Cancers of the lips, oral cavity, oropharynx, nasopharynx, hypopharynx, larynx, nasal and paranasal sinuses, neck, ear, and salivary glands, as well as those of regional soft tissues and supporting bones, are customarily grouped as cancers of the head and neck. Consequently, a large variety of neoplasms may be encountered, including sarcomas of any type, adenocarcinomas of major or minor salivary gland origin, and squamous cell carcinomas of the mucosal lining of the upper aerodigestive tract. Squamous cell carcinoma (SCCHN) is the most common head and neck cancer, occurring in over 90% of cases. Thus, the term head and neck cancer is most often equated with squamous cell carcinoma.
Estimates provided by the American Cancer Society suggest that more than 48,000 new cases of head and neck cancer were diagnosed in the United States in 2008, and approximately 30% of these patients will die of their disease ( ). SCCHN represents approximately 5% of cancers in men and 2% of cancers in women. Even though more than 70% of patients present with disease apparently confined to the head and neck region, 5-year survival rates for whites and blacks are 56% and 34%, respectively, for cancers of the oral cavity and pharynx. Because disease of the larynx tends to become apparent at an earlier stage, 5-year survival rates at this site are slightly better—66% and 53% for whites and blacks, respectively. Squamous cell carcinoma of the aerodigestive tract is directly related to tobacco and alcohol use. Tobacco is the more important of these two substances, but they appear to be synergistic. Individuals who consume substantial quantities of both tobacco and alcohol are 20 times more likely to develop SCCHN than nonusers of these substances ( ). The cessation of both alcohol and tobacco consumption is associated with a decreased subsequent risk for all upper aerodigestive neoplasms.
Additional risk factors for cancer of the upper aerodigestive tract include exposure to ionizing radiation and occupational and environmental exposure to carcinogens other than tobacco. For instance, workers involved in plastic fabrication, metal working, and textile processing, as well as individuals occupationally or environmentally exposed to asbestos, show an increased incidence of head and neck cancers. Similarly, nasal and sinus cancers are more common in workers in the furniture industry exposed to the dust of hardwoods. Given that the entire mucosa of the upper aerodigestive tract is exposed in such instances, multicentric lesions are not uncommon and may occur either simultaneously or sequentially. With time, the risk for a second, related cancer can exceed that for direct recurrence of the original tumor. The concept that a focus of cancer is but part of a generally “sick mucosa” is essential to the management of these tumors. The term “field cancerization” is often used to describe this phenomenon.
Viral etiologies are well described in head and neck cancer. Indeed it is accepted now that more than 50% of oropharyngeal cancers are linked to human papillomavirus (HPV) infection, in particular HPV-16. These HPV tumors seem to respond better to treatment than other oropharyngeal cancers and thus have a favorable prognosis ( ). Nasopharyngeal cancer is also linked to Epstein-Barr virus (EBV) and is endemic in various parts of the world.
Histology
EPITHELIAL LESIONS
Two types of premalignant epithelial lesions are recognized based on their clinical appearance: leukoplakia and erythroplasia. Leukoplakia, commonly referred to as smoker’s keratosis, is marked by raised, white patches that microscopically represent hyperkeratosis or parakeratosis with varying degrees of atypia and associated mucosal atrophy. The atypia can be graded, in a manner similar to changes seen in the uterine cervix, as mild, moderate, or severe, based on the degree of mucosal involvement by disorderly cells with nuclear abnormalities. There are many causes of leukoplakia. Although the majority of such lesions are innocent and secondary to chronic irritation such as tobacco exposure or dental trauma, 3% to 15% of persistent raised white lesions within the oral cavity and oropharynx represent premalignant dysplastic leukoplakia. Therefore, even leukoplakia on mucous membranes should be considered dysplastic and potentially premalignant until proved otherwise. These lesions always contain some degree of cellular atypia, the severity of which is impossible to determine without biopsy.
Erythroplasia is a mucosal abnormality of greater concern. Visually, the lesions consist of superficial or slightly depressed areas of denuded mucosa where cellular atypia has reached the mucosal surface; they appear red and velvet-like. It should be assumed that they represent at least carcinoma in situ, because over 80% of such lesions at the time of biopsy exhibit pleomorphic squamous cells with full-thickness atypia of the mucosa. With in situ lesions the basement membrane separating the mucosa from the underlying stroma is intact, but early microinvasion of the basement membrane with extension into the adjacent stroma can also clinically present as erythroplasia. Locations at high risk for erythroplasia include the central floor of the mouth, the ventrolateral surface of the tongue, the buccal mucosa, the anterior tonsillar pillars, and the soft palate.
In the vast majority (90%) of cases, malignant neoplasms of the head and neck arise from the surface epithelium and are therefore squamous cell carcinomas or one of its many variants, including undifferentiated carcinoma, lymphoepithelioma, spindle cell carcinoma, and verrucous carcinoma ( Table 4.1 ). Malignant transformation is accompanied by varying degrees of differentiation of the squamous cells, ranging from well- and moderately well differentiated to poorly differentiated and anaplastic lesions. As a general rule, less differentiated tumors have a higher incidence of infiltration into neighboring glands, muscles, and loose connective tissue, as well as regional spread to lymph nodes of the neck. Bone, cartilage, ligaments, vessels, and nerves, however, offer higher resistance to tumor infiltration. In advanced cancers the morphologic picture may vary considerably in different regions of the lesion. Therefore, surface biopsy specimens may not necessarily represent the entire lesion.
| Tumor Type | Typical Location |
|---|---|
| Premalignant Lesions | |
| Leukoplakia | Floor of mouth |
| Ventral and lateral surfaces of tongue | |
| Buccal space | |
| Erythroplasia | Same as for leukoplakia |
| Malignant Lesions | |
| Squamous cell carcinoma | |
| Well differentiated | All sites, especially oral cavity |
| Moderately well differentiated | All sites |
| Poorly differentiated | All sites |
| Verrucous carcinoma (variant of well differentiated) | Oral cavity |
| Spindle cell carcinoma * | All sites |
| Undifferentiated carcinoma (includes lymphoepithelioma) | Nasopharynx and Waldeyer’s ring |
| Nonkeratinizing epithelial carcinoma | Nasopharynx and Waldeyer’s ring |
* Spindle cell carcinoma is also known as pleomorphic carcinoma, pseudosarcoma, and sarcomatoid squamous cell carcinoma.
Salivary Gland Tumors
Salivary gland neoplasms may arise from any of the paired major salivary glands—the parotid, submandibular, or sublingual glands—or from one of the many minor salivary glands present throughout the mucosal surfaces of the upper aerodigestive system. These tumors may be benign or malignant, and the probability that a given lesion is malignant varies among sites ( Table 4.2 ). Parotid gland tumors, which are relatively common, are usually benign, pleomorphic adenomas being the most frequently encountered. Other benign salivary tumors include papillary cystadenoma lymphomatosum (Warthin’s tumor or adenolymphoma) and benign lymphoepithelial lesions, such as Godwin’s tumor. Malignant parotid tumors are less common, but a spectrum of malignant histologic types may be encountered ( Table 4.3 ). Histologic classification of salivary gland cancers is difficult because of the wide extent of morphologic variation within each tumor type ( ). The same spectrum of benign and malignant histologic types of neoplasms as are found in the parotid gland may be encountered in the other major salivary glands and the minor salivary glands.
| Type | Location | Type | Frequency (%) |
|---|---|---|---|
| Major | Parotid | Benign | 75 |
| Malignant | 25 | ||
| Submandibular | Benign | 40 | |
| Malignant | 60 | ||
| Sublingual | Benign | 15 | |
| Malignant | 85 | ||
| Minor | Throughout mucous membranes of upper aerodigestive tract | Benign | 45 |
| Malignant | 55 |
| Type | Frequency (%) |
|---|---|
| Mucoepidermoid carcinoma | 29 |
| High grade (26%) | |
| Low grade (74%) | |
| Adenocarcinoma | 14 |
| Adenoid cystic carcinoma | 13 |
| Malignant mixed tumor | 13 |
| Undifferentiated carcinoma | 11 |
| Squamous cell carcinoma | 8 |
| Acinic cell carcinoma | 6 |
| Malignant lymphoma | 2 |
| Melanoma | <1 |
| Other | 3 |
Less Frequently Encountered Neoplasms
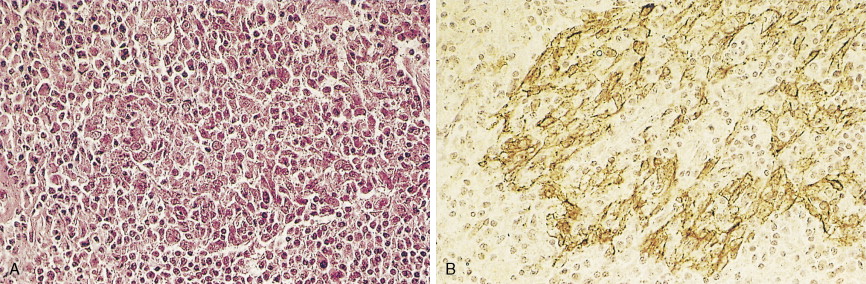
Esthesioneuroblastomas (olfactory neuroblastomas) arise from the respiratory epithelium about the cribriform plate and nasal septum. They may occur at any age but are most commonly seen in the second and third decades. Histologically, these lesions are composed of rather uniform small blue cells of neuroectodermal origin. They may be confused with undifferentiated carcinoma, undifferentiated lymphoma, or rhabdomyosarcoma. (See also discussions and illustrations of PNET in Chapter 12 and non-CNS PNET in Chapter 16 .)
Chemodectomas or non-chromaffin paragangliomas are a group of uncommon, slow-growing neoplasms that may originate wherever glomus bodies are found. Most often they arise from the carotid artery and temporal bone and only rarely from the orbit, nasopharynx, larynx, nasal cavity, paranasal sinuses, tongue, jaw, and trachea. In 10% to 20% of cases, glomus tumors may occur in multiple sites, especially in families with a history of this tumor. The histologic picture of these benign lesions is marked by nests of epithelioid cells within stroma containing thin-walled blood vessels and nonmyelinated nerve fibers; the relative amounts of epithelioid and vascular tissue may vary. The criterion of malignancy is based on the clinical progress of the disease rather than the histologic appearance. Metastases are infrequent (<5% of cases). Endocrine activity has been reported, and serotonin has been identified on histochemical staining, thus confirming the tumor’s origin from primitive neuroectodermal cells.
Extramedullary plasmacytomas may occur throughout the body, but 80% of these malignancies are located in the head and neck, most notably in the upper air passages and associated structures: nasopharynx, tonsil, maxillary sinus, nasal vestibule, and trachea. The majority of patients are in their sixth to eighth decades. Up to 25% of patients present with cervical lymph node involvement, and 10% to 40% eventually progress to multiple myeloma. Histologically identical to the osseous form of plasmacytoma, these extramedullary tumors consist of sheets and aggregates of plasma cells.
Inverting papillomas are low-grade neoplasms that are generally considered to be benign. Most frequently affecting the nasal cavity and paranasal sinuses, they are clinically aggressive lesions characterized by extensive bone destruction, intracranial extension, and multiple recurrences. There is an association with squamous cell carcinoma in 10% to 15% of cases. The histologic picture is that of a papilloma that is growing into the stroma rather than outward.
Mucosal melanomas of the head and neck represent 0.5% to 2% of all malignant melanomas. The most common location within the head and neck is the nasal cavity, where melanomas represent up to 18% of all malignant tumors. Though varying in their gross appearance, they are usually solid, polypoid lesions about 3.5 cm in diameter. Approximately one third are amelanotic. The histologic picture is similar to that of cutaneous melanoma, although lymphocytic infiltration is rare.
Midline lethal nonhealing granuloma is a nonspecific term encompassing a variety of histologic and clinical entities that lead to progressive destruction of the nose, paranasal sinuses, hard palate, and contiguous structures. Midline lethal granuloma has been subdivided into three different entities: midline malignant (polymorphic) reticulosis, malignant lymphoma (usually diffuse large cell lymphoma), and Wegener’s granulomatosis. (See “ Staging of Head and Neck Cancers ” and “ Clinical Manifestations .”)
Molecular Biology
The molecular biology of head and neck cancers has not been defined systematically except for lesions arising from squamous epithelium. Efforts are being made to determine the genetic events associated with, and perhaps responsible for, the conversion of normal squamous epithelium to dysplastic leukoplakia or erythroplasia, to carcinoma in situ, and finally, to invasive cancer. These studies attempt to define environmental and genetic differences between patients with isolated mucosal lesions and those with multiple neoplastic abnormalities, so-called “field carcinogenesis.” Related studies are in progress to define the incidence of known oncogenes and tumor suppressor genes in invasive epithelial tumors and to determine their influence on clinical events such as tumor growth rate, potential for metastatic spread, response to therapy, and prognosis after treatment.
The most commonly deleted chromosomal region in SCCHN is 9p21. This region encodes the tumor suppressor p16 (INK4A/MTS-1/CDKN2A), a cyclin-dependent kinase inhibitor ( ). The loss of p16 is seen early in the evolution of SCCHN, suggesting it may play a part in the early carcinogenic process. Additionally, mutations of the tumor suppressor gene TP53 are also common both in malignant and premalignant mucosal lesions of the head and neck, as well as the histologically normal mucosa of patients with treated squamous cell cancers of the oral cavity and oropharynx ( ).
Mutations of TP53 within invasive head and neck squamous carcinomas are frequent. Loss of p53 function due to mutations results in a progression from premalignant lesions to invasive cancer, and approximately half of all SCCHNs contain a mutation of the TP53 gene located at 17p13. Gene amplifications of known oncogenes, though less frequently encountered than TP53 mutations, have also been associated with a poor prognosis.
Cytogenetic evidence for genetic instability and gene amplification of DNA markers on chromosome 11 band q13 has been identified in many invasive head and neck cancers. Moreover, the presence of 11q13 rearrangements has been associated with poor clinical outcome and younger patient age at presentation. This is of interest, in that amplification of several oncogenes located in 11q13, such as int -2, bcl -1, prad -1, and cyclin D1, has been reported in this cancer. Amplification of cyclin D1 in particular has been identified in up to 20% of head and neck cancers and is independently associated with tobacco exposure and poor prognosis ( ).
Several studies have reported overexpression of epidermal growth factor receptor (EGFR) and transforming growth factor α in many invasive head and neck squamous cancers. These findings suggest growth stimulation in these tumors by autocrine or paracrine mechanisms, and overexpression of EGFR family members is associated with a worse prognosis ( ). EGFR inhibitors have been developed and are used in treating head and neck cancer. Whereas tobacco and alcohol environmentally induce the majority of head and neck cancers, viral infection has been suggested as contributory in many patients. HPV-16 has been detected by polymerase chain reaction in more than 50% of oropharyngeal cancers ( ). The molecular profile of HPV-positive tumors is different from the HPV-negative tumors, reflecting what is probably a different disease. EBV DNA has similarly been detected in most nasopharyngeal carcinomas and in some non-nasopharyngeal squamous head and neck cancers ( ). In addition, human herpesvirus 6, previously isolated from patients with lymphoproliferative disorders and acquired immunodeficiency syndrome, can be detected in up to 80% of oral SCCHNs ( ). Oral carcinogenesis is probably a multistep process with a multifactorial etiology. Some oncogenic viruses, perhaps acting synergistically with chemical carcinogens and a patient’s underlying genetic predisposition, could facilitate the transformation process to carcinoma.
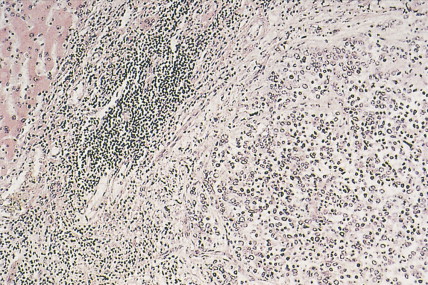
Staging of Head and Neck Cancers
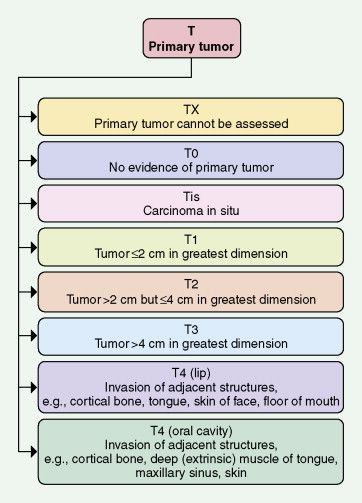
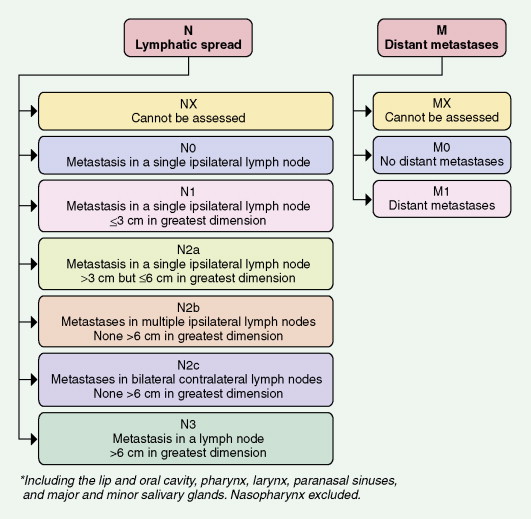
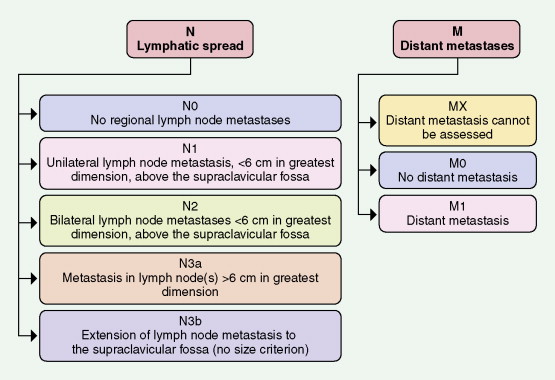
Tumors of the head and neck are staged according to a site-specific TNM system (see Figs. 4.26, 4.27, 4.28 ) ( ). The multiple TNM combinations are ultimately grouped into four stages ( Table 4.4 ), each having a progressively lower survival rate. At best, this anatomically dependent system provides a rapid estimate of a patient’s prognosis, facilitates the formation of a treatment plan, and assures the uniform reporting of treatment outcomes. Unfortunately, TNM staging is complex, and the concordance in staging between any two physicians is low. The greatest utility of this imperfect measure of disease potential is restricted to the definition of patients with limited lesions and a good prognosis.
| Stage | T (Primary Tumor) | N (Regional Nodes) | M (Metastases) |
|---|---|---|---|
| 0 | Tis | N0 | M0 |
| I | T1 | N0 | M0 |
| II | T2 | N0 | M0 |
| III | T3 | N0 | M0 |
| T1, T2, or T3 | N1 | M0 | |
| IV | T1, T2, or T3 | N2 or N3 | M0 |
| T4 | Any N | M0 | |
| Any T | Any N | M1 |
Recommended staging procedures for determining a patient’s TNM classification include direct and indirect (mirror or fiberoptic) inspection and palpation of all accessible mucosal surfaces in the head and neck and radiologic investigation. Radiographs or radionuclide scanning of the mandible may be appropriate in selected cases; however, computed tomography (CT) or magnetic resonance imaging (MRI) is standard for exact anatomic localization of primary lesions and facilitates quantification of regional lymph node involvement and parapharyngeal spread of disease. In addition, CT scanning and MRI may be helpful in identifying the site of an occult primary lesion in patients with a solitary neck mass.
There is considerable debate about the necessity for “triple endoscopy” in staging patients with head and neck cancers. The addition of bronchoscopy and esophagoscopy to direct laryngoscopy may be appropriate for patients with tumors of the oropharynx, hypopharynx, or larynx that are inadequately evaluated by indirect means. The time, risk, and expense of these additional procedures are negligible given the incidence (as high as 5% in some series) for multiple synchronous primary tumors of the head and neck, lung, and esophagus. On the other hand, the value of triple endoscopy remains controversial in most patients with localized lesions of the oral cavity that can adequately be staged by indirect, noninvasive means ( ). Triple endoscopy is particularly important for patients with intraoral lesions associated with diffuse mucosal abnormalities such as leukoplakia or erythroplasia, who are more likely to have multiple primary tumors.
Clinical Manifestations
The clinical manifestations and natural history of head and neck cancers depend on the site from which they arise. Thus, considerable variability exists and certain characteristic features for the several primary anatomic locations are discussed separately.
Occasionally, patients present with solitary, asymptomatic cervical adenopathy, usually in the upper neck. The primary cancer may be asymptomatic but will usually be discovered on a detailed head and neck examination. Pain may be the first manifestation, usually representing more locally advanced disease. Surprisingly, some patients have large lesions that cause minimal, if any, symptoms.
Lips
Cancer of the lips accounts for 15% of all head and neck cancers. In 95% of cases, the lesion occurs on the lower lip and may involve the vermilion and mucosal surfaces. It typically presents as a recurrent scab or a persistent or slow-growing sore, blister, or ulcer. Because of its location, it is discovered early and lymphatic spread of squamous cell carcinoma to submental and submaxillary lymph nodes occurs in only 5% to 10% of patients. The approximate 5-year survival after standard treatment is greater than 90% for all stages, because most lesions are quite limited. Survival falls to 65% for patients with locally advanced lesions of the upper lip or with regional adenopathy.
Oral Cavity
The oral cavity encompasses the floor of the mouth, oral tongue, buccal mucosa, gingiva, retromolar trigone, and hard palate. Cancers involving these structures account for 20% of all head and neck malignancies. Typically, cancers of the oral cavity present as asymptomatic lesions noted on routine dental examination, as painful ulcers with or without referred otalgia or in association with ill-fitting dentures, difficulty in swallowing or chewing, or altered speech. The principal sites of involvement are the ventrolateral surface of the tongue and the floor of the mouth. In as many as 40% of patients, adenopathy may be present in the upper jugular and submandibular lymph nodes. Carcinomas of the middle and posterior thirds of the mobile tongue have the greatest metastatic potential.
Nasopharynx
Cancers of the nasopharynx are unique in their histology (frequently an undifferentiated or nonkeratinizing epithelial cancer), biology, and epidemiology. They are minimally associated with tobacco use but are 25 times more prevalent in patients of southern Chinese descent. The EBV serum titer is typically elevated, and molecular biologic techniques reveal EBV incorporation into the genome in the majority of tumors. These lesions occur in a younger population than patients with SCCHN at other sites and are best known for their propensity for early spread to regional and distant sites and their relative sensitivity to chemotherapy and radiation therapy.
Nasopharyngeal cancers of epithelial origin typically arise from the lateral pharyngeal wall adjacent to the orifice of the eustachian tube. Cancers similar to those of the nasopharynx may occur in other areas of Waldeyer’s ring (a ring of lymphoid tissue encircling the nasopharynx and oropharynx). Although many of these lesions are asymptomatic and found incidentally during evaluation of an upper neck mass of unknown origin, limited lesions may present in association with epistaxis, nasal obstruction, or unilateral hearing loss due to eustachian tube obstruction. More advanced lesions may present with headache, direct osseous involvement of the parasphenoid region, or multiple cranial neuropathies due to tumor extension behind the sphenoid (cranial nerves II through VI) or along the base of the skull about the hypoglossal foramen (cranial nerves XI and XII). The metastatic potential of these lesions is well known. Malignant adenopathy involving the retropharynx and lateral pharyngeal wall is present in 80% of patients at presentation. Bilateral adenopathy is common, and involvement of the posterior cervical chain is characteristic.
| Stage | T (Primary Tumor) | N (Regional Nodes) | M (Metastases) |
|---|---|---|---|
| 0 | Tis | N0 | M0 |
| I | T1 | N0 | M0 |
| IIA | T2a | N0 | M0 |
| IIB | T1, T2a, or T2b | N1 | M0 |
| T2b | N0 | M0 | |
| III | T1, T2a, or T2b | N2 | M0 |
| T3 | N0, N1 or N2 | M0 | |
| IVA | T4 | N0, N1, or N2 | M0 |
| IVB | Any T | N3 | M0 |
| IVC | Any T | Any N | M1 |
The nasopharynx may also be involved in nonepithelial malignancies, including sarcomas, minor salivary gland carcinomas, esthesioneuroblastomas, or other neuroectodermal lesions, and unusual tumors such as angiofibromas. Presenting features may mimic those of the more common squamous cell carcinoma, and adequate biopsy specimens must therefore be obtained in all cases.
Oropharynx
Accounting for 10% of all head and neck malignancies, oropharyngeal cancers may involve the tonsillar fossa or pillars, soft palate, base of the tongue, or lateral or posterior pharyngeal wall. They may be clinically silent until they present as advanced tumors with local pain, odynophagia, dysphagia, referred otalgia, or trismus. Tonsillar and base-of-tongue carcinomas have the greatest metastatic potential, with upper jugular (subdigastric or jugulodigastric) lymphadenopathy present in up to 70% of cases. These areas therefore require careful visual inspection and digital palpation during evaluation of a patient with either pharyngeal symptoms consistent with carcinoma or an upper neck mass of unknown origin. CT scanning is standard for tumors presenting at this site.
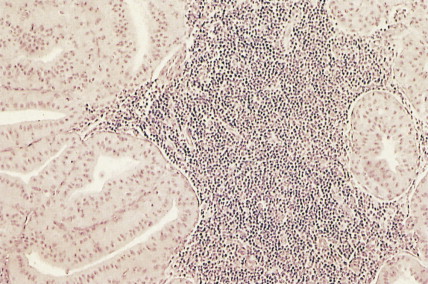
The oropharynx is rich in lymphatic tissue belonging to Waldeyer’s ring. Occasionally, primary Waldeyer’s ring lymphomas arise, typically non-Hodgkin’s lymphomas of the diffuse large cell type. These tumors are often difficult to distinguish from epithelial tumors on clinical grounds. After histologic and immunologic confirmation, staging procedures for a Waldeyer’s ring lymphoma should include bone marrow aspiration and biopsy; CT scan of the chest, abdomen, and pelvis; and, given the high incidence of concurrent gastric involvement, radiographic or endoscopic visualization of the stomach.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree