Cancer of the Nasal Cavity and Paranasal Sinuses
 ANATOMY
ANATOMY
Nasal Cavity
The nasal cavity extends from the hard palate inferiorly to the base of the skull superiorly. It is above and behind the vestibule and is defined anteriorly by the transition from skin to mucous membrane and posteriorly by the choanae, which open directly into the nasopharynx.1 The nasal cavity consists of four subsites: the nasal vestibule, the lateral walls, the floor, and the septum.
The nasal vestibule is the triangular space located inside the aperture of the nostril as a slight dilatation that extends as a small recess toward the apex of the nose. It is defined laterally by the alae; medially by the membranous septum, the distal end of the cartilaginous septum, and columella; and inferiorly by the adjacent floor of the nasal cavity. It is lined by skin containing hairs and sebaceous glands; therefore, tumors at this location are often those that arise from the skin, usually squamous cell cancers2 but may occasionally be basal cell carcinoma3 sebaceous carcinoma,4 melanoma,5 or non-Hodgkin lymphoma.6
The lateral walls correspond with the medial walls of the maxillary sinuses and consist of thin bony structures that have three shell-shaped projections (superior, middle, and inferior conchae or turbinates) into the nasal cavity. The floor extends from the vestibule to the nasopharynx above the hard palate of the maxilla. The septum divides the nasal cavity into right and left halves.
Paranasal Sinuses
The paranasal sinuses are named according to the bones in which they are located: the ethmoid, maxilla, sphenoid, and frontal.
Ethmoid Sinuses
The ethmoid sinuses are composed of several small cavities, the ethmoid air cells, within the ethmoid labyrinth located below the anterior cranial fossa and between the nasal cavity and the orbit. They are separated from the orbital cavity by a thin, porous bone, the lamina papyracea, and from the anterior cranial fossa by a portion of the frontal bone, the fovea ethmoidalis. They are in close proximity to the optic nerves laterally and the optic chiasm posteriorly. The ethmoid sinuses are divided into anterior, middle, and posterior groups of air cells. The middle ethmoid cells open directly into the middle meatus. The anterior cells may drain indirectly into the middle meatus via the infundibulum. The posterior cells open directly into the superior meatus.
Maxillary Sinuses
The maxillary sinuses, the largest of the paranasal sinuses, are pyramid-shaped cavities located in the maxillae. The lateral walls of the nasal cavity form the base and the roofs correspond to the orbital floors, which contain the infraorbital canals. The floors of the maxillary sinuses are composed of the alveolar processes. The apices extend toward and frequently into the zygomatic bones. Secretions drain by mucociliary action into the middle meatus via the hiatus semilunaris through an aperture near the roof of the maxillary sinus. Ohngren’s line is a theoretical plane dividing each maxillary sinus into the suprastructure and infrastructure; it is defined by connecting the medial canthus with the angle of the mandible.
Sphenoid Sinus and Frontal Sinuses
The sphenoid bone forms a midline inner cavity that communicates with the nasal cavity through an aperture in its anterior wall. It is directly apposed superiorly to the pituitary gland and optic chiasm, laterally to the cavernous sinuses, anteriorly to the ethmoid sinuses and nasal cavity, and inferiorly to the nasopharynx. The paired, typically asymmetric frontal sinuses are located between the inner and outer tables of the frontal bone. They are anterior to the anterior cranial fossa, superior to the sphenoid and ethmoid sinuses, and superomedial to the orbits. They usually communicate with the middle meatus of the nasal cavity.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Cancers of the nasal cavity and paranasal sinuses are relatively uncommon. Fewer than 4,500 cases are diagnosed each year in the United States, an incidence of 0.75 per 100,000.7 Cancers of the maxillary sinus are twice as common as those of the nasal cavity; cancers of the ethmoid, frontal, and sphenoid sinuses are extremely rare. They generally develop after the age of 40 years, except for esthesioneuroblastoma, which has a unique bimodal age distribution8 and occurs twice as often in men than in women.9 These tumors are most common in Japan and South Africa.
The etiologic factors vary by tumor type and location. Adenocarcinomas of the nasal cavity and ethmoid sinus have been reported to occur more frequently in carpenters and sawmill workers who are exposed to wood dust,10,11,12 Synthetic wood, binding agents, and glues may also be involved as cocarcinogens.13 Squamous cell carcinomas of the nasal cavity have been seen more often in nickel workers.14 Maxillary sinus carcinomas have been associated with radioactive thorium-containing contrast material (Thorotrast) used for radiographic visualization of the maxillary sinuses in the past. Occupational exposure in the production of chromium, mustard gas, isopropyl alcohol, and radium also may increase the risk of sinonasal carcinomas.
Cigarette smoking is reported to increase the risk of nasal cancer, with a doubling of risk among heavy or long-term smokers and a reduction in risk after long-term cessation. After adjustment for smoking, a significant dose–response relationship has also been noted between alcohol consumption and risk of nasal cancer.15
 NATURAL HISTORY
NATURAL HISTORY
Nasal Vestibule
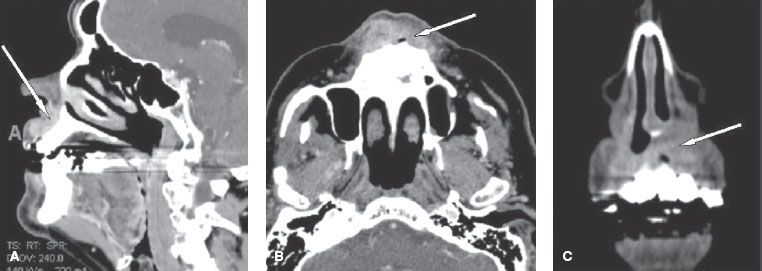
Nasal vestibule carcinomas can spread by direct invasion of the upper lip, gingivolabial sulcus, premaxilla (early events), or nasal cavity (late events), as shown in Figure 42.1. Vertical invasion may result in septal (membranous or cartilaginous) perforation or alar cartilage destruction. Lymphatic spread from nasal vestibule carcinomas is usually to the ipsilateral facial (buccinator and mandibular) and submandibular nodes. Large lesions extending across the midline may spread to the contralateral facial or submandibular nodes. The incidence of nodal metastasis at diagnosis is approximately 5%.16,17 Without elective nodal treatment, approximately 15% of patients develop nodal relapse. Hematogenous metastases are rare.
FIGURE 42.1. Computed tomography scans of a nasal vestibule squamous cell carcinoma that has spread by direct invasion of the upper lip (arrow in A) and gingivolabial sulcus and premaxilla (arrow in B and C).
Nasal Cavity and Ethmoid Sinuses
The pattern of contiguous spread of carcinomas varies with the location of the primary lesion. Tumors arising in the upper nasal cavity and ethmoid cells can extend to the orbit through the thin lamina papyracea and to the anterior cranial fossa via the cribriform plate, or they may grow through the nasal bone to the subcutaneous tissue and skin. Lateral wall primaries invade the maxillary antrum, ethmoid cells, orbit, pterygopalatine fossa, and nasopharynx. Primaries of the floor and lower septum may invade the palate and maxillary antrum. Perineural extension (typically involving branches of the trigeminal nerve) is seen most often with adenoid cystic carcinomas.
Lymphatic spread of nasal cavity primaries is uncommon, although spread to retropharyngeal and cervical lymph nodes is possible. In a series of 51 patients reported by the University of Texas MD Anderson Cancer Center,18 only 1 patient had palpable subdigastric nodes at diagnosis. Of the 36 patients who did not receive elective lymphatic irradiation, 2 (6%) experienced subdigastric nodal relapse. Hematogenous dissemination is rare. In the MD Anderson Cancer Center series, for example, distant metastasis to bone, brain, or liver occurred in only 4 of 51 patients.18
The olfactory region is the site of origin of esthesioneuroblastoma and, occasionally, adenocarcinomas. Esthesioneuroblastoma is a tumor of neural crest origin first reported by Berger and Luc in 1924 as esthesioneuroepithelioma olfactif19; other names include olfactory neuroblastoma and esthesioneurocytoma. Esthesioneuroblastoma constitutes approximately only 3% of all intranasal neoplasms. About 250 cases have been reported between 1924 and 1990.20 The tumor typically is composed of round, oval, or fusiform cells containing neurofibrils with pseudorosette formation and diffusely increased microvascularity.21
Esthesioneuroblastoma may be mistaken for any other “small round-cell tumor,” that is, a group of aggressive malignant tumors composed of small and monotonous undifferentiated cells that includes Ewing’s sarcoma, peripheral primitive neuroectodermal tumor (also known as extraskeletal Ewing’s), rhabdomyosarcoma, lymphoma, small cell carcinoma (undifferentiated or neuroendocrine), and mesenchymal chondrosarcoma. The clinical presentations of these entities often overlap, but clinicopathologic features and immunohistochemical staining may help in distinguishing among them.
The route of contiguous spread of esthesioneuroblastomas is similar to that of ethmoid carcinomas. Lymph node involvement and distant metastasis are uncommon at diagnosis.22,23
Maxillary Sinuses
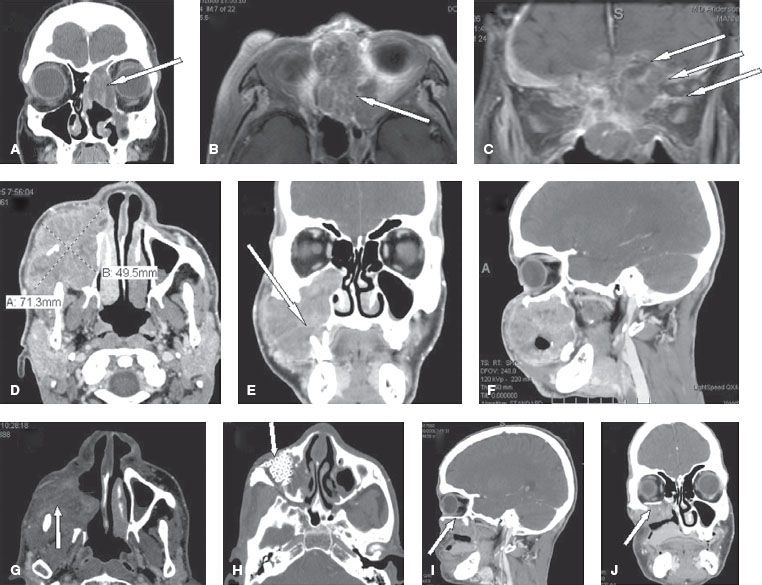
The pattern of spread of maxillary sinus cancers varies with the site of origin. Suprastructure tumors extend into the nasal cavity, ethmoid cells, orbit, pterygopalatine fossa, infratemporal fossa, and base of skull (Fig. 42.2A–C). Invasion of these structures gives lesions of the suprastructure a poorer prognosis. Their treatment is also associated with greater morbidity as a consequence of craniofacial resection or radiation of intracranial and ocular structures. Infrastructure tumors often infiltrate the palate, alveolar process, gingivobuccal sulcus, soft tissue of the cheek, nasal cavity, masseter muscle, pterygopalatine space, and pterygoid fossa (Fig. 42.2D–J).
The maxillary sinuses are believed to have a limited lymphatic supply24 and a correspondingly low incidence of lymphadenopathy at diagnosis.25,26 Only 6 of the 73 patients (8%) in the MD Anderson Cancer Center series had palpable lymphadenopathy at diagnosis. The incidence of nodal spread, however, varies with the histologic type (17%, or 5 of 29 patients with squamous cell or poorly differentiated carcinomas vs. 4%, or 1 of 27 for patients with adenocarcinoma, adenoid cystic carcinoma, or mucoepidermoid carcinoma). The incidence of subclinical disease, as reflected in the rate of nodal relapse in patients who did not receive elective neck treatment, also varies with histologic type (38%, or 9 of 24 patients with squamous cell or poorly differentiated carcinomas vs. 8%, or 2 of 26 patients with adenocarcinoma, adenoid cystic carcinoma, or mucoepidermoid carcinoma). The cumulative incidence of nodal involvement (gross and microscopic) for patients with squamous cell and poorly differentiated carcinomas is about 30%. The risk of regional recurrence after treatment is 20% to 30% or higher, depending on the extent of disease and elective neck treatment.27 Ipsilateral subdigastric and submandibular nodes are most often involved. Hematogenous spread is uncommon.
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
Nasal Vestibule
Carcinomas of the nasal vestibule usually present as asymptomatic plaques or nodules, often with crusting and scabbing. Advanced lesions may extend beyond the vestibule and may cause pain, bleeding, or ulceration. Large ulcerated lesions may become infected, leading to severe tenderness that requires anesthesia for complete clinical assessment.
FIGURE 42.2. The pattern of spread of maxillary sinus cancers. A–C: Suprastructure tumors are shown, with arrows indicating the involvement of the nasal cavity and ethmoid cells (A), the orbit (B), and the base of skull (C). D–J: Advanced tumor is shown, with arrows indicating alveolar process destruction with loosening of a tooth (E) and abutment of the orbital floor without frank intraorbital invasion (F). The patient had a maxillectomy and orbital floor resection with an anterolateral thigh (ALT) flap (arrow in G), and titanium mesh reconstruction of the orbital floor (H, I, and J).
Nasal Cavity
Nasal cavity tumors present with symptoms and signs of nasal polyps (e.g., chronic unilateral discharge, ulcer, obstruction, anterior headache, and intermittent epistaxis), hence delaying the diagnosis. Additional symptoms and signs develop as the lesion enlarges: medial orbital mass, proptosis, expansion of the nasal bridge, diplopia resulting from invasion of the orbit, epiphora due to obstruction of the nasolacrimal duct, anomaly of smell or anosmia from involvement of the olfactory region, or frontal headache due to extension through the cribriform plate.
The common presenting symptoms of esthesioneuroblastomas are nasal obstruction and epistaxis. Spaulding et al.28 found that anosmia could precede diagnosis by many years. Other symptoms are related to contiguous disease extension into the orbit (proptosis, visual-field defects, orbital pain, epiphora), paranasal sinuses (medial canthus mass, facial swelling), or anterior cranial fossa (headache) or are due to inappropriate antidiuretic hormone secretion.28
Ethmoid Sinuses
The presenting symptoms and signs of ethmoid sinus tumors are central or facial headaches and referred pain to the nasal or retrobulbar region, a subcutaneous mass at the inner canthus, nasal obstruction and discharge, diplopia, and proptosis. In one study of 34 patients with ethmoid sinus cancers treated at MD Anderson Cancer Center between 1969 and 1993,29 nasal cavity symptoms (nasal obstruction, epistaxis, discharge) were reported in 25 patients (74%), orbital symptoms (diplopia, orbital pain, vision loss, proptosis, inner canthus mass, tearing) in 12 (35%), headache in 6 (18%), and hyposmia or anosmia in 5 (15%).
Maxillary Sinuses
Maxillary sinus cancers usually are diagnosed at advanced stages. Symptoms and signs are facial swelling, pain, or paresthesia of the cheek induced by disease extension to the premaxillary region, epistaxis, nasal discharge and obstruction related to tumor spread to the nasal cavity, ill-fitting dentures, alveolar or palatal mass, unhealed tooth socket after extraction from spread to the oral cavity, and proptosis, diplopia, impaired vision, or orbital pain due to orbital invasion.30
 DIAGNOSTIC WORKUP
DIAGNOSTIC WORKUP
The recommended pretreatment physical, diagnostic, and staging evaluations are listed in Table 42.1.
TABLE 42.1 PRETREATMENT EVALUATION FOR TUMORS OF THE NASAL CAVITY AND PARANASAL SINUSES

Physical Examination
Inspection and palpation of the orbits, nasal and oral cavities, and nasopharynx can provide preliminary determination of tumor extent. Bimanual palpation is important in assessing contiguous extension of nasal vestibule lesions and in identifying buccinator and submandibular nodal involvement. Careful examination of cranial nerves is required. Fiberoptic nasal endoscopy after mucosal decongestion and topical analgesia allows assessment of local extent and facilitates biopsy of tumor involving the nasal cavity or nasopharynx.
Radiographic Evaluation
Imaging has a crucial role in the staging of sinonasal tumors. Magnetic resonance imaging (MRI) and computed tomography (CT) scans are complementary.31 MRI is superior at detecting direct intracranial or perineural or leptomeningeal spread.32 T2-weighted MRI can be helpful in distinguishing tumor (low signal) from obstructed secretions (bright).33 CT is superior for detecting early cortical bone erosion or extension through the cribriform plate or orbital walls.
Certain features provide clues as to the nature of the tumors in this region. Slowly progressive lesions tend to deform instead of destroy bony structures. Intermediate-grade tumors can cause sclerosis of adjacent bone. Lymphomas tend to permeate bone without frank destruction, and carcinomas and sarcomas infiltrate and destroy adjacent bone.
Biopsy
Transnasal biopsy is preferred for tumors arising from or extending into the nasal cavity or nasopharynx. Some paranasal sinus tumors may be more easily sampled using transoral procedures or an open Caldwell-Luc approach.
Laboratory Studies
Complete blood counts and serum chemistries can be used to screen for the presence of distant metastases. Abnormalities of these tests can be further investigated as necessary.
 STAGING
STAGING
The seventh edition of the American Joint Committee on Cancer’s (AJCC) AJCC Cancer Staging Manual tumor-node-metastasis (TNM) classification includes staging for cancers of the maxillary sinus, ethmoid sinus, and the nasal cavity.34 Significant updates from the sixth edition affect classifications of T4 lesions and hence stage IV disease. Specifically, T4 lesions are now considered either T4a (moderately advanced local disease) or T4b (very advanced local disease), which leads to stratification of stage IV disease as either IVA (moderately advanced local or regional disease, IVB (very advanced local or regional disease), or IVC (distant metastatic disease). Definitions of anatomic stage prognostic groupings and TNM classifications are given in Table 42.2.
 PATHOLOGIC CLASSIFICATION
PATHOLOGIC CLASSIFICATION
Most nasal vestibule cancers are squamous cell carcinomas; the remaining tumors are basal cell or adnexal carcinomas. Most cancers of the nasal cavity and paranasal sinuses are also squamous cell carcinomas, although minor salivary gland neoplasms (adenocarcinoma, adenoid cystic carcinoma, and mucoepidermoid carcinoma) account for 10% to 15% of lesions in these locations. Melanoma accounts for 5% to 10% of nasal cavity malignancies but is rare in the paranasal sinuses. Neuroendocrine carcinomas of the sinonasal region (including small cell carcinoma, esthesioneuroblastoma, and sinonasal undifferentiated carcinomas), lymphomas, sarcomas, and plasmacytomas are even less common.
 PROGNOSTIC FACTORS
PROGNOSTIC FACTORS
Patient-specific factors (primarily prognostic for survival) include age and performance status. Disease-specific factors (primarily prognostic for locoregional control) include location, histology, and locoregional extent (reflected in TNM stage), and perineural invasion. Extensive local disease involving the nasopharynx, base of skull, or cavernous sinuses markedly increases surgical morbidity as well as the risk of subtotal surgical excision. Tumor extension into the orbit may require enucleation, but minimal invasion of the floor or medial wall may be dealt with through resection and reconstruction, sparing the globe.
 GENERAL MANAGEMENT
GENERAL MANAGEMENT
Nasal Vestibule Tumors
Nasal vestibule tumors can be treated definitively with surgery, primary radiation therapy, or postoperative (adjuvant) radiation therapy when indicated because of tumor size or positive surgical findings. For small superficial tumors, standard treatment approaches are surgery or primary radiation therapy. Depending on the location and size of the primary tumor, radiation can be delivered as external beam radiation therapy, brachytherapy, or a combination of the two. Primary radiation therapy may be preferable for nasal vestibule carcinoma for better cosmetic outcome, although surgery can yield high control rates with excellent cosmetic results for selected small superficial tumors. Adjuvant radiation is indicated for cases involving positive surgical margins, positive lymph nodes, or perineural invasion. Cartilaginous invasion is not a contraindication for radiation therapy because fractionated treatment carries a low risk of necrosis.35 For large invasive tumors with extensive tissue destruction and distortion, the combination of surgery and radiation therapy, with the radiation given either before or after surgery, is the mainstay of treatment. However, some clinicians favor primary radiation with salvage surgery for this situation.36 Cosmesis can be enhanced by having experienced prosthodontists design aesthetically satisfactory custom-made nasal prostheses after radical surgery. Patients who are older or who have poor performance status can be treated with radiation therapy alone. No role for systemic chemotherapy has been established for tumors of this type.
Nasal Fossa Tumors
Either surgery or primary radiation therapy can produce similarly high control rates for early-stage nasal fossa lesions. The choice of treatment modality is generally guided by the size and location of the tumor as well as the anticipated cosmetic outcome. Posterior nasal septum lesions or locally advanced lesions are generally treated surgically, but small anterior-inferior septal lesions (≤1.5 cm) can be treated effectively with interstitial brachytherapy (iridium-192 [192Ir] implant). For lateral wall lesions extending to the nasal ala, primary external beam radiation therapy may produce the best cosmetic results.
TABLE 42.2 2010 AMERICAN JOINT COMMITTEE ON CANCER STAGING SYSTEM FOR CANCER OF THE NASAL CAVITY AND PARANASAL SINUSES


Paranasal Sinus Tumors
Surgery can produce excellent control rates for T1 and T2 tumors and is generally the mainstay of treatment. The combination of surgery and postoperative radiation therapy is the treatment of choice for patients with more advanced but resectable disease who are medically fit to undergo surgery. Maxillary sinus and ethmoid sinus tumors often present as locally advanced disease (large T3 or T4) and are commonly managed with surgery and postoperative radiation therapy. Ethmoid sinus carcinomas can be treated with radiation alone or with concurrent chemotherapy to avoid structural or functional deficits.37 Surgery generally involves medial maxillectomy and en bloc ethmoidectomy; a craniofacial approach is required if tumor extends superiorly to the ethmoid roof or olfactory region.38,39 Primary radiation therapy, with or without concurrent chemotherapy, can be considered for patients who are not fit to undergo surgery owing to significant comorbid conditions or poor performance status, or for patients who decline radical surgery.
For patients presenting with Kadish stage A esthesioneuroblastoma, either surgery or radiation therapy ultimately yields locoregional control rates exceeding 90%.8 Single-modality therapy has also been used for lesions involving the nasal cavity and one or more paranasal sinuses (stage B), as has surgery followed by adjuvant radiation therapy. However, the optimal therapy for stage B lesions is not clear because of the heterogeneity of these tumors. Surgery with adjuvant radiation is generally used for disease that extends beyond the nasal cavity and paranasal sinuses (stage C). Overall, local therapy with surgery and postoperative radiation therapy yields excellent results at 5 years with regard to both overall survival (93.1%) and local control (96.2%).36 Elective nodal irradiation is not generally recommended because the incidence of nodal relapse is <15%. Distant metastasis is uncommon (10%) even among patients presenting with locally advanced disease.
 CHEMOTHERAPY: NEOADJUVANT AND CONCOMITANT
CHEMOTHERAPY: NEOADJUVANT AND CONCOMITANT
Neoadjuvant chemotherapy (i.e., chemotherapy given before surgery) can reduce tumor volumes, which may allow a less extensive surgical resection than would be possible otherwise. Similarly, chemotherapy given before primary radiation therapy can also reduce tumor volumes and facilitate radiotherapy planning by increasing the distance between tumor borders and critical organ structures such as brain, chiasm, optic nerve, or spinal cord. Investigations are ongoing to determine whether the response (or lack of same) to neoadjuvant chemotherapy can help in the choice of definitive treatment. For example, if neoadjuvant chemotherapy produces a complete response, then primary radiation therapy, with or without chemotherapy, can be considered; a less-than-complete response would prompt surgical excision of the lesion followed by adjuvant radiation therapy.
Concurrent chemoradiation therapy can also be used for patients with medical conditions that preclude surgery if those patients have good performance status. Depending on the patient’s performance status and renal function, single-agent cisplatin or carboplatin can be used concurrently with external beam radiation for locally advanced, unresectable squamous cell carcinoma. Neoadjuvant chemotherapy or concurrent chemoradiation with etoposide and cisplatin or carboplatin can be used to treat sinonasal undifferentiated carcinoma, neuroendocrine carcinoma, or small cell carcinoma. Chemotherapy is not used routinely for esthesioneuroblastoma, and its role in the management of this disease is under investigation. Chemotherapy may have a role in the management of Kadish stage C disease, and although responses to chemotherapy have been reported, they are usually of limited duration.40 Concurrent chemotherapy during radiation may be considered for inoperable cases.
 PALLIATION
PALLIATION
Symptoms of incurable sinonasal cancer are particularly distressing. Multidisciplinary input is required even for very advanced cases, as palliation may involve limited surgery, radiation therapy, chemotherapy, investigational studies, or best supportive care. The morbidity of each modality must be balanced with the potential benefits in symptom control and improved quality of life. Particular attention is required to address the control of pain and discomfort as a first priority, and the impact of disfigurement and dysfunction, which is often present.
Chemotherapy can be given as single-agent therapy in investigational settings. If radiation therapy is given, large doses per fraction are usually given to reduce the duration of treatment. However, if concurrent chemotherapy is added, treatment with 2-Gy fractions should be considered to avoid severe acute effects. Radiation or chemotherapy is often effective in reducing tumor bulk and relieving symptoms associated with disfiguring masses, proptosis, discomfort or neuropathic pain, headache, epistaxis or other bleeding, nasal obstruction or discharge, and trismus.
 RADIATION THERAPY TECHNIQUES
RADIATION THERAPY TECHNIQUES
Tumors of the Nasal Cavity
Nasal Vestibule Tumors
Target Volumes
For small, well-differentiated lesions that are ≤1.5 cm in diameter, small fields with a 1- to 2-cm margin are appropriate. The initial target volume for all poorly differentiated tumors and well-differentiated primary tumors larger than 1.5 cm without palpable lymphadenopathy should include both nasal vestibules with at least 2- to 3-cm margins around the primary tumor (wider margins for infiltrative tumor) as well as bilateral facial, submandibular, and subdigastric nodes. When lymph node involvement is present at diagnosis, the lower neck is also irradiated. For larger nasal vestibule lesions, the lower half of the nose and the upper lip are treated as well as the regional lymphatics, including the facial lymphatics and upper neck nodes. For postoperative radiation therapy, the initial target volume includes the operative bed plus a 1- to 1.5-cm margin and the elective nodal regions.
Treatment Techniques
External Beam Radiation. Thin superficial nasal vestibule lesions can be treated with orthovoltage x-rays or electrons with skin bolus, whereas thicker lesions are generally treated with electrons. In definitive therapy, the target volume is treated to a dose of 66 to 70 Gy, with a small reduction in the treatment fields after 50 Gy to boost the dose to the gross disease. For patients presenting with palpable neck adenopathy, the entire neck is treated with at least a subclinical dose of 50 Gy, and the gross disease plus a 1- to 2-cm margin is then treated with an additional 16 to 20 Gy. A technique for external beam irradiation using electrons is illustrated in Figure 42.3. The patient lies supine, immobilized with the neck slightly flexed by using a custom mask to align the anterior surface of the maxilla parallel with the top of the couch. For larger nasal vestibule lesions, the lower half of the nose and the upper lip are treated with an anterior appositional field using 20-MeV electrons and 6-MV photons weighted 4 to 1. Skin collimation is used to minimize scatter irradiation to the eye and reduce the penumbra of the beam and reduce the field size required. Custom beeswax bolus material is prepared to allow a relatively flat surface contour onto which the electron beam is incident, avoiding inhomogeneity due to oblique incidence and surface irregularity. A bolus is also used to fill the nares to avoid the dose perturbation from the air cavity with electron beams. In photon treatments, the bolus is removed to spare the skin unless the overlying skin is involved. An intraoral Cerrobend-containing stent is used to displace the tongue posteriorly and partially shield the upper alveolar ridge.
When indicated, the right and left facial lymphatics are irradiated with appositional fields; these require an approximately 15-degree gantry rotation to the respective side with 6-MeV electron fields, each abutting the appositional primary lesion portal and the upper neck fields. The medial border is matched to the lateral border of the anterior primary field. The anterior border extends down from the oral commissure to the middle of the horizontal ramus of the mandible, whereas the posterior border extends from the upper edge of the anterior field to just above the angle of the mandible. The inferior border splits the horizontal ramus of the mandible and is matched to the upper neck field. The junctions are moved twice during the course of treatment to reduce dose heterogeneity. The submandibular and subdigastric nodes are treated with lateral parallel-opposed photon fields. For patients with involved nodes, these upper neck fields are matched inferiorly to an anterior portal treating the middle and lower neck nodes.
For definitive treatment, the external beam radiation schedule for lesions up to 1.5 cm in diameter for which a combination of electrons and photons is used is typically 50 Gy in 25 fractions followed by a boost of 10 to 16 Gy in 5 to 8 fractions (prescribed at the 90% isodose line). Larger lesions to be treated by external beam radiation alone receive 50 Gy in 25 fractions plus a boost of 16 to 20 Gy in 8 to 10 fractions. The schedule for elective nodal irradiation is 50 Gy in 25 fractions. Palpable nodes are given a boost to a total dose of 66 to 70 Gy in 33 to 35 fractions, depending on the size. For postoperative treatment, the volume is reduced off the undissected nodal regions after 50 Gy (25 fractions) to deliver an additional 6 Gy to the surgical bed. At 56 Gy, a final “cone down” is done to include a 4-Gy dose to the preoperative tumor bed, for a total dose of 60 Gy. If the excision was limited or positive margins are present, the final cone-down dose is 10 Gy for a total dose of 66 Gy.
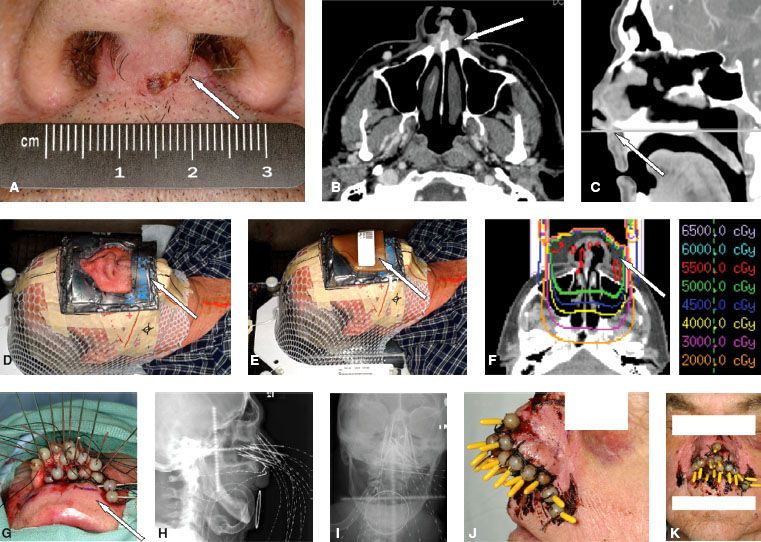
FIGURE 42.3. Nasal vestibule squamous cell carcinoma. A: Arrows indicate tumor expanding the columella. B,C: Arrows indicate invasion downward into the upper gingivobuccal sulcus on computed tomography (CT) imaging. D: Arrow shows setup for electron-beam phase of therapy with custom lead skin collimation in situ. E: Arrow shows the beeswax bolus in situ. F: Dosimetry to 50 Gy resulting from an appositional electron beam with beeswax bolus (arrow) to compensate for surface obliquity. The primary tumor, facial, and level II nodes were treated to 50 Gy. This was followed by 25 Gy administered by an interstitial low-dose-rate iridium needle implant at 0.55 Gy per hour. G: Dummy wires are inserted into each hollow tube. Each tube has a ball anchor at the distal end of the needles, which is pushed snugly against the skin and sutured to the skin. Note the placement of transverse “moustache” needles. H,I: Orthogonal x-ray (anteroposterior, lateral) films taken to document the placement of the needles. CT-based planning was performed. J,K: Live sources in situ.