Cancer of the Kidney, Renal Pelvis, and Ureter
 ANATOMY
ANATOMY
The kidneys are retroperitoneal structures located at the level between the 11th rib and the transverse process of the 3rd lumbar vertebral body. Usually, the right kidney is inferior to the right hepatic lobe and slightly more inferior than the left kidney. The renal axis runs parallel to the lateral margin of the psoas muscle. Each kidney is approximately 11 to 12 cm in length. The kidney is encased by a fibrous capsule and surrounded by perinephric fat, which is enveloped by Gerota’s fascia. At the renal hilus are the pelvis, ureter, renal artery, and vein. The organs adjacent to the right kidney include the liver superiorly, the duodenum and the vertebral bodies medially, and the transverse colon and small bowel anteriorly. On the left, the kidney abuts the spleen laterally; the stomach, pancreas, and vertebral bodies medially; and the small bowel and colon anteriorly.
The kidney consists of the cortex (glomeruli, convoluted tubules) and the medulla (Henle’s loops, collecting ducts, and pyramids of converging tubules). Each papilla opens in the minor calices, which unite in the major calices and drain into the renal pelvis. The caliceal collecting systems lie on the anteromedial surface of each kidney. The ureteropelvic junction is variable in position but serves as the landmark to separate the renal pelvis and the ureter. The ureters course posteriorly and inferiorly, paralleling the lateral border of the psoas muscle until they curve anteriorly to join the bladder at the trigone. The mucosal surfaces of the renal collecting tubules, calyces, renal pelvis, ureter, bladder, and urethra all have the same embryologic origin. The renal pelvis and ureter have the following layers: epithelium, subepithelial connective tissue, and muscularis, which is continuous with a connective tissue adventitial layer.
The lymphatics of the kidney and renal pelvis drain along the renal vessels. The right kidney drains predominantly into the paracaval and interaortocaval lymph nodes, and the left kidney drains exclusively to the paraaortic lymph nodes.1 The lymphatic drainage of the ureter is segmented and diffuse and may involve any of the renal hilar, abdominal para-aortic, paracaval, common iliac, internal iliac, or external iliac lymph nodes.
 EPIDEMIOLOGY AND RISK FACTORS
EPIDEMIOLOGY AND RISK FACTORS
The lesions discussed in this chapter are limited to adult renal cell carcinoma (RCC; e.g., hypernephroma, Grawitz’s tumor) and urothelial carcinoma of the renal pelvis and ureter. Lymphomas, primary retroperitoneal sarcomas, and Wilms’ tumors are discussed in Chapters 78, 83, and 85, respectively. Approximately 88% of solid renal masses are malignant, and the probability of malignancy is proportional to the size of the lesion.2 RCCs comprise 80% to 85% of primary kidney tumors, whereas urothelial (transitional cell) carcinomas of the renal pelvis account for 7% of kidney tumors.
Renal Cell Carcinoma
Globally in 2008, the male kidney cancer incidence and mortality age-standardized rate per 100,000 (ASR) was 11.8 and 4.1 in more developed areas, and 2.5 and 1.3 in less developed areas, respectively. The estimated new kidney cancer cases in males in developed countries were 111,100, with 43,000 deaths. In contrast, the female kidney cancer incidence and mortality ASR was 5.8 and 1.7 in more developed areas, and 1.4 and 0.8 in less developed areas, respectively.3
In the United States in 2011, the estimated number of new cases of kidney and renal pelvis cancer was 60,920, with 13,120 deaths.4 These figures represent approximately 4% of all new cancers and 2% of cancer-related deaths. The incidence of RCC has been increasing in the United States, whereas the size of primary RCCs has been gradually decreasing.5 This is partly because the increased use of abdominal computed tomography (CT) and ultrasound for nonmalignant medical illnesses has increased the number of incidental RCCs.
The median age of RCC diagnosis is 65 years, and males are affected more commonly than females, with a ratio of 1.5:1. Occupations associated with a higher risk of RCC are employment in the blast-furnace, coke-oven, or iron and steel industry, as well as exposure to asbestos, cadmium, dry-cleaning solvents, gasoline, and other petroleum products.6 In addition, several other environmental (e.g., exposure to thorium dioxide), hormonal (e.g., diethylstilbestrol), dietary (e.g., high total energy intake and fried meats increase the risk, whereas vegetables, fruits, and alcohol are protective), cellular, and genetic factors have been associated with the development of RCC.7–9
Long-term cigarette smoking is associated with an increased risk of developing RCC. Obesity, diabetes, hepatitis C, and hypertension are also associated with a higher relative risk for development of these tumors.10–12 Cytotoxic chemotherapy may predispose childhood cancer survivors to translocation RCC, bearing TFE3 or TFEB gene fusions.13
Acquired cystic kidney disease (ACKD), which occurs in up to 50% of patients on dialysis for >3 years, is associated with a 50-fold increased risk of developing RCC.14,15 ACKD-associated RCC is seen mostly in males, occurs approximately 20 years earlier than in the general population, and is frequently bilateral (9%) and multicentric (50%).15
Several inherited cancer syndromes affect the kidney: von Hippel-Lindau (VHL) disease, hereditary papillary renal cancer (HPRC), hereditary leiomyomatosis and renal cell carcinoma (HLRCC), Birt-Hogg-Dubé (BHD), and constitutional chromosome 3 translocation. VHL is autosomal dominant and is caused by germline mutations of the VHL tumor suppressor gene, located on chromosome 3p25–26. The VHL protein is involved in cell cycle regulation and angiogenesis. In patients with VHL disease, loss of the sole functioning VHL allele in somatic tissues causes a situation similar to hypoxia, with elevated levels of HIF-1alpha, despite the presence of normal oxygen tension.16 The renal manifestations of VHL are kidney cysts and clear cell RCC. The mean age onset for VHL associated clear cell RCC is 37 years, and periodic screening with magnetic resonance imaging (MRI) should start after the age of 10 years.17
HPRC is autosomal dominant with high penetrance and is characterized by multiple, bilateral, late-onset papillary RCCs. HLRCC is autosomal dominant with a predisposition to papillary type 2 RCC. BHD is autosomal dominant with incomplete penetrance and is associated with multiple chromophobe and clear cell RCCs, papillary RCCs, and oncocytomas. Constitutional chromosome 3 translocation is associated with multiple, bilateral clear cell RCCs.18 Autosomal dominant polycystic kidney disease does not appear to increase the incidence of RCC; however, the tumors are more often multicentric (28% vs. 6%), bilateral (12% vs. 1% to 5%), and sarcomatoid in type (33% vs. 1% to 5%) than in the general population.19
Renal Pelvis and Ureter Carcinoma
Urothelial carcinoma of the upper urinary tract accounts for 7% of all kidney tumors and 5% of all urothelial malignancies.20 The incidence of bilateral upper urinary tract tumors is 1.5% to 2% for synchronous and 6% to 8% for asynchronous presentations.21 Renal pelvis tumors are found two to three times more commonly in men than in women, and the peak incidence is in the fifth and sixth decades of life. Because the mucosal surfaces of the renal pelvis, ureter, and bladder have the same embryologic origin, many of the etiologic factors in renal pelvis and ureter tumors also apply to tumors of the urinary bladder. Urothelial carcinomas of the upper urinary tract tend to be multifocal owing to field cancerization, which may be caused by exposure of the urothelium to potential carcinogens. Urothelial tumors can also spread to urothelial structures that are either distal or proximal to the primary tumor and are referred to as drop metastases. About 40% to 50% of patients with upper urinary tract tumors will have a synchronous or metachronous bladder cancer.22,23
Cigarette smoking is the most important factor contributing to the overall incidence of urothelial cancer in Western countries. Patients with Lynch syndrome, an autosomal dominant genetic condition attributable to inherited mutations that impair DNA mismatch repair, have an increased risk of developing urinary tract cancer.24
Exposure to aristolochic acid has been associated with acute, near end-stage renal disease. Aristolochic acid is commonly found in the Aristolochiaceae family of plants commonly used in Chinese herbal medicine. A high incidence of cellular atypia and urothelial carcinoma of the renal pelvis, ureter, and bladder has been associated with aristolochic acid nephropathy.25 Arsenic-contaminated water has been associated with a high incidence of upper urinary tract urothelial carcinoma in Taiwan.26 Prolonged heavy phenacetin-containing analgesic use can lead to urothelial carcinomas of the renal pelvis, ureter, and bladder (which may be multiple and bilateral).27
Balkan endemic nephropathy (BEN) is a chronic tubulointerstitial disease of unknown etiology most commonly reported in southeastern Europe. A high frequency of urothelial atypia, occasionally progressing to tumors of the renal pelvis and urethra, but also involving the bladder, is associated with BEN.27
 NATURAL HISTORY
NATURAL HISTORY
Renal Cell Carcinoma
Primary renal cell tumors may spread by local infiltration through the renal capsule to involve the perinephric fat and Gerota’s fascia. The tumor may grow directly along the venous channels to the renal vein or vena cava. Lymph node metastases occur with an incidence of 9% to 27%, and most often involve the renal hilar, para-aortic, and paracaval lymph nodes.28,29 The renal vein is invaded by tumor in 21% of cases, and the inferior vena cava is invaded in as many as 4% of cases.30
Approximately 45% of patients with RCC have localized disease, 25% have regional disease, and about 30% have evidence of distant metastases at the time of diagnosis.29,31 Of patients with metastases, about 1% to 3% have solitary lesions.32 About half of the patients with RCC eventually develop metastatic disease.33
Among patients presenting with metastatic RCC, the sites of metastases include lung, bone, brain, liver, and adrenal gland. Patients with metastatic disease at diagnosis have an extremely poor prognosis, with an expected survival <5 years regardless of the site of metastasis.29,31
Renal Pelvis and Ureter Carcinoma
Upper urinary tract carcinoma is frequently a multifocal process. Patients with cancer at one site in the upper urinary tract are at significant risk for the development of tumors elsewhere along the urothelium. The probability of multifocal occurrence is greatest in patients with large tumors and those with carcinoma in situ. Ureteral tumors tend to occur in the distal third of the ureter.
Urothelial carcinoma of the upper urothelial tract may spread by direct extension, and by hematogenous and lymphatic metastases. Implantation of tumor cells in the bladder has been demonstrated, especially in previously traumatized areas. The incidence of lymph node metastases highly depends on the grade of the primary tumor. Low-grade tumors have a very low metastatic propensity. In a series of 94 patients, none of 43 low-grade tumors had lymph node metastases, compared with 3 of 22 grade 3 or 4 tumors.34 Lymph node metastases were reported in 9 of 26 patients selected to receive adjuvant radiotherapy.35
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
Renal Cell Carcinoma
Patients with RCC may present with an occult primary tumor, or with signs and symptoms attributable to a local mass or systemic paraneoplastic syndromes. Gross hematuria, palpable flank mass, and pain describe a classic triad that occurs only in 5% to 10% of patients.36,37 Indeed, a finding of the classic triad often suggests advanced disease with a poor prognosis. The most frequent symptom associated with RCC is hematuria, either gross or microscopic, when there is invasion of the collecting system.38 Scrotal varicoceles, mostly left-sided, are observed in as many as 11 % of men with RCC.39 Other symptoms include anemia, hepatic dysfunction in the absence of liver metastases (called Stauffer’s syndrome and attributable to a paraneoplastic elevation in alkaline phosphatase), secondary AA amyloidosis, fever, hypercalcemia, cachexia, erythrocytosis, thrombocytosis, and a syndrome resembling polymyalgia rhumatica.39,40,41–44,45 RCC presenting as an incidental mass on a diagnostic imaging study ordered for other purposes accounts for 61% of all diagnoses.46
A wide range of paraneoplastic syndromes has been associated with RCC. Parathyroidlike hormones, erythropoietin, renin, gonadotropins, placental lactogen, prolactin, enteroglucagon, insulinlike hormones, adrenocorticotropic hormone, and prostaglandins have been identified in patients with RCC.47,48
Renal Pelvis and Ureter Carcinoma
Gross or microscopic hematuria occurs in 70% to 95% of patients with renal pelvis or ureter tumors.20 The other less common symptoms include pain (8% to 40%), bladder irritation (5% to 10%), or other constitutional symptoms (5%). About 10% to 20% of patients may present with a flank mass secondary to tumor or hydronephrosis.
 DIAGNOSTIC WORKUP
DIAGNOSTIC WORKUP
Renal Cell Carcinoma
Renal masses are not uncommon, and most of them are benign. A central renal mass may suggest urothelial carcinoma; if so, urine cytology or ureteroscopy should be considered. Renal masses are frequently diagnosed as an incidental finding during abdominal imaging for metastatic evaluation of an unrelated malignancy or other disease.
An algorithm for the workup of renal masses has been proposed.49 If CT or ultrasound clearly identify the mass as a cyst, no further workup is necessary. If a solid lesion is identified, then tumor removal by nephrectomy should be considered. In the case of small lesions, a follow-up CT scan to evaluate potential growth of the mass may raise the suspicion of malignancy. The diagnostic and staging workup for RCC is given in Table 63.1. The diagnosis of RCC is established clinically and radiographically in most cases. Pathologic confirmation often is made at the time of nephrectomy.
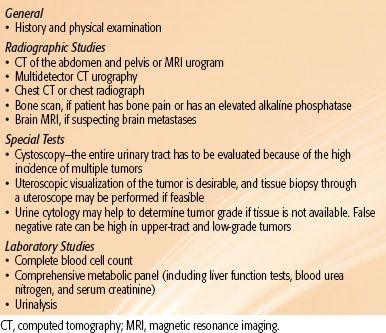
Once a radiographic diagnosis is made, a staging evaluation should be undertaken, which should include: a complete history and physical examination, complete blood count, and liver and kidney function tests. A metastatic workup should include a chest CT and an abdominal/pelvic CT (preferred) or abdominal MRI scan. Patients with symptoms suggestive of bone metastases and those with an elevated alkaline phosphatase level should undergo a bone scan. If metastatic lesions are detected, histologic confirmation should be made by biopsy of either the metastatic focus or the primary tumor. MRI can be valuable when evaluating the extent of involvement of the collecting system or inferior vena cava, or radiographic contrast cannot be administered. Renal arteriography is sometimes helpful in planning surgery.
Renal Pelvis and Ureter Carcinoma
The diagnostic workup for renal pelvis and ureter carcinoma is listed in Table 63.2. Staging includes a complete history and physical examination, complete blood count, and liver and kidney function tests. CT urography is now used to evaluate patients with renal pelvis carcinoma. CT or MRI of the abdomen and pelvis before and after contrast administration gives useful information regarding the possible extension of tumor outside the collecting system. Uteroscopic visualization of the tumor is desirable, and tissue biopsy through a uteroscope should be performed if feasible. Cystoscopy is very important because of the high incidence of multiple tumors. Urine cytology may help to determine tumor grade if tissue is not available; however, false-negative rates can be high for upper tract and low-grade tumors.
TABLE 63.1 DIAGNOSTIC WORKUP FOR RENAL CELL CARCINOMA
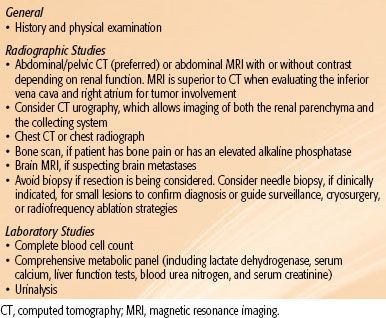
TABLE 63.2 DIAGNOSTIC WORKUP FOR RENAL PELVIS AND URETER CARCINOMA
 STAGING
STAGING
Renal Cell Carcinoma
The American Joint Committee on Cancer (AJCC) system is utilized to stage patients with RCC50 (Table 63.3). T1 and T2 cancers are limited to the kidney. T3 tumors extend into major veins or perinephric tissues, although not into the ipsilateral adrenal gland, and not beyond Gerota’s fascia. T4 tumors invade beyond Gerota’s fascia (including contiguous extension into the ipsilateral adrenal gland). Regional lymph node metastases may involve spread to the renal hilar, paracaval, aortic, or retroperitoneal drainage sites. Metastasis in regional lymph node(s) is classified as N1. This staging system underwent significant modifications in the 2010 AJCC 7th edition of its Cancer Staging Manual: T2 lesions were divided into T2a (>7 cm but ≤10 cm) and T2b (>10 cm); ipsilateral adrenal involvement was reclassified as T4 if contiguous invasion and M1 if not contiguous; renal vein involvement was reclassified as T3a; and nodal involvement was simplified to N0 versus N1.
TABLE 63.3 AMERICAN JOINT COMMITTEE ON CANCER 2010 STAGING CLASSIFICATION FOR KIDNEY TUMORS
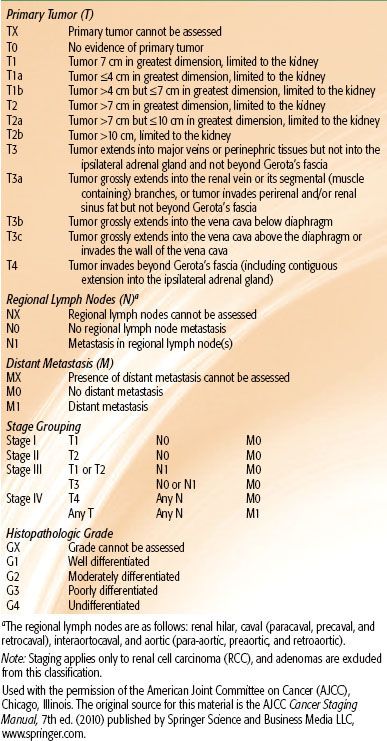
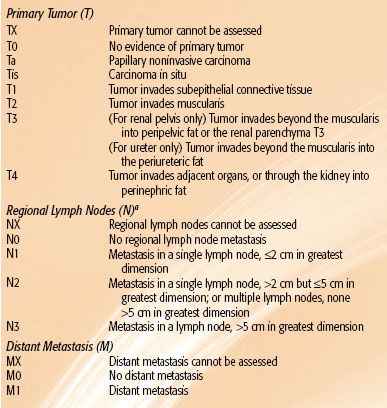
TABLE 63.4 AMERICAN JOINT COMMITTEE ON CANCER 2010 STAGING CLASSIFICATION FOR RENAL PELVIS AND URETER TUMORS
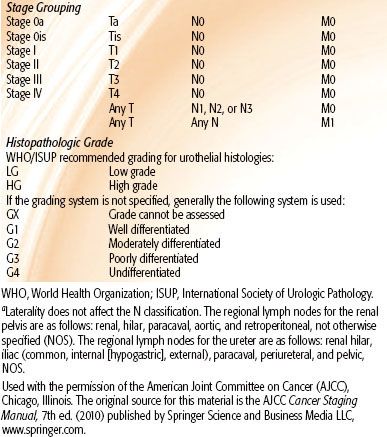
Renal Pelvis and Ureter Carcinoma
Tumors of the renal pelvis and ureter have a natural history that is not too dissimilar from that of other urothelial malignancies originating in the bladder. Their prognoses depend on tumor invasiveness and pathologic grade. The 2010 AJCC 7th edition staging classification for renal pelvis and ureter carcinoma is shown in Table 63.4.51
 PATHOLOGIC CLASSIFICATION
PATHOLOGIC CLASSIFICATION
Renal Cell Carcinoma
RCC is a group of malignancies arising from the epithelium of the renal tubules and comprises 90% of all malignancies in the kidney.18 The World Health Organization (WHO) classifies renal cell tumors as clear cell RCC, multilocular clear cell RCC, papillary RCC, chromophobe RCC, carcinoma of the collecting ducts of Bellini, renal medullary carcinoma, Xp11 translocation carcinomas, carcinoma associated with neuroblastoma, mucinous tubular and spindle cell carcinoma, papillary adenoma, oncocytoma, and RCC unclassified.18
Clear cell RCC is the most common (80% to 90% of tumors), followed by papillary RCC (10% to 15%) and chromophobe RCC (4% to 5%). Papillary RCC can be subdivided into type 1, which tends to be low grade and have a better prognosis, and type 2, which is the opposite. Renal medullary carcinoma is a very aggressive malignancy mostly associated with young black patients with sickle cell trait and, less commonly, sickle cell disease.52
Renal Pelvis and Ureter Carcinoma
More than 90% of malignant tumors arising from the renal pelvis and ureter are urothelial (also called transitional cell) carcinomas. The WHO classifies urothelial tumors as infiltrating urothelial carcinoma (with squamous differentiation, glandular differentiation, trophoblastic differentiation, nested variant, microcytic variant, micropapillary variant, lymphoepitheliomalike carcinoma, lymphomalike variant, plasmacytoid variant, sarcomatoid variant, with giant cells, and undifferentiated carcinoma).18 The most common histologic variant is squamous differentiation followed by glandular.18 Squamous cell carcinomas account for only 7% to 8% of renal pelvis and ureter carcinomas, and are often associated with chronic calculus disease and infection. Squamous cancers of the renal pelvis and ureter are often locally advanced and associated with a high local recurrence rate.53
 PROGNOSTIC FACTORS
PROGNOSTIC FACTORS
Renal Cell Carcinoma
The 5-year survival rate of patients with kidney cancer has doubled over the past 50 years, from 34% in 1954 to 70.9% in 2007.54,55 The stage at initial presentation remains the most important prognostic factor for RCC survival. Using the current 7th edition AJCC staging, the 5-year kidney cancer survival for stage I is 80.9%, 73.7% for stage II, 53.3% for stage III, and 8.2% for stage IV. Prognostic features for RCC are tumor, patient, and laboratory related. Tumor-related prognostic factors include stage, tumor size, tumor grade, histologic type, tumor necrosis, sarcomatoid transformation, and more than two sites of organ metastases. Patient-related factors include asymptomatic versus local symptoms versus systemic symptoms, weight loss, paraneoplastic syndromes, and an interval <1 year from original diagnosis to start of systemic therapy. Laboratory prognostic factors include thrombocytosis as well as elevated erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP).50,56
For patients with metastatic RCC, the following factors were predictive of survival in a retrospective study of 670 patients: low Karnofsky performance status (KPS; <80), high lactate dehydrogenase (LDH; >1.5 times upper limit of normal), low hemoglobin (less than the lower limit of normal), high “corrected” serum calcium (>10 mg/dL or 2.5 mmol/L), and absence of prior nephrectomy.57
Lymph node metastases are associated with increased rates of local recurrence and distant metastasis.36,58–59,60 Nuclear grade, sarcomatoid component, tumor size, stage, and the presence of tumor necrosis increase the likelihood of lymph node involvement.61 The overall risk of lymph node metastases is 20%.62,63 Patients with lymph node metastases in radical nephrectomy specimens have a local failure rate of 21%, compared with only 4% in patients without lymph node metastases (p = .0002).60 A select group of patients with solitary metastases may have a 5-year survival rate of 25% to 35%.64,65
Nuclear grade, after stage, is the most important prognostic feature of clear cell carcinoma. Fuhrman et al.66 developed a four-tier grading system that is based on nuclear and nucleolar size, shape, and content. Fuhrman’s grade is the most widely used grading system. Grade is also an independent prognostic factor for papillary RCC and chromophobe RCC especially when using standardized criteria.67 Worsening pathologic grade is associated with a poor 5-year disease-free survival.31,36
Papillary RCC has a 5-year survival rate that approaches 90% and metastasizes less frequently than clear cell RCC. The spindle cell or sarcomatoid variants of RCC are associated with statistically significant inferior 5-year survival rates, compared with pure clear, or clear and granular, histologic variants.31,36
Nuclear morphology is a strong predictor of tumor stage and prognosis.66 High nuclear grade is associated with an increased incidence of advanced tumor stage, lymph node involvement, distant metastases, renal vein involvement, tumor size, and perirenal fat involvement. In a series of 190 patients reported by Bretheau et al.,68 the 5-year actuarial survival rates of patients with grade I, II, III, and IV tumors were 76%, 72%, 51%, and 35%, respectively. Sarcomatoid differentiation carries a significantly poorer prognosis than the clear cell or granular cell subtypes. Almost half of patients with sarcomatoid RCC have bone metastases at presentation. The median survival time of patients with sarcomatoid renal cell cancer is only 6.6 months, compared with 19 months for other histologic types.69
Nomograms and algorithms have been described to facilitate the determination of cancer-free survival in patients with RCC. Based on 601 patients treated at Memorial Sloan-Kettering Cancer Center with radical nephrectomy, Kattan et al.70 used variables including patient symptoms (incidental, local, or systemic), histology (chromophobe, papillary, or conventional), tumor size, and pathologic stage to predict risk of recurrence after surgery. (Note: Kattan et al.70 uses the older 1997 staging and is available at the Memorial Sloan-Kettering Cancer Center website, http://nomograms.mskcc.org/Renal/PostSurgery.aspx.) Frank et al.71 from the Mayo Clinic developed a predictive algorithm based on 1,801 patients treated with radical nephrectomy. This system combines stage, size, grade, and necrosis (SSIGN) to predict patient survival. Finally, Zisman et al.72 from the University of California–Los Angeles (UCLA) have developed an algorithm that utilizes the AJCC TNM stage, Fuhrman’s grade, and Eastern Cooperative Oncology Group (ECOG) performance status to divide patients into low-, intermediate-, and high-risk groups. This model is also known as the UISS, or the UCLA integrated staging system.
Several molecular markers are being explored for their prognostic significance, including lack of B7H1 expression,73 immunohistochemical detection of carbonic anhydrase IX (CAIX),74 the proliferative marker Ki67,74 immunohistochemical expression of IMP3,75 and others.
Renal Pelvis and Ureter Carcinoma
The major prognostic factors in patients with renal pelvis or ureter carcinoma are initial stage and grade of the tumor. There is no significant difference in prognosis between urothelial carcinomas originating in the ureter compared to those arising in the renal pelvis.76 Using the current 7th edition AJCC staging, the 5-year renal pelvis and ureter cancer survival is as follows: stage 0a, 72.3%; stage 0is, 70.0%; stage I, 63.9%; stage II, 56.7%; stage III, 36.5%; and stage IV, 10.2%.
High-grade tumors are associated with a higher incidence of metastases and worse survival. Corrado et al.77 reported 5-year survival rates of 83%, 75%, 52%, and 0% for grades 1 through 4, respectively. These results are comparable to those described by Heney et al.,78 who reported 100% survival for grade 1, 81% for grade 2, and 0% for grade 3. Local recurrence was identified in 3 of 24 patients with grade 3 tumors. No survival differences were seen for patients with papillary versus solid tumors. In the series of Charbit et al.,23 lymph node metastases were seen exclusively in patients with high-grade tumors. Of tumor-related deaths, 90% were in patients with high-grade tumors. Hall et al.79 reported a retrospective series of 252 patients treated surgically for upper urinary tract urothelial cancers. Significant factors for recurrence included high tumor grade and advanced clinical stage. Older patients and patients treated with parenchymal-sparing surgical procedures had higher rates of recurrence. In their series of 77 patients, Akdogan et al.80 reported from a multivariate analysis that higher recurrence rates were associated with tumor location, higher grade, and advanced T-stage. Tumors in the ureters were more likely to recur than tumors involving the renal pelvis. In a series of 86 patients, Park et al.81 also reported a higher rate of recurrence in ureteral tumors, compared to those arising in the renal pelvis.
A prior history of bladder cancer has been reported to worsen the prognosis of patients with second urothelial cancers involving the upper tracts.80,82 From the Memorial Sloan-Kettering Cancer Center series of 129 patients, a multivariate analysis demonstrated that patients with advanced primary tumors and a prior history of bladder cancer were associated with worse disease-free survival.82
Flow cytometry may aid in estimating long-term prognosis. In a multivariate analysis, Corrado et al.77 demonstrated that although stage and grade were the most important prognostic indices, DNA pattern (diploid vs. nondiploid) and the number of lesions (unifocal vs. multifocal) identified at initial diagnosis also determined prognosis. Patients with diploid tumors had a 79% survival rate, compared with only 46% in patients with nondiploid tumors (p = .0003). Recent data suggest that hypermethylation of the promoter region of patients with urothelial cancers is associated with a worse prognosis. Tumors of the renal pelvis and ureters demonstrate hypermethylation in 94% of cases compared to 76% of similar-appearing tumors in the bladder (p <0.0001). Hypermethylation was also associated with higher tumor stage, tumor progression, and mortality.83
 GENERAL MANAGEMENT
GENERAL MANAGEMENT
Renal Cell Carcinoma
Surgery is the therapeutic foundation for the management of kidney cancer. Radiotherapy has an important and growing role in the palliative management of RCC. Although RCC is traditionally considered to be radioresistant, it has a clear dose response to radiation.84,85 As long as sufficient radiation dose is delivered to the tumor while respecting normal tissue dose constraints, RCC “radioresistance” can be overcome with modern techniques. At present, no effective, clinically proven, adjuvant therapy exists for RCC. The kidney cancer National Comprehensive Cancer Network (NCCN) guidelines (version 1.2013) offers the following surgical options depending on the stage86:
• Stage IA: Partial (preferred) or radical nephrectomy, active surveillance in selected patients, or ablative techniques for nonsurgical candidates
• Stage IB: Partial or radical nephrectomy
• Stage II and III: Radical nephrectomy
• Stage IV: Nephrectomy and surgical metastasectomy for a solitary metastasis if feasible, followed by systemic first-line therapy; cytoreductive nephrectomy if feasible when multiple metastatic sites, followed by systemic first-line therapy; or systemic first-line therapy if surgery is not feasible.
Active surveillance should be considered for patients with localized disease and short life expectancy, or significant comorbidities placing them at a surgical risk.
Surgery
A radical nephrectomy includes a perifascial resection of the kidney, perirenal fat, regional lymph nodes, and ipsilateral adrenal gland. It is the preferred treatment if the tumor extends into the inferior vena cava and usually requires the assistance of a cardiovascular surgeon if there is a caval or atrial thrombus. An experienced team should be involved in the context of a thrombus, as treatment-related mortality can reach 10%.86 This operation is undertaken by a thoracoabdominal or transabdominal approach. Improved preoperative assessment with CT can identify patients who have no significant risk of adrenal gland involvement.87 In 76% of cases, the adrenal gland can be spared at the time of surgery. The European Organisation for Research and Treatment of Cancer88 (EORTC) conducted a randomized trial of radical nephrectomy with or without an elective lymph node dissection, and there was no survival advantage between the two study groups. The incidence of unsuspected lymph node metastases was low (4%).88 Nevertheless, lymph node dissection does provide valuable prognostic information.
Radical nephrectomies should be avoided if nephron-sparing surgery is feasible for T1a and T1b renal tumors. In this setting, nephron-sparing surgery has shown equivalent outcomes to radical nephrectomy.89,90 Radical nephrectomy–induced chronic renal insufficiency is associated with an increased risk of cardiovascular death and death from any cause.91 For this reason, nephron-sparing surgery is preferred in T1a and T1b tumors. Nephron-sparing surgery is also preferred in patients with hereditary RCC to preserve renal function and decrease the risk of cardiovascular events.92 In a matched pair analysis of 164 patients undergoing nephron-sparing surgery at the Mayo Clinic, the disease-free survival was 79%, which compared favorably to 77% in patients undergoing radical nephrectomy.93 In 117 patients with renal tumors ≤4 cm undergoing partial nephrectomy at the Memorial Sloan-Kettering Cancer Center, the 5-year freedom from recurrence was 98.6% compared to 96.4% in a similar group of 173 patients undergoing radical nephrectomy. Compared to patients undergoing partial nephrectomy, those undergoing radical nephrectomy were at a higher risk of chronic renal insufficiency.94 There is some risk that sparing of the renal parenchyma may leave microscopic residual tumor or inadequately treat multifocal cancers.95–96,97 Bilateral RCC occurs in 2% to 3% of patients. In these patients, nephron-sparing surgery is an attractive option because bilateral radical nephrectomy sentences the patient to a lifetime of renal dialysis or the need for a renal transplant.
Following surgery, 20% to 30% of patients with localized tumors relapse, with a median time to relapse of 1 to 2 years, and most occurring within 3 years.86 Although at present there is no role for adjuvant therapy after surgery, several recent trials are exploring the role of targeted therapy.
Patients who have local symptoms, such as hematuria, pain, hypertension, or other paraneoplastic syndromes, may benefit from palliative nephrectomy. Spontaneous regression of metastatic renal cell cancer after nephrectomy has been reported. In an extensive literature review, the incidence of regression of metastatic foci induced by nephrectomy was 0.8% (4 of 474 patients).33 Cytoreductive surgery performed to prolong or increase the response of metastatic disease in response to systemic therapy may be beneficial.98,99–100,101
Thermal Ablation
Recently, the minimally invasive ablative technologies of cryoablation and radiofrequency ablation (RFA) have emerged as potential treatment options for clinically localized RCC, especially in the elderly or in patients with a solitary kidney or comorbidities impeding surgery. Long-term oncologic efficacy for these modalities remains to be established. The most favorable lesions for this approach are <4 cm and in the periphery of the kidney. Relative contraindications for RFA and cryoablation include distant metastases, tumors >5 cm, tumors in the hilum or central collecting system, and life expectancy <1 year. A meta-analysis comparing cryoablation and RFA suggested that cryoablation results in fewer re-treatments and improved local tumor control, and that cryoablation may be associated with a lower risk of metastatic progression compared with RFA.101
Renal Stereotactic Body Radiotherapy
Renal stereotactic body radiotherapy (SBRT) as an alternative to thermal ablation is in its infancy. Beitler et al.102 identified nine RCC patients with primary kidney tumors who received SBRT to 8 Gy é five fractions. The tumors ranged from 1.5 to 10 cm in diameter. With a median follow up of 26.7 months, four of the nine patients were alive. One of the nine patients failed in the ipsilateral kidney, away from the initial radiation treatment volume, while the other eight patients had durable local control. One patient had a radiation injury to the stomach resulting in a 30 lb weight loss in 1 month.102 Wersall et al.103 reported eight patients treated with stereotactic radiotherapy to medically inoperable primary tumors using a radiation treatment schedule of 8 Gy é five fractions. Seven of the eight patients were locally controlled, and the median survival exceeded 58 months.103 Ponsky et al.104 also reported their initial experience on three patients treated with 4 Gy é four fractions. Two of three patients had evidence of residual disease at these low doses at the time of partial nephrectomy 8 weeks later.104
Svedman et al.105 reported their SBRT experience with seven patients who were treated for metastases from a malignant kidney to its contralateral counterpart. Dose/fractionation schedules varied between 10 Gy é three fractions and 10 Gy é four fractions depending on target location and size. Local control was obtained in six of seven patients and regained after retreatment in the one patient whose lesion progressed. Side effects were generally mild, and in five of the seven patients kidney function remained unaffected after treatment. In two patients, the creatinine levels remained moderately elevated but dialysis was not required.105
TABLE 63.5 SURVIVAL AFTER NEPHRECTOMY OR NEOADJUVANT RADIOTHERAPY AND NEPHRECTOMY FOR RENAL CELL CARCINOMA, PROSPECTIVE RANDOMIZED TRIALS





