblastoma
Pancreatoblastoma is the most common pancreatic malignancy in children and usually occurs in the first 8 years of life. These tumors have been associated with the Beckwith-Wiedmann and familial adenomatous polyposis syndromes. Elevated levels of serum α-fetoprotein and hormones have been described. Cures are often achievable with resection in children, although one-third of patients present with metastatic disease. Cases have been reported in adults as well, with survival after resection that is comparable to conventional PDA.65 Microscopically, these tumors contain acinar cells, but other cell types (e.g., neuroendocrine, ductal) are often present. Pancreatoblastomas have been whole-exome sequenced in only two adult cases.64 These tumors acquire relatively few mutations (~15 per tumor), and SMAD4 and CTNNB1 are typical. Kras mutations have not been reported.
Intraductal Papillary Mucinous Neoplasms
Intraductal papillary mucinous neoplasms (IPMN) are mucin-producing cystic neoplasms that arise from (and therefore communicate with) pancreatic ducts.66 Similar to PanIN lesions, they follow a progression pattern: low-grade dysplasia, moderate-grade dysplasia, high-grade dysplasia, and frank invasive carcinoma. Benign IPMNs are subgrouped according to their papillary appearance on microscopy (see Fig. 49.1), with each type associated with a particular mucin expression pattern: intestinal (MUC5AC, MUC2), gastric-foveolar (MUC5AC, MUC6), pancreatobiliary (MUC5AC, MUC1), and intraductal oncocytic neoplasm (MUC1, MUC6). Clinically, IPMNs are classified as involving the side-branch ducts or the main-pancreatic duct, with some tumors involving both of these. IPMNs are the most common pancreatic cystic neoplasms and with increased usage of high-resolution cross-sectional imaging, are believed to develop in roughly 1% to 5% of the general population.67 Although all benign IPMNs are technically premalignant, there is a wide range of malignant potential amongst encountered cysts. For instance, main duct IPMNs harbor associated cancers 40% of the time, whereas small side-branched IPMNs without any concerning radiographic features have a cancer risk closer to 5% or less.68 Thus, there has been considerable effort to try to determine which IPMNs harbor invasive cancer or are at highest risk for malignant transformation and warrant resection. The Sendai guidelines recommend resection for IPMNs that are either symptomatic, >3 cm in diameter, contain solid components (e.g., mural nodules), have malignant cells on cytology, or involve the main pancreatic duct.68 Invasive cancer associated with IPMNs are most commonly either tubular (conventional PDA) or colloid subtypes. The former is typically more aggressive and develops from pancreatobiliary IPMNs, whereas the latter usually develops from intestinal subtypes. Although IPMN-associated cancers have a more favorable prognosis than conventional PDA, this has been attributed to the lower stage at diagnosis; when matched stage for stage, the two pancreatic cancer types are associated with similar outcomes.69 Moreover, a recent multi-institution analysis of IPMNs with small foci of invasion (all <2 cm invasive component) indicates a significant recurrence risk (~20%) regardless of the size of the invasive component (Jordan M. Winter, MD, unpublished, July 2014).
Whole-exome sequencing of IPMN lesions revealed roughly half the number of mutations (~27 per tumor) compared to PDA. There was a high incidence of RNF43 mutations, which is a tumor suppressor with E3 ubiquitin ligase activity (involved in protein degradation).70 Additionally, Kras and GNAS mutations occurred with high frequency.
Mucinous Cystic Neoplasms
Mucinous cystic neoplasms (MCN) are typically large cysts lined by tall, columnar epithelium (see Fig. 49.1). Benign MCNs are categorized similar to IPMNs from low- to high-grade dysplasia, and roughly one-third of cases harbor invasive cancer. Additionally, RNF43 and Kras mutations are frequently present (as with IPMNs). Distinguishing features of MCNs (from IPMNs) include a large predominance in woman, the cysts are typically localized on the left side of the pancreas, ovarian stroma underlying the epithelial component is pathognomonic (even in men), there is no communication with the pancreatic duct, and GNAS mutations have not been identified.26,70
Solid-Pseudopapillary Neoplasms
Solid-pseudopapillary neoplasms (SPNs) solid and cystic tumors are eponymously referred to as Hamoudi or Franz tumors. They typically occur in young women and may arise throughout the gland. Histologically, they consist of noncohesive polygonal cells that form solid masses, but develop cystic components over time with frequent intracystic hemorrhage. The tumors are considered low-grade malignancies, with a 10% risk of lymph node spread in resected specimens, and a 95% lifetime recurrence-free survival rate after resection.71 SPNs develop fewer than five somatic mutations per tumor, but virtually all SPNs harbor CTNNB1 (β-catenin) mutations. This molecular abnormality is believed to contribute to the poor cohesion between cells apparent on microscopy (wild type β-catenin interacts with E-cadherin at cell–cell junctions).
The classification of pancreatic neuroendocrine tumors (PNET) has been challenging based on the heterogenous biology of this tumor type. Recent staging systems have been adopted based on size and lymph node metastases, with different versions in the United States (AJCC 7th ed. 2010, same as exocrine pancreatic cancer) and Europe (European Neuroendocrine Tumor Society [ENETS]).72 The ENETS system also considers biologic parameters and has simplified the World Health Organization classification according to the following scheme: G1 (well differentiated, <2 mitoses per 10 High power field (HPF), <3% KI67 index); G2 (well differentiated, intermediate grade: 2 to 20 mitoses per 10 HPF, 3% to 20% KI67 index); and G3 (high grade or poorly differentiated: >20 mitoses per 10 HPF, >20% KI67 index).73 Typically, G1 tumors are considered benign and cured with resection, whereas G2 tumors are considered malignant. However, the distinction (benign versus malignant) is not always clear. Older studies observed that half of PNETs were nonfunctional and half were functional due to the presence of measurable hormones in serum.74 The balance has shifted dramatically toward nonfunctional tumors being more common, in large part due to small, incidentally discovered PNETs identified on cross-sectional imaging obtained for nonpancreatic reasons.
Pancreatic neuroendocrine cancers have an incidence of roughly 0.2 out of 100,000 (versus 12 out of 100,00 for PDAs), yet autopsy studies show that small PNETs are very common (~10% of deceased individuals).75 They comprise roughly 5% of all resected pancreatic cancers (on par with IPMN associated cancers).27 PNETs are typically well demarcated and hypervascular. Therefore, they focally enhance on the arterial phase in imaging studies with intravenous contrast. Microscopically, neoplastic cells are arranged in a nested fashion with a high density of intratumoral microvessels (in contrast to PDA). The majority of well-differentiated PNETs have an indolent course and are cured with surgery alone. Even patients with metastatic disease have a 5-year survival around 50%.72 Insulinomas are the most common functional PNETs (30% to 45%) and symptoms are associated with excessive endogenous insulin production (Whipple triad: documented hypoglycemia, symptoms of hypoglycemia, improved symptoms with correction of hypoglycemia).76 Gastrinomas comprise roughly 20% of functional PNETs, and cause peptic ulcer disease and diarrhea from hypergastrinemia. Less common functional PNETs include glucagonomas (associated with glucose intolerance and migratory necrolytic erythema), VIPomas (watery diarrhea), and somatostatinomas (steatorrhea, diabetes mellitus, and gallstones). Familial syndromes associated with the development of PNETs include multiple endocrine neoplasia 1 (MEN1 gene), von Hippel-Lindau disease (VHL), neurofibromatosis (NF1), and tuberous sclerosis (TSC1 or TSC2).
The genetic landscape of PNETs has now been defined through whole-exome sequencing.77 Compared to PDAs, there are fewer somatic mutations per tumor (~16). Kras mutations have not been observed. Commonly mutated genes included MEN1 (44% of PNETs), chromatin remodeling genes (43%: DAXX and ATRX), and mTOR pathway genes (15%: phosphatase and tensin homolog [PTEN], PIK3CA, and TSC2). This latter gene group likely renders many PNETs vulnerable to systemic targeted therapy using mTOR inhibitors. Although surgery is the principal therapy for local disease, treatment options have greatly expanded for advanced disease, and include local liver-directed therapies (radiofrequency ablation), regional liver-directed therapies (bland, chemo, and radioembolization), and systemic therapies (cytotoxic drugs, targeted agents, somatostatin analogs including radio-labeled drugs). The complex management of PNETs is discussed in other reviews on the subject.78
STAGE I AND II: LOCALIZED PANCREATIC DUCTAL ADENOCARCINOMA
Anatomy
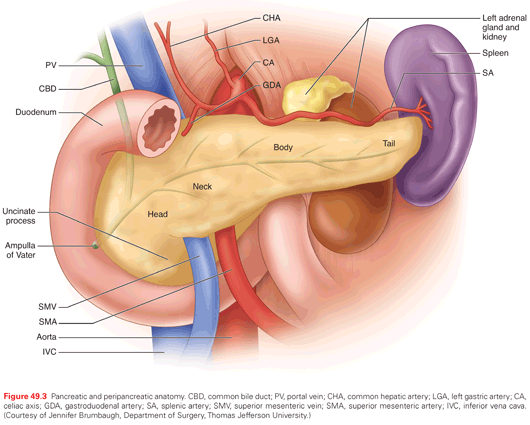
The evaluation and treatment of localized pancreatic cancer requires a strong understanding of the anatomy of the pancreas and nearby structures. The pancreas is an elongated gland in the retroperitoneum that crosses the midline at the L2 spinal level (Fig. 49.3). It is bounded anteriorly by the stomach and posteriorly by the inferior vena cava, aorta, left adrenal gland, and left kidney. The descriptive anatomy of the gland is grossly separated into four components. The head is the right-most portion, and sits within the duodenal C-loop. It includes parenchyma to the right of the superior mesenteric vessels and contains the uncinate process, which projects inferomedially, extending to the right lateral border of the superior mesenteric artery. The common bile duct runs within (or rarely just posterior) to the pancreatic head and enters the duodenum at the ampulla of Vater with the main pancreatic duct. Moving leftward, the neck of the pancreas lies anterior to the superior mesenteric vein–portal vein axis. The superior mesenteric vessels run posterior to the pancreatic neck, and course inferiorly across the anterior border of the third portion of the duodenum. The body of the pancreas extends to the left of the superior mesenteric vessels. The gland transitions distally into the pancreatic tail anterior to the left kidney, and courses toward the splenic hilum.
Presentation
The most common presenting symptoms associated with PDA located in the right side of the pancreas (head, neck, or uncinate process) include jaundice (75%), weight loss (50%), abdominal pain (40%), or nausea (10%).27 Jaundice occurs as a result of obstruction of the common bile duct and is often associated with pruritus. Other symptoms or findings associated with an obstructed bile duct include acholic stools and tea-colored urine. An obstructed pancreatic duct may induce acute pancreatitis and result in exocrine insufficiency associated with steatorrhea. Patients with left-sided PDAs (body or tail) typically experience abdominal pain, back pain, and nausea. New onset DM (within 1 year of diagnosis) occurs in roughly 10% of patients with PDA, based on our own institutional experience and others (unpublished).79
Evaluation and Assessing Resectability
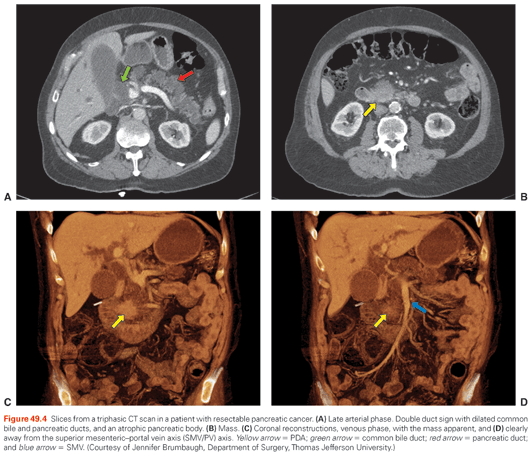
Although periampullary cancer (adenocarcinoma of the pancreas, ampulla of Vater, bile duct, or periampullary duodenum) should be considered in any patient who presents with a conjugated hyperbilirubinemia, the likelihood is highest in older patients (e.g., >55 years). In individuals with an expected pancreatic cancer, a thorough history and physical should be performed, followed by appropriate imaging for staging and to assess resectability. Critical findings on physical exam include scleral icterus, jaundice, and lymphadenopathy (e.g., Sister Mary Joseph or Virchow nodes). A chest x-ray or chest CT scan is performed to assess for pulmonary metastases and as a baseline study. Either a high-quality MRI or CT scan of the abdomen is performed to evaluate the pancreas and measure the extent of disease. We typically prefer CT for its superior resolution and detailed depiction of the relevant vasculature. Water is administered as oral contrast, and nonionic intravenous contrast is rapidly injected. Slices are captured at 1-mm intervals from the diaphragm to the iliac crests at three different times or phases: early arterial, late arterial, and venous. Multiplanar reformatting and three-dimensional (3D) surface rendering is performed during the early arterial and venous phases (Fig. 49.4).80 Positron-emission tomography (PET)/CT scans do not add much additional value and are not routinely performed in the initial assessment for resectability.
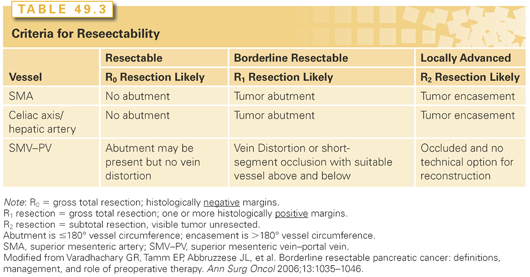
Resection is attempted if (1) patients are medically fit for a pancreatectomy, (2) there is no evidence of metastases, and (3) patients are believed to have resectable disease. Resectability is ultimately decided by the operating surgeon, but general guidelines have been proposed and are based on the likelihood of achieving a complete, margin-negative resection.81,82 Resectability equates to a high probability of an R0 resection; borderline resectability equates to a likely result of an R1 resection (positive microscopic margins); and unresectable (or locally advanced) PDA is likely to result in R2 resection (residual macroscopic disease). Resectable lesions do not invade the superior mesenteric artery (SMA), celiac axis (CA), common hepatic artery (CHA), or superior mesenteric–portal vein axis (SMV-PV). In contrast, locally advanced lesions encase (i.e., >180° invasion) any of the previously mentioned arteries or occlude the SMV-PV such that no reconstructive options remain. Borderline resectable lesions involve the visceral vessels to a lesser extent; they can abut the visceral arteries (<180° invasion), distort the visceral veins, or even occlude the SMV-PV, but with venous reconstruction still technically feasible (Table 49.3).83 Most pancreatic surgeons offer patients with resectable lesions an attempt at resection (although some centers advocate neoadjvuant treatment prior to resection even for resectable lesions84). Patients with locally advanced lesions are recommended to undergo palliative treatment without the intent to cure. Considerable debate remains for borderline PDAs; resection may be offered, but increasingly, neoadjuvant treatment is recommended for even abutment or narrowing of the SMV-PV.81 Neoadjuvant chemotherapy (± radiation) can facilitate resection, and may improve the likelihood of a complete resection with negative margins, even in the absence of a radiographic response.85
Obtaining a tissue diagnosis is not essential for all cases. Indications include instances when (1) neoadjuvant treatment is advised or (2) the pretest probability of an alternative diagnosis is considerable (e.g., suspicion for benign causes of pancreatitis, medically managed neoplasms such as lymphoma, or a benign stricture). In these instances, an EUS with fine-needle aspiration (FNA) biopsy is an effective method for obtaining tissue and has an accuracy in excess of 90%.86 When the diagnosis is clear based on imaging and history, it is appropriate to proceed to attempted resection without a preoperative tissue diagnosis. Similarly, placement of an endoscopic biliary stent is frequently performed in jaundiced patients preoperatively, but is essential in only selected cases. A multicenter prospective and randomized trial compared routine preoperative biliary drainage with delayed resection to early surgery without stenting in jaundiced patients with pancreatic cancer. Serious complications were increased nearly twofold in the routine biliary drainage group (74% versus 39%; p <0.001), suggesting that biliary decompression be reserved for jaundiced patients unable to undergo resection in a short-time frame (1 to 2 weeks).86 A pancreaticoduodenectomy is safely performed, even when the total bilirubin is markedly elevated. Patients, who are otherwise healthy, with normal renal function and clotting parameters will usually tolerate a safely performed pancreaticoduodenectomy with a total bilirubin as high as 20 mg/dL.
Surgery
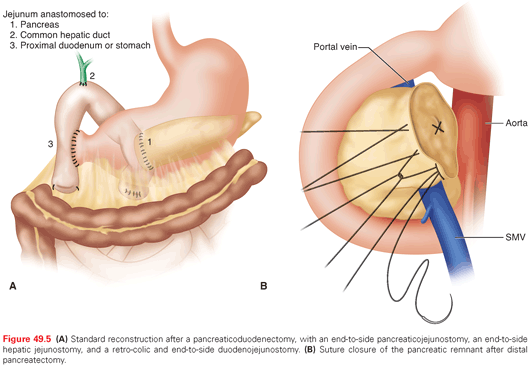
Technical aspects of a pancreatectomy are detailed elsewhere and are well beyond the scope of this chapter, hence will only be reviewed briefly here. For right-sided pancreatic cancers, a pancreaticoduodenectomy (PD) is performed. The specimen includes the gallbladder, duodenum, head of the pancreas (the pancreatic transection typically is at the level of the neck), proximal jejunum, and distal common bile duct. The most proximal retained jejunum is brought up into the right upper quadrant, and three anastomoses to the pancreas remnant, common hepatic duct, and proximal duodenum (or stomach) are performed (Fig. 49.5A). A distal pancreatectomy for PDA involves resection of the pancreatic body and tail, with an en bloc splenectomy performed to ensure a proper lymphadenectomy. The transected surface of the pancreatic remnant is closed with suture (Fig. 49.5B) or staples. A central pancreatectomy or local excision for a PDA is seldom if ever performed for PDA due to inadequate lymph node harvest.
A minimally invasive pancreatectomy using laparoscopy or a robotic-assisted approach may be safely performed. Although a minimally invasive approach is more common for benign and premalignant lesions, a presumed diagnosis of PDA is not a contraindication.87 A meta-analysis comparing open versus laparoscopic distal pancreatectomies for PDA revealed similar oncologic and pathologic outcomes in the two groups. Patients undergoing laparoscopy had a shorter postoperative stay by 4 days, less blood loss, and fewer surgical site infections.88 Importantly, studies comparing the two techniques have not been prospective and randomized, and are, therefore, all subject to selection bias, with the more difficult resections falling into the open group. Although most high-volume pancreatic centers offer minimally invasive left-sided resections, laparoscopic PD is more technically challenging, and only a handful of centers have a significant experience.89 These centers report comparable outcomes with minimally invasive versus open pancreatic surgery in their own experience, although an advantage of laparoscopic PD has not yet been proven in a prospective and randomized study.
International Study Group of Pancreatic Surgery Contributions
Surgically related mortality after PD has improved dramatically over the last 3 decades, and is lower than 5% at most high volume centers.27 However, morbidity remains high (~40%). The most common complications include pancreatic leak (20%), delayed gastric emptying (15%), and wound infection (10%). Bile and duodenal leaks occur in roughly 3% and 1% of patients, respectively.27 The greatest limitation to studies focused on pancreatectomy-related complications has been a lack of standard definitions across institutions, making comparisons between institutions’ reports difficult. The formation of an International Study Group of Pancreatic Surgery (ISGPS) to address this issue has been a great advance in pancreatic surgery–related outcomes research. The group has published consensus criteria and definitions for complication grading on the following pancreatic-specific morbidities: postoperative pancreatic fistula (leak),90 delayed gastric emptying,91 and postpancreatectomy hemorrhage.92 In addition, the group established concrete guidelines on reporting features and management of the pancreatic remnant/anastomosis (e.g., duct size, gland texture, mobilization distance, type of anastomosis, suture used, use of stent).93 Definitions of postpancreatectomy-related mortality, bile leak,94 and wound infection also vary in the literature, and a strategy to standardize terms and complication grading for these outcomes would enhance the surgical literature.
Surgical Trials
There have been numerous prospective and randomized surgical trials that have shaped the surgical management of PDA. A trial unique to PDA, compared to other common cancers, is a study demonstrating superiority of resection over best nonoperative therapy for the management of resectable disease.95 A total of 42 patients in Japan with resectable PDA were randomized to either standard pancreatectomy without adjuvant treatment or to chemoradiation alone (50.4 Gy with continuous fluorouracil [5-FU] at 200 mg/m2 per day) without surgery. Randomization occurred at laparotomy and patients randomized to chemoradiation had only an open biopsy and abdominal closure. The only multivariate predictor of survival in the study was the treatment group (hazard ratio [HR] = 0.4; p = 0.02), with the group undergoing resecting having superior survival (median overall survival (OS), 13 versus 9 months).
Pancreaticoduodenectomy
A comprehensive list of surgical trials in patients undergoing pancreaticoduodenectomy is provided in Table 49.4. Regarding technical aspects of the operation, one of the more studied questions compares pylorus preserving pancreaticoduodenectomy (PPPD) to distal gastrectomy plus pancreaticoduodenectomy (classic PD).96–102 Karanicolas et al.97 performed a meta-analysis of six randomized trials including 574 patients. PPPD was associated with a reduced operative time by more than 1 hour (p <0.001), less blood loss (284 mL; p <0.001), and fewer blood transfusions. There was a nonsignificant trend toward improved mortality in the same direction (0.4; p = 0.09). There was not a significant difference in delayed gastric emptying. Multiple randomized studies have also examined the location of the enteroenterostomy (antecolic versus retrocolic), and no significant differences were observed.103–107
A postoperative pancreatic fistula (POPF) remains the most challenging complication after PD. Clinically significant leaks (e.g., requiring treatment intervention) occur in roughly 10% of cases. Risk factors include soft pancreatic texture, a fatty pancreas, a small pancreatic duct, high intraoperative blood loss, and a high postoperative serum amylase level.108,109 Numerous randomized trials have attempted to reduce the pancreatic leak rate, although few have succeeded. Ineffective interventions include fibrin glue,110,111 octreotide,112–119 and internal pancreatic stenting.27 Mixed results have been reported with pancreaticogastrostomy (versus standard pancreaticojejunostomy).120–126 The pancreaticojejunostomy technique was recently examined, comparing a two-layered invagination technique with interrupted outer stitches and a continuous inner layer, against a duct-to-mucosa technique. The pancreatic leak rate was more than twofold higher with the latter approach (7% grade B/C versus 17%; p = 0.03).127 A binding pancreaticojejunostomy (versus invagination) is a second technique shown to have a decreased pancreatic leak rate. With this technique, an end-to-end pancreaticojejunostomy is performed; 3 cm of the pancreatic remnant is mobilized and telescoped within the jejunum. No leaks were observed in 106 patients in the latter group, although these data have not yet been replicated.128 Interestingly, there now have been three randomized trials showing a lower pancreatic leak rate associated with external pancreatic duct stenting (the pancreatic duct stent is brought out through the bowel and skin as a drain),129–131 whereas a fourth study revealed no benefit.132 In the past, trials using somatostatin or its analogs have had mixed outcomes in lowering pancreatic leak rates. Recently, a phase III trial using pasireotide (SOM230, a potent somatostatin analog) was completed at Memorial Sloan-Kettering Cancer Center, and revealed a statistically significant lower pancreatic leak rate in patients receiving subcutaneous injections of the study drug (Peter J. Allen, email communication, January 2014). Roux-en-Y reconstruction performed to isolate the pancreaticojejunostomy (PJ) from the other two anastomoses did not decrease the overall leak rate, but the proportion of clinically significant leaks was substantially reduced (74% versus 29% of all leaks; p = 0.01).133
In addition to these important technical trials relevant to the management of right-sided pancreatic neoplasms, other studies have informed the management of patients undergoing PD. A group of studies established that a radical retroperitoneal lymphadenectomy does not confer improved cancer-specific survival (four randomized trials).134–137 Additionally, routine adjuvant parenteral nutrition has proven to be detrimental.138 A previous single-institution randomized trial suggested that routine nondrainage after a pancreatic resection is safe,139 whereas a recent multi-institution and randomized study revealed a much higher mortality rate in patients without drains,140 leaving this issue unresolved.
Distal Pancreatectomy
A distal pancreatectomy and splenectomy for left-sided PDA is a simpler operation than pancreaticoduodenectomy, yet also carries significant risk. POPF is the principal morbidity associated with distal pancreatectomy. Clinically significant leaks occur in roughly 10% of cases. In 10% to 20% of cases, a leak is appreciable as amylase-rich fluid in surgical drains, but does not add morbidity. There are now roughly a half-dozen randomized clinical trials examining the effect of pancreatic stump management on POPF, ranging from staplers to mattress sutures. In summary, no positive interventions have been discovered. The DISPACT trial was the largest of these trials. This European multi-institution study analyzed 352 patients, and compared stapled and hand-sewn closures. The pancreatic leak rate was roughly 30% in both groups (p = 0.56).141 Fibrin sealant and falciform ligament patches have also been tested, with no observed benefit.142,143 Interestingly, ultrasonic dissection of the pancreas with ligation of all visualized ducts in nonfibrotic glands was associated with improved pancreatic leak rates compared to conventional division and suturing (n = 58, 4% versus 26%, p = 0.02). Roughly 20 4-0 silk ligatures were used per neck transection in the ultrasonic dissection group, and transection took 10 extra minutes, on average.144
Palliative Surgery
There have been five randomized trials directly comparing hepaticojejunostomy with endoscopic or percutaneous biliary decompression for patients with malignant periampullary obstruction.145 A meta-analysis revealed that OS is the same between the groups, although total hospital days after randomization was twofold more in the stenting group, principally related to recurrent biliary obstruction requiring intervention. The authors recommend that for patients projected to live more than 6 months, a surgical biliary (as well as gastric bypass) be considered. Two separate randomized studies have demonstrated that prophylactic gastrojejunostomy in nonobstructed patients decreased the absolute risk of subsequent gastric outlet obstruction by roughly 20%, but did not affect OS.146,147 Finally, there is evidence that celiac neurolysis with alcohol injected at laparotomy in patients with unresectable disease improves quality of life and OS.148
Operative and Surgical Pathology Reporting
Just as the ISGPS has sought to standardize complication reporting, there have been efforts to standardize operative and pathology reporting to facilitate collaboration between institutions, establish a minimum standard of quality, and provide consistency to the literature. With regard to the operative dictation, a description of the clinical stage (relationship to mesenteric vessels and evidence of metastatic disease) is important. Specific mention of the liver, peritoneum, and small intestines is necessary. Blood loss and the need for transfusion are recorded. The surgical technique used to dissect the uncinate process should be described, as well as the management of the SMA and SMV (e.g., were they skeletonized? resected? the method of visceral vessel repair?). The anastomotic techniques need to be described. The important elements of the pancreaticojejunostomy have been addressed by the ISGPS, as mentioned previously.93 If a frozen section analysis was performed, the results should be stated. Similarly, any gross residual disease must be reported.149
Standards for pathology reporting have been established by the College of American Pathologists, and protocols are available at the Web site (http://www.cap.org/apps/cap.portal). On the pathology review, tumor site (e.g., head, uncinate, body, tail), maximum tumor diameter (in centimeters), histologic type or subtype, and histologic grade (well, moderate, or poor) are provided. Extension into extrapancreatic tissue is described. Margins are assessed (e.g., involvement or distance), including the uncinate, pancreatic neck, common bile duct, and duodenum. The distance to the margin (in millimeters) from the tumor should be stated. The posterior pancreatic surface margin is also reported, although this is not a transected or surgical margin. Microscopic invasion of the lymphatics, small vessels, and nerves are indicated. The TNM stage and the component elements are provided according to the AJCC 7th edition staging system. A minimum of 12 lymph nodes should be evaluated. Treatment effect is described if the patient has received neoadjuvant therapy.
Assessing Prognosis
Although PDA is widely viewed as an aggressive cancer, like other common cancers, tumors display a wide range of biology. The median survival after resection for PDA is around 18 months in large series27; roughly 20% of patients survive more than 5 years, and a comparable number suffer cancer-specific mortality within just 1 year of surgery.5 There would be substantial value in accurately predicting patients’ cancer-specific outcome; at the present time, there are no reliable tests to predict prognosis. The most frequently considered adverse prognostic factors include conventional pathologic features (lymph node metastases, poor differentiation, tumor size >3cm, and positive resection margins). The approximate proportions of resected PDAs that fulfill each criterion are 75%, 40%, 50%, and 40%, respectively.27 It must be remembered that each of these individual prognostic factors are weak predictors of outcome, with multivariate Cox proportional hazards ratios only around 1.5. Winter et al.,162 studied 137 patients who underwent resection for PDA and died within 1 year from their disease or survived more than 30 months (patients with intermediate survival were excluded). Adverse pathologic features were frequently present in long-term survivors; for instance, 65% of patients in the long survivor group had lymph node metastases in the resected specimen. Conversely, lymph node metastases were not detected in 17% of the short survivor group, yet they still recurred early after resection and succumbed to their disease.
Perhaps the most robust prognostic marker routinely used in patients with localized and resectable PDA is the postoperative CA 19-9 level. CA 19-9 is a high–molecular-weight glycolipid that is a sialylated derivative of the Lewis a antigen (normally expressed by epithelial cells and absorbed onto the surface of erythrocytes). The oligosaccharide epitope is also present on mucins secreted by pancreatic cancer cells and detectable in the serum. A markedly elevated preoperative CA 19-9 level has some prognostic value (on par with conventional pathologic features). Preoperative CA 19-9 has also been used by some surgeons as a predictor of unresectable disease when the lesion appears resectable on imaging. Roughly 30% of patients with serum values above 300 U/mL were found to have a contraindication to resection on staging laparoscopy.150 However, preoperative CA 19-9 is particularly limited in patients with biliary obstruction because the antigen is falsely elevated in this setting.151–153 In addition, the sensitivity suffers because 5% to 10% of the population are unable to express CA 19-9 due to Lewis antigen variability (related to the presence or absence of a fucosyltransferase).154–156 As noted previously, CA 19-9 is most informative after resection when biliary obstruction is no longer a confounding variable and there has been macroscopic clearance of the disease. In a landmark ad hoc analysis of the Radiation Therapy Oncology Group (RTOG) 9704 adjuvant trial, an elevated postoperative CA 19-9 level (≥180 U/mL, drawn 1 to 2 months after resection) was associated with a multivariate proportional HR of 3.6.154 Interestingly, Lewis antigen-negative individuals (who cannot express elevated serum CA 19-9) had the same survival as patients with low CA 19-9 levels for unclear reasons.
Patterns of Failure
Although OS after resection for PDA is roughly 18 months,27 patients typically recur by 1 year,159 indicating a short survival time once a recurrence is detected (comparable in survival to patients with metastatic disease). Recurrences have been reported in virtually every organ site, but most commonly occur in the retroperitoneum (57%), liver (51%), peritoneum (35%), and lung (15%). Interestingly, lung recurrences are typically delayed, and rarely occur early after resection. A study of recurrence patterns out of Memorial Sloan-Kettering revealed that 12% of resected patients developed a local-only recurrence pattern; 33% had metastatic disease only and 46% had both local and metastatic disease (recurrence status was unknown in the remaining patients).160
Identifying biomarkers to predict patterns of recurrence could help select the patients who are most likely to benefit from intensive local therapy (radiation). The most intriguing biomarker to date in this area of research has been SMAD4. A study of pancreatic adenocarcinomas from an autopsy series (n = 65) revealed that a local predominant pattern was associated with intact SMAD4 expression (7 of 9 cases), whereas a disseminated pattern was associated with absent SMAD4 expression (16 of 22 cases).49 This pattern was confirmed in tissue samples of patients from a phase II trial of locally advanced PDA.161 Out of 15 patients with SMAD4-positive tumors, 11 had progression in a local-predominant pattern. In contrast, 10 of 14 patients with absent SMAD4 had significant distant spread. These studies suggest that patients having tumors with intact SMAD4 are most likely to benefit from radiation, whereas those patients with absent SMAD4 should only receive systemic therapy. However, a study of resected PDA samples revealed that resection alters the natural history of PDA recurrence, such that SMAD4 status is no longer predictive of recurrence pattern in this setting.160 Other biomarkers162 were examined as part of that study, and MUC1 expression was associated with a metastatic recurrence pattern. In a multivariate analysis, lymph node spread was the only variable (including the tested biomarkers) associated with pattern of failure. Patients without regional lymph node metastases had an increased risk of a local-only recurrence, typically in a relatively delayed fashion.160 These data highlight that some patients would likely benefit from intensified local therapy, whereas others are best suited to receive systemic therapy only. Future studies are needed to define a personalized approach.
Adjuvant Therapy
According to the National Comprehensive Cancer Network (NCCN) guidelines, adjuvant chemotherapy is recommended for patients who recover well from pancreatic resection. Acceptable strategies include chemotherapy alone (gemcitabine, 5-FU, or capecitabine monotherapy), or chemoradiation plus chemotherapy (gemcitabine or 5-FU monotherapy, either given before or after chemoradiation).157 Unfortunately, these regimens all consist of single-agent therapy with minimally effective agents. A meta-analysis of five randomized adjuvant trials including 951 patients concluded that adjuvant therapy provides a 3-month median survival advantage, and a 3% absolute improvement in 5-year survival. To put these findings in perspective, patients typically require a 6-month course of adjuvant treatment to achieve a 3-month survival.158 Although these figures may appear unappealing to many patients, the reality is more complex; many patients receive no benefit at all, and may even be harmed with treatment, whereas a subset of patients receive a robust and durable survival benefit with these adjuvant treatments. Identifying which patients are most likely to experience a survival benefit is as important as discovering superior treatment regimens.
Adjuvant Trials
There have been eight prospective and randomized adjuvant trials that have shaped current treatment recommendations, including: five European trials, two in the United States, and one in Japan (Table 49.5).163–170 Out of these eight trials, only four were considered positive with respect to the planned primary end point (one in the United States and three in Europe).164,165,168,169, The continent where these trials were performed has played an important factor in defining the standard of care adjuvant treatment strategy around the world; chemoradiation is commonly performed in addition to chemotherapy in the United States, whereas chemotherapy (without radiation) is the standard in Europe. The principal adjuvant trials will briefly be reviewed, emphasizing the strengths and weaknesses of the studies.
The Gastrointestinal Tumor Study Group (GITSG) trial was the first of the adjuvant trials and was a small phase III trial (by today’s standards, n = 49 patients treated between 1974 and 1982) performed in the United States. Patients in the experimental arm received 40 Gy (split course with 20 Gy in each course, and a 2-week break in the middle). Bolus 5-FU was administered weekly for 2 years as maintenance therapy (500 mg/m2), but was given daily for the first 3 days of each radiation course. The treatment arm was compared to an observation only arm, and the chemoradiation group had superior survival (20 versus 11 months; p = 0.04). The study has been criticized for its small sample size. Furthermore, it is not known whether the observed benefit was attributable to chemoradiation, to maintenance chemotherapy, or to both. Nevertheless, this landmark study established a role for adjuvant chemoradiation as an acceptable treatment for resected PDA in the United States.
Two small trials (in Norway169 and Japan167) were performed over the last 15 years that provided equivocal results for chemotherapy alone, compared to surgery only. In the Norwegian trial, authored by Bakkevold et al.,169 61 patients with periampullary cancer (only 47 with PDA) were randomized to receive doxorubicin, mitomycin C, and 5-FU (six cycles), or observation. Patients receiving adjuvant chemotherapy had an improved median survival (23 versus 11 months; p = 0.04), but 2-year survival was similar (43% versus 32%; p = 0.1). In the Japanese trial, 89 patients were randomized to just two cycles (separated by 4 to 8 weeks) of 5-FU (500 mg/m2 as a continuous infusion over 5 days) plus cisplatin (80 mg/m2 on day 1 of each cycle), versus observation. OS was similar in the two groups (12.5 versus 15.8, respectively; p = 0.9).
The European Organization for Research and Treatment of Cancer (EORTC) trial was Europe’s response to the GITSG trial and proved to be the largest adjuvant trial for PDA at that time.163 A total of 218 patients with either PDA or other periampullary cancer were randomized to chemoradiation or observation. The treatment arm received split-course radiation for 40 Gy (as with GITSG). However, 5-FU was given as a continuous infusion (as opposed to bolus) on days 1 through 5 of each radiation course (for a total of 125 mg/m2). No maintenance chemotherapy was administered. OS was 21.6 months with treatment and 19.2 months with observation (p = 0.5).
The European Study Group for Pancreatic Cancer (ESPAC-1) was the next large randomized adjuvant trial.165 A total of 289 patients were randomized to a 2×2 design, with one of the four groups receiving no treatment. Treatment groups included (1) chemoradiation (40 Gy split-course radiation with bolus 5-FU at 500 mg/m2 on days 1 through 3 of radiation, as with GITSG) followed by chemotherapy (bolus leucovorin at 20 mg/m2 and 5-FU at 425 mg/m2 daily for 5 days, every 28 days for six cycles); (2) adjuvant chemotherapy only (without chemoradiation) as previously defined for group 1; (3) chemoradiation only (without chemotherapy) as previously defined for group 1; and (4) no treatment. The two groups receiving chemotherapy (groups 1 and 2) had superior survival as compared to those who did not (20.1 versus 15.5 months; p = 0.009). When chemoradiation was analyzed separately, the OS were 15.9 months with the two chemoradiation groups (groups 1 and 3) and 17.9 months without chemoradiation (groups 2 and 4; p = 0.05). When patients who received chemotherapy only were excluded from the no chemoradiation group (leaving just patients in the observation group), there was still a strong trend toward improved survival without chemoradiation. The results have been widely questioned because of the complex study design and because patients apparently received suboptimal radiation therapy (split course, no central quality of radiation control, 9% protocol violation, a very high [62%] local failure rate compared to recent trials). Although this study established chemotherapy alone as an acceptable standard of care in Europe and other parts of the world, oncologists in North America remain largely divided on the role of chemoradiation.
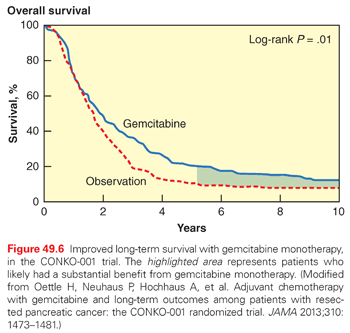
The Charité Onkologie (CONKO-001) trial primarily took place in German centers between 1998 and 2004,159 with updated results recently reported.164 The trial was an appropriate follow-up to ESPAC-1, which was interpreted in Europe as evidence in favor of adjuvant chemotherapy (without chemoradiation). Moreover, only 5-FU–based adjuvant chemotherapy had been tested up to that point, whereas the superiority of gemcitabine over 5-FU had been established for patients with advanced PDA (discussed in the Stage IV: Metastatic Disease section).171 A total of 368 patients were randomized to receive six cycles of gemcitabine (4-week cycles) at a weekly dose of 1,000 mg/m2 every 3 weeks with a 1 week break versus observation alone. Chemoradiation was not included in the treatment arm. The initial report reached its primary end point of disease-free survival (13.4 versus 6.9 months; p <0.001), but OS was not significantly different.159 However, the updated analysis demonstrated improved OS with gemcitabine (22.8 versus 20.2 months; p = 0.01).164 The small absolute difference in survival could be explained, in part, by the fact that 38% of patients did not receive the planned dose of gemcitabine and 13% did not receive at least one full cycle. In addition, most patients in the control arm received palliative chemotherapy once a recurrence was detected. Grade 3 through 4 toxicities were extremely rare in the treatment arm (no specific toxicity occurred in more than 3% of patients in the gemcitabine group). Although the median survival advantage is modest, it should be emphasized that the 5-year survival advantage was 10% (20.7% versus 10.2%; p <0.05) and the 10-year survival advantage was 5% (12.2% versus 7.7%). These data provide very strong evidence that, in a small subset of patients (i.e., 1 in 10), gemcitabine monotherapy is highly effective (Fig. 49.6). The opportunity to achieve long-term survival due to gemcitabine (albeit uncommon) is an important consideration for patients contemplating adjuvant treatment, despite the meager improvement in median survival.
The European ESPAC-3 trial is the largest adjuvant trial to date, and directly compared chemotherapy with gemcitabine (1,000 mg/m2 over 30 minutes weekly, 3 out of 4 weeks) versus 5-FU (bolus folinic acid at 20 mg/m2 plus bolus 5-FU at 425 mg/m2 on days 1 to 5, every 28 days for six cycles as administered in ESPAC-1). As with CONKO-001, no adjuvant chemoradiation was given. Median OS were 23.6 and 23.0 months, respectively (p = 0.4), demonstrating equivalence between the two agents. However, this study did not change the emerging paradigm of frontline gemcitabine monotherapy at most centers, because toxicity was less with gemcitabine (14% serious adverse events with 5-FU versus 7.5% with gemcitabine; p <0.001). The increased toxicity due to 5-FU was primarily related to increased stomatitis and diarrhea.
Finally, RTOG 9704 was the second United States adjuvant trial, completed in 2002172 and updated in 2011.170 The trial’s treatment arms paralleled the ESPAC-3 study (5-FU versus gemcitabine), except patients in both treatment groups received 5-FU–based chemoradiation in the middle of their adjuvant chemotherapy course. Total treatment duration in both groups was 6 months, and the study question related to chemotherapy (5-FU versus gemcitabine), and not the role of chemoradiation. A total of 451 patients were randomized to chemotherapy with 5-FU at 250 mg/m2 per day given as a continuous infusion (different from ESPAC-3) or gemcitabine 1,000 mg/m2 given weekly. Patients received one chemotherapy cycle prior to chemoradiation (3 weeks) and 12 additional weeks afterwards (two cycles of 5-FU consisting of 4 weeks on and 2 weeks off; three cycles of gemcitabine with 3 weeks on and 1 week off). Chemoradiation was given to all study patients as 50.4 Gy with continuous infusion 5-FU (250 mg/m2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree