There are no capillary lymphatics in the epithelium. The tumor must penetrate the lamina propria before lymphatic invasion can occur. One can predict the richness of the capillary network in a given head and neck site by the relative incidence of lymph node metastases at presentation. The nasopharynx and pyriform sinus have the most profuse capillary lymphatic networks. The paranasal sinuses, middle ear, and vocal cords have few or no capillary lymphatics. Muscle and fat contain few capillary lymphatics, as do bone and cartilage within the periosteum or perichondrium. There are no capillary lymphatics in the eye, and few in the orbit.
Most head and neck malignant neoplasms arise from the surface epithelium and are squamous cell carcinoma (SCC) or one of its variants, including lymphoepithelioma, spindle cell carcinoma, verrucous carcinoma, and undifferentiated carcinoma. Lymphomas and a wide variety of other malignant and benign neoplasms make up the remaining cases.14–16
Lymphoepithelioma is an SCC with a lymphoid stroma and occurs in the nasopharynx, tonsillar fossa, and base of tongue; it may also occur in the salivary glands. In the spindle cell variant, there is a spindle cell component that resembles sarcoma intermixed with SCC. It is generally managed like other high-grade SCCs. Verrucous carcinoma is a low-grade SCC found most often in the oral cavity, particularly on the gingiva and buccal mucosa. It usually has an indolent growth pattern and is often associated with the chronic use of snuff or chewing tobacco.
Small cell neuroendocrine carcinoma occurs rarely throughout the head and neck. Upper aerodigestive tract lymphomas almost always show a diffuse non-Hodgkin histologic pattern.
Patterns of Spread
Primary Lesion
SCCs usually begin as surface lesions. Superficial tumors arising in the Waldeyer ring may be difficult to distinguish from normal lymphoid tissue. Very early surface lesions may show only erythema and a slightly elevated mucosa.
Spread is dictated by local anatomy, and thus varies by each site. Muscle invasion is common, and the tumor may spread along muscle or fascial planes a surprising distance from the palpable or visible lesion. The tumor may attach early to the periosteum or perichondrium, but bone or cartilage invasion is usually a late event. Bone and cartilage usually act as a barrier to spread; the tumor that encounters these structures will often be diverted and spread along a path of less resistance. Slow-growing gingival neoplasms may produce a smooth pressure defect of the underlying bone without bone invasion.
Tumor extension into the parapharyngeal space allows superior or inferior spread from the skull base to the low neck.
Spread inside the lumen of the sublingual, submandibular, and parotid gland ducts is uncommon. The nasolacrimal duct, however, is often invaded in ethmoid sinus and nasal carcinomas.
Perineural invasion (PNI) is observed in SCCs as well as salivary gland tumors, especially adenoid cystic carcinomas. When advanced, PNI may produce neurologic symptoms and is associated with a poorer rate of local control.17 Tumors may track along a nerve to the skull base and central nervous system (CNS) or peripherally.
Vascular space invasion is associated with an increased risk for regional and distant metastases.
Lymphatic Spread
The differentiation of the tumor, the size of the primary lesion, the presence of vascular space invasion, and the density of capillary lymphatics predict the risk of lymph node metastasis. Recurrent lesions have an increased risk. Histology also impacts the likelihood of lymphatic spread. Low-grade minor salivary gland tumors and sarcomas have a lower risk of lymph node metastases than SCCs arising in similar mucosal sites.
A patient may present with SCC in a cervical lymph node, and despite an extensive work-up, the site of origin may remain undetermined in approximately 50% of patients.18 If only the neck is treated, a primary lesion may appear later, but sometimes is never found.19
The relative incidence of clinically positive lymph nodes on admission is determined by primary site and T stage.20 Well-lateralized lesions spread to ipsilateral neck nodes.21 Lesions on or near the midline, tongue base, and nasopharyngeal lesions (even when lateralized), may spread to both sides of the neck, although the risk is higher to the side occupied by the bulk of the lesion. Patients with clinically positive ipsilateral neck nodes are at risk for contralateral disease, especially if the nodes are large or multiple; obstruction of the lymphatic pathways by surgery or radiotherapy (RT) also shunts the lymphatic flow to the opposite neck.21 When lymph node metastases appear at an unusual site, a careful search must be made for a second primary. The likelihood of retropharyngeal adenopathy is related to the presence of clinically involved lymph nodes and the primary site, and is particularly high for NPCs.22
Distant Spread
The risk of distant metastasis is related more to N stage and the location of involved nodes in the low neck, rather than to T stage.23 The risk is less than 10% for N0 or N1 disease and rises to approximately 30% for N3 disease as well as N1 or N2 nodes with disease below the level of the thyroid notch. Distant metastases are found most often in the lung.24
A general medical evaluation is performed, including a thorough head and neck examination. The location and extent of the primary tumor and any clinically positive lymph nodes is documented. Almost all patients undergo contrast enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI) to further define the extent of local–regional disease. The scan(s) should be obtained prior to biopsy so that biopsy changes are not confused with the tumor. A chest radiograph is obtained to determine the presence of distant metastases and/or a synchronous primary lung cancer. Patients with N3 neck disease, as well as those with N2 disease with nodes below the level of the thyroid notch, have a 20% to 30% risk of developing distant metastases and are considered for a chest CT or positron emission tomography (PET).
Tumors amenable to transoral biopsy may be biopsied using local anesthetics in the clinic. Otherwise, direct laryngoscopy under anesthesia is performed to determine the extent of the tumor and to obtain a tissue diagnosis. Given the risk of synchronous cancers, some advocate routine triple endoscopy (i.e., laryngoscopy/pharyngoscopy, bronchoscopy, and esophagoscopy). The additional yield is low, unless diffuse mucosal abnormalities or a malignant lymph node without an identified primary site, particularly in the low neck, are present. Patients presenting with a metastatic node from an unknown primary site undergo fine-needle aspiration (FNA) of the node. An excisional biopsy is not routinely performed unless lymphoma is suspected or FNA results are equivocal. If SCC is a consideration, the excision should be done in a manner to facilitate subsequent management, including neck dissection. Occasionally, the diagnosis may be made by clinical and radiographic evaluation, and a biopsy should be avoided in situations where the treatment is definitive for RT and where obtaining a tissue sample is risky (e.g., paragangliomas, juvenile nasopharyngeal angiofibromas).14,25
Before the initial treatment, the patient should be evaluated by members of the team who may be involved in the initial management as well as possible salvage therapy. Head and neck surgeons, radiation oncologists, medical oncologists, diagnostic radiologists, plastic surgeons, pathologists, dentists, speech and swallowing therapists, and social workers may all play a role.
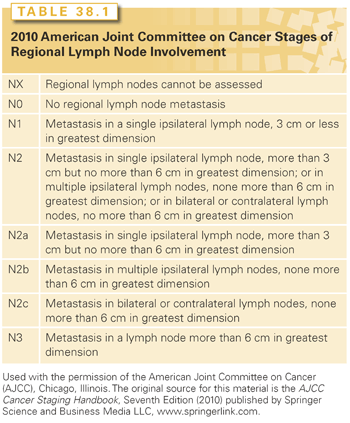
The staging system of the American Joint Committee on Cancer (AJCC) is used.26 In general, excisional biopsy (TX) indicates that the primary tumor cannot be assessed; T0 indicates no evidence of primary tumor; and Tis indicates carcinoma in situ. For tumors of the oral cavity and oropharynx, further staging of the primary lesion is based primarily on size criteria: 2 cm or less for T1; greater than 2 cm but no more than 4 cm for T2; greater than 4 cm for T3; and T4 tumors involve major invasion or encasement of surrounding structures (e.g., bone, carotid artery, deep musculature). For the other primary sites, further staging is less easily generalized because the anatomic extent of spread and/or functional criteria (e.g., vocal cord mobility) are used and, for certain sites, are combined with tumor size (e.g., hypopharynx, major salivary glands), and so will be given in the discussion of each respective primary site.26 Neck staging is common to all head and neck sites, except the nasopharynx (Table 38.1).26 Lesions may be clinically or pathologically staged. Clinical staging is more commonly used for treatment planning and the reporting of results. The format for combining T and N stages into an overall stage is depicted in Table 38.2 and is common to all sites except the nasopharynx.26
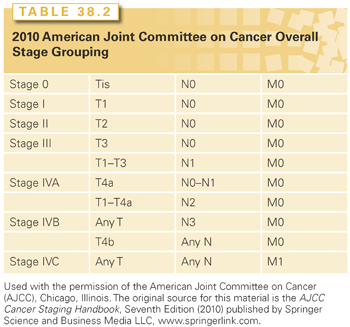
Stage IV represents a wide spectrum of disease. One patient may have a T1, T2, or T3 lesion with low-volume N2 neck disease and a high probability of cure (stage IVA), whereas another may have a T4b primary cancer and/or N3 neck disease and a relatively poor prognosis (stage IVB)27; distant metastases indicate stage IVC disease, and the treatment intent is typically palliative.
PRINCIPLES OF TREATMENT FOR SQUAMOUS CELL CARCINOMA
General Principles for Selection of Treatment
Surgery and RT are the only curative treatments for head and neck carcinomas. Although chemotherapy alone is not curative, it enhances the effects of RT, and thus is routinely used as part of combined modality treatment in patients with stage III or IV disease.
The advantages of surgery compared with RT, assuming similar cure rates, may include the following: (1) a limited amount of tissue is exposed to treatment, (2) the treatment time is shorter, (3) the risk of immediate and late RT sequelae is avoided, and (4) RT is reserved for a head and neck SPT, which may not be as suitable for surgery.
The advantages of RT may include (1) the risk of a major postoperative complication is avoided, (2) no tissues are removed so that the probability of a functional or cosmetic defect may be reduced, (3) elective neck RT can be included with little added morbidity, and (4) the surgical salvage of RT failure is probably more likely than the salvage of a surgical failure.
Salvage of a surgical failure may be attempted by operation, RT, or both. Surgical recurrences usually develop at the resection margins, in or near the suture line. It is difficult to distinguish the normal surgical scarring from recurrent disease, and the diagnosis of recurrence is often delayed. Tumor response to RT under these circumstances is poor. Surgery, RT, or both, however, may salvage small mucosal recurrences and some neck recurrences. For bulkier recurrences treated with RT, concurrent chemotherapy is often incorporated.
Primary Site
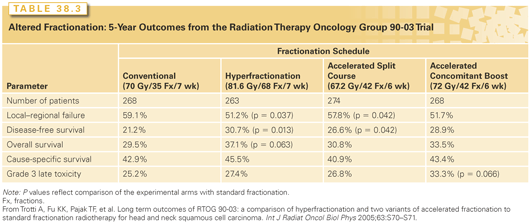
The management of the primary cancer will be considered separately for each anatomical site. Patients who are in poor nutritional condition may require a nasogastric (NG) tube or a percutaneous gastrostomy (PEG) before initiating RT, particularly if concomitant chemotherapy is used. Opinions vary regarding the role of prophylactic NG or PEG placement in anticipation of RT-based local toxicity in patients without significant baseline dysphagia or weight loss; a reactive strategy is preferred by many and may facilitate swallowing recovery.28 If external-beam radiotherapy (EBRT) is selected, it may be given with either conventional once-daily fractionation, 66 to 70 Gy at 2 Gy per fraction, 5 days a week in a continuous course, or with an altered fractionation schedule. Whether an altered fractionation schedule is better than conventional fractionation depends on the altered fractionation technique that is selected. Two altered fractionation schedules shown to result in improved local–regional control rates are the University of Florida hyperfractionation and the M.D. Anderson concomitant boost techniques.29 The results of a prospective randomized Radiation Therapy Oncology Group (RTOG) trial comparing these schedules with conventional fractionation and the Massachusetts General Hospital accelerated split-course schedule are shown in Table 38.3. Acute toxicity is increased with altered fractionation; late toxicity is comparable with conventional fractionation.30
Conventional EBRT techniques and/or brachytherapy will be discussed in the subsequent site-specific sections. EBRT may also be delivered with intensity-modulated radiation therapy (IMRT) to produce a more conformal dose distribution and to reduce the dose to the normal tissues.31–33 The disadvantages of IMRT are that it is more time consuming to plan and treat the patient, the dose distribution is often less homogenous so that “hot spots” may increase the risk of late complications, the risk of a marginal miss may be increased because the fields are more conformal, the total body RT dose is higher because of increased “beam on” time and scatter irradiation, and it is more costly. Therefore, a clear reason for using IMRT versus conventional RT must be identified. The usual indication for IMRT is to reduce the dose to the contralateral parotid gland, and thus limit long-term xerostomia.34 Another indication is to reduce the CNS dose in patients with NPC. Finally, it may be used to avoid a difficult low neck match in patients with laryngeal or hypopharyngeal cancers and a low-lying larynx. Proton therapy, which offers potential targeting and dosing advantages for selected tumors,35 is useful for reducing the dose to the brain and the visual apparatus for patients with nasal cavity and paranasal sinus malignancies.
In a classic radical neck dissection, the superficial and deep cervical fascia with its enclosed lymph nodes (levels I to V) is removed in continuity with the sternocleidomastoid muscle, the omohyoid muscle, the internal and external jugular veins, cranial nerve XI, and the submandibular gland. The incisions used by the surgeon will be governed largely by the primary lesion. The radical neck dissection can be modified to spare certain structures with the intent of decreasing morbidity and improving functional outcome without compromising disease control. There are three main types of modified radical neck dissections: type I, CN XI is spared; type II, CN XI and the internal jugular vein are spared; and type III (functional), CN XI, the internal jugular vein, and the sternocleidomastoid muscle are spared. Selective neck dissections are more limited and include the resection of lymph node levels that are at greatest risk for nodal metastatic spread. Examples include the lateral, posterolateral, and supraomohyoid, which include resections of lymph node levels II through IV, II through V, and I through III, respectively.
A modified or selective neck dissection is recommended for the cN0 neck, for selected clinically positive necks (mobile, 1 to 3 cm lymph nodes), and for removing residual disease after RT when there has been excellent regression of N2 or N3 disease.36,37
The more extensive the neck dissection, the higher the risk of complications. Complications after neck dissection include hematoma, seroma, lymphedema, wound infections and dehiscence, damage to the 7th, 10th, 11th, and 12th cranial nerves, carotid exposure, and carotid rupture. The last-mentioned complication can be minimized by covering the carotid artery with a dermal graft at the time of surgery.36 Pain and dysfunction in the neck or shoulder may occur. Rehabilitation and anti-inflammatory medication are commonly utilized with varying benefits; acupuncture had demonstrated a benefit compared to the usual care in one randomized study.38
Clinically Negative Neck
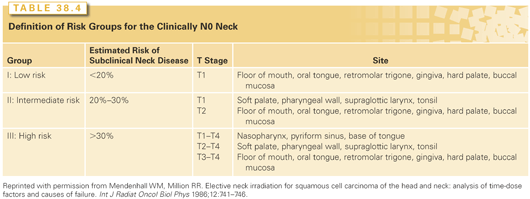
The estimated incidence of subclinical disease in the regional lymphatics when the neck is cN0 is presented in Table 38.4.39 Both RT and neck dissection are approximately 90% efficient at eradicating subclinical regional disease.36 Alternatively, a policy of close observation may be adopted for the cN0 neck to avoid unnecessary treatment, and the neck is managed by surgery and/or RT if cervical metastases develop. The salvage rate for patients developing clinically positive lymph nodes with the primary lesion controlled is 50% to 60%.39
Elective neck irradiation (ENI) and elective neck dissection are equally effective in the management of the N0 neck, with control rates exceeding 90%.39,40 Treatment of the entire neck is advised for primary lesions with a high rate of subclinical disease, such as the base of tongue, soft palate, supraglottis, and hypopharynx. Patients with lateralized T1 to T2 tonsillar cancers do not require elective treatment for the contralateral N0 neck41; T3 or T4 cancers or those with significant extension into the tongue and/or soft palate should receive bilateral neck treatment to the entire neck.
When the primary tumor is to be treated surgically, an elective neck dissection should be performed when the risk of regional lymph node metastasis is 10% to 15% or greater. Modified neck dissection has a good rate of disease control; patients who are found to have multiple positive nodes or extracapsular extension (ECE) are then referred for postoperative RT,42 and concurrent chemotherapy is recommended in the latter circumstance.43–46 If the primary lesion is to be treated with EBRT, ENI adds relatively little cost and modest morbidity.
Clinically Positive Neck Lymph Nodes
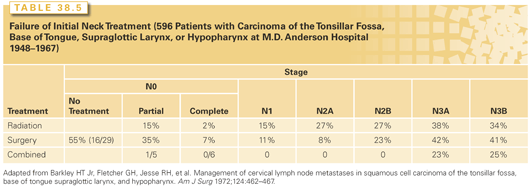
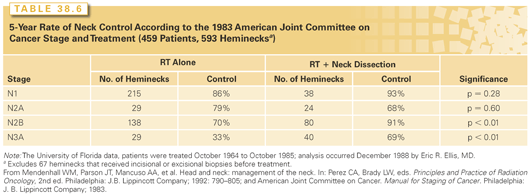
The rates of neck failure by N stage and treatment group reported from the M.D. Anderson Cancer Center and the University of Florida are shown in Tables 38.5 and 38.6, respectively.40,47 In general, RT precedes surgery if the primary site is to be treated by RT or if the node was fixed. The operation precedes RT if the primary site is to be treated surgically.
Modified neck dissection is sufficient treatment for the ipsilateral neck for patients with N1 or N2A disease without ECE. RT, often combined with concurrent chemotherapy, is added for those with more advanced neck disease.42
When the primary lesion is to be managed by RT or chemoradiotherapy (chemoRT), then RT-based therapy alone is sufficient for patients in whom the node(s) regress completely as documented on CT obtained 4 weeks post-RT.37,48 RT is followed by a neck dissection for patients with residual nodes that are 1.5 cm or larger, as well as those that demonstrate focal defects, enhancement, and/or calcification.48 A PET scan done 3 months after RT is completed is an alternative to CT to assess whether there is persistent disease.49
McGuirt and McCabe50 compared results of definitive surgery with and without a prior open neck biopsy and concluded the risks of neck failure, distant metastases, and complications were all increased. Ellis et al.51 studied the results of therapy following open biopsy of a lymph node before treatment. Patients received definitive RT to the primary site and neck; a subset of patients underwent a neck dissection after RT. Open biopsy had no adverse impact on these patients compared with those who did not undergo an open biopsy.51 Therefore, after open biopsy of the neck, RT-based therapy is recommended as the initial treatment, particularly if the primary tumor is to be managed by RT or chemoRT. Under these circumstances, no further neck treatment is needed if the neck node had been removed; if there was residual gross tumor in the neck after open biopsy, a planned neck dissection should be added depending on the results of radiologic reassessment.48
Drug therapy may be administered to prevent the development of SPTs (chemoprevention), to palliate symptoms in patients with incurable disease, to improve the odds of cure or organ preservation when combined with definitive local-regional therapy, or to decrease treatment toxicity. The first two indications are discussed here; the last two are discussed in a subsequent section.
Chemoprevention
Chemoprevention is the administration of natural or synthetic agents to reduce the risk of developing SPTs. Patients who have a head and neck SCC have an increased risk of developing an upper aerodigestive tract SPT because of exposure to carcinogens and/or genetic predisposition.3 The risk of developing an SPT is approximately 2.7% to 4% per year52 and may impact survival. Current data indicate the risk of SPTs is lower among patients with HR-HPV–related head and neck cancers.6,8 Analogs of vitamin A, particularly of the retinoids, have been a particular focus of clinical investigations. At present, there is no standard role for the use of chemopreventive agents in the management of head and neck cancer.
Retinoids and beta-carotene both may cause regression of oral leukoplakia; the former appear more efficacious.53 Lesions commonly recur after cessation of drug therapy. Chemoprevention agents do not reduce the risk of recurrence of the index cancer.
High-dose13-cis-retinoic acid (100 mg/m2 daily for 12 months) has been shown in a randomized, placebo-controlled trial to reduce the risk of SPTs in patients previously treated for stage I to IV, M0, head and neck cancer.54 However, a large, placebo-controlled, randomized trial in 1,190 survivors of stage I and II head and neck SCC found no difference in the rate of SPTs or survival after 3 years of a low-dose schedule of this agent (30 mg per day).55 Outcomes were better among nonsmokers and those who quit compared to smokers, emphasizing the important role of tobacco cessation as part of head and neck cancer management and strategies to decrease SPTs. Similarly, etretinate was not shown to be efficacious in decreasing SPTs.56
With regard to other agents, vitamin A, N-acetylcysteine, both, or neither were evaluated using a factorial design in the EUROSCAN study. No significant improvement in survival or SPTs was observed.57 Bairati et al.58 randomized head and neck cancer survivors to 3 years of therapy with alpha-tocopherol and beta-carotene versus placebo; the rate of SPTs was actually higher during the period of treatment, a difference that did not persist with longer follow-up. A randomized phase II, placebo-controlled trial demonstrated no significant benefit of celecoxib at 100 mg or 200 mg, both twice daily, on the control of oral premalignant lesions.59
Targeting the epidermal growth factor receptor (EGFR) pathway is receiving attention, because there is an association between progressive EGFR dysregulation and the transition from normal mucosa to dysplasia to SCC.60 The concept of bioadjuvant therapy,55 whereby drug combinations intended to reduce both the risk of SPTs and relapse from the index cancer, as well as the potential role of natural extracts are also of interest.61
There is no standard role for the use of HR-HPV vaccination in the prevention of head and neck cancer at this time,62 although the impact of current vaccination programs on the incidence of head and neck cancer warrants follow-up.
Chemotherapy for Recurrent or Metastatic Disease
Single Agents
Patients with recurrent or metastatic head and neck SCCs have a median survival of 6 to 9 months, and a 1-year survival rate of 20% to 40% when treated with chemotherapy alone.63,64 Although selected patients may derive apparent significant prolongations in survival, average survival improvements appear small at best. Morton et al.65 reported a 2-month improvement in median survival after treatment with cisplatin, with or without bleomycin, compared to no treatment. The duration of responses is typically measured in weeks to months, not years; survival beyond 2 years is infrequent; and cures are anecdotal. Thus, the primary intent of chemotherapy in this setting is to achieve tumor regression with the hope that the potential palliative benefit and possible modest survival improvement will outweigh the side effects of treatment.
A number of drugs have been demonstrated in clinical trials to have activity in head and neck SCCs, and the list is well summarized in prior reviews.63,64 The most commonly used include methotrexate, cisplatin, carboplatin, 5-fluorouracil, paclitaxel, and docetaxel, with reported major response rates ranging from 15% to 42%. Among other drugs with reported major response rates of 15% or greater are bleomycin, cyclophosphamide, doxorubicin, hydroxyurea, ifosfamide, irinotecan, oral uracil, ftorafur (with leucovorin), pemetrexed, vinblastine, and vinorelbine. Some of these agents (e.g., cyclophosphamide, doxorubicin, hydroxyurea) have their activity based on reported assessments in a limited number of patients from over 2 decades ago, an era when methods and criteria for response assessments may have differed from current standards. Anticipated response rates and toxicity profiles may vary based on patient selection and drug schedule. A poor performance status is associated with both lower response rates and greater potential for toxicity. The larger the amount of prior treatment also adversely affects response rates.64
Methotrexate is a historic standard drug used in the recurrent or metastatic disease setting. The typical standard dosing is 40 mg/m2 intravenously weekly, with dose attenuation or increase (up to 60 mg/m2) based on toxicity, with mucositis being a frequent reason for dose adjustment. The favorable side effect profile and convenience of administration of methotrexate make it well-suited for use in this patient population in which medical comorbidity is common, as is more advanced age. In randomized trials, higher doses increase response rates and toxicity without a significant improvement in overall survival.66,67 Newer analogs of methotrexate (e.g., edatrexate) have not been shown to offer a therapeutic advantage in phase III trials.68
Cisplatin is a cornerstone drug in the modern management of head and neck cancer. Cisplatin is customarily dosed at 75 to 100 mg/m2 intravenously every 3 to 4 weeks. The potential for renal (i.e., increase in creatinine, electrolyte abnormalities), otologic (i.e., high frequency hearing loss, tinnitus), neurologic (i.e., peripheral neuropathy), and gastrointestinal (i.e., nausea and vomiting) toxicity are widely appreciated, but these risks are manageable if patients are appropriately screened for therapy, monitored closely, and state of the art supportive care measures are applied. Further dose escalation of cisplatin has not been established to improve outcome. A randomized trial comparing 60 mg/m2 versus 120 mg/m2 of cisplatin failed to demonstrate a significant improvement in response or survival.69 Carboplatin is the best studied and most commonly used platinum analog in head and neck cancer. Although generally less toxic and easier to administer than the parent drug, it is more bone marrow suppressive and may be somewhat less active. This last issue is more of a concern in the definitive treatment setting in which cure is a central endpoint, as opposed to the palliative setting, when patients often seek a less toxic alternative treatment.
Although taxanes as a class have significant activity in head and neck SCCs, hopes of clinically significant improvement in survival in the palliative setting with the introduction of these agents have yet to be realized. Neither paclitaxel or docetaxel has been demonstrated in random assignment trials to be clearly superior to methotrexate with regard to survival as an endpoint.70 Paclitaxel dosed at 250 mg/m2 intravenously over 24 hours with growth factor support in an Eastern Cooperative Oncology Group (ECOG) trial, yielded a major response in 12 of 30 patients (40%) including four complete responses; grade 3 or greater neutropenia occurred in 91% of patients, and there were two deaths.71 Less cumbersome to administer and less toxic schedules are commonly used in practice (e.g., 135 to 225 mg/m2 intravenously over 3 hours every 3 weeks; 80 to 100 mg/m2 weekly), although their relative efficacies have not been well evaluated. A paclitaxel schedule that provides more prolonged exposure to the drug may be more efficacious,72 although a phase II trial of 120 to 140 mg/m2 over 96 hours yielded disappointing results even in treatment-naïve patients (major response rate, 13%).65 Other toxicities besides myelosuppression include sensory neuropathy, alopecia, allergic reactions, and arrhythmia, although cardiac monitoring is not required.
Docetaxel appears less neuropathic than paclitaxel, but fluid retention and hematologic toxicity may be more problematic. A typical dose is 60 to 100 mg/m2 intravenously over 1 hour. Initial studies evaluated the efficacy of the 100 mg/m2 dose level, with major response rates ranging from 21% to 42%73; an excellent performance status is required for this higher dose. Lower doses may offer similar efficacy and better tolerance.74 As with paclitaxel, weekly schedules are applied in practice, but the relative efficacy of weekly versus a schedule of every 3 weeks is not well-studied.
Although initial studies evaluated a bolus schedule for 5-fluorouracil, an infusional program of 1,000 mg/m2 per day over 96 to 120 hours appears more efficacious in head and neck cancer.75 Infusional 5-fluorouracil is associated with more mucositis and diarrhea than a bolus schedule, so the shorter infusion (i.e., 96 hours) is typically applied in patients who are pretreated and have received prior head and neck RT.
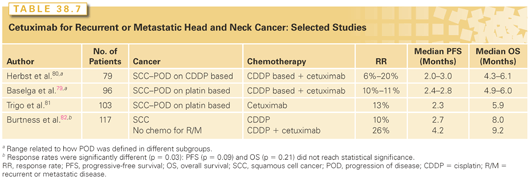
EGFR is highly expressed in most head and neck SCCs, and the degree of expression is inversely associated with prognosis.76–78 As such, there has been a keen interest in drugs that target the receptor itself or steps downstream. Cetuximab, a chimeric immunoglobulin G antibody that binds the receptor, has been approved by the U.S. Food and Drug Administration for use in patients with disease refractory to platin-based therapy. As summarized in Table 38.7, the response rates in this refractory setting are similar (10% to 13%) whether cetuximab is used alone or combined with platin-based therapy; median survivals remained disappointing, ranging from 5.2 to 6.1 months.79–82 Another EGFR antibody, zalutumumab, was compared in a randomized trial to best supportive care alone in patients with cisplatin-refractory squamous cell head and neck cancer. Among 286 entered patients, there was no significant improvement in the primary endpoint of overall survival (median 6.7 versus 5.2 months, p = 0.0648) although a significant difference was found on an exploratory post hoc analysis done 12 months after the last patient was randomized. Response rate (6.3% versus 1.1%) and progression-free survival (median 9.9 versus 8.4 weeks, p = 0.0012) were improved with zalutumumab.83
The small molecule tyrosine–kinase inhibitors, gefitinib and erlotinib, offer no efficacy advantage in similar refractory patients. Major response rates and median survivals ranged from 0% to 15% and 5.9 to 8.1 months, respectively.78,84–86 A large randomized trial (486 patients) compared gefitinib (250 or 500 mg daily) to methotrexate and demonstrated no survival improvement with either gefitinib dose.87
With both of these classes of agents, the development of rash was associated with clinical benefit, but this association is not fully explained by simple pharmacokinetics.78 There is no established molecular predictor of response to these agents currently in head and neck SCC.
The successful development and approval of cetuximab in head and neck SCC highlights the potential for therapies to exploit specific molecular pathways with therapeutic effect. A number of other new agents, often with multitarget capability, are entering clinical trials. There is a good rationale for agents that target angiogenesis in head and neck SCC.88 However, the development of bevacizumab has been cautious given the reported toxicity concerns, specifically bleeding, in patients with squamous cell lung cancer.89 Alterations in the PI3K/Akt/mammalian target of rapamycin (mTOR) pathway are common in head and neck cancer, and activation appears independent of EGFR activation, making targeting of this pathway of great interest.90 Cancer gene therapy, whereby genetic sequences are introduced via viral or nonviral vectors, is well-suited to head and neck tumors given the local–regional character of head and neck tumors that facilitates direct injection and the monitoring of gene expression. The tumor suppressor gene p53 has been one target, because somatic mutations of it are common in head and neck cancers, particularly among patients who have smoked cigarettes and used alcohol.91 For example, in a phase II study of Onyx-015, a replication-competent adenovirus absent the E1B gene, major responses, including some complete regressions, occurred in 10% to 14% of treated patients.92 In other studies, treatment with Onyx-015, or a similar virus such as H101, improved the efficacy of chemotherapy.93,94
Combination Therapy
Given the disappointing track record for single-agent therapy in the palliative setting, combinations of drugs have been extensively evaluated. In the early 1980s, investigators from Wayne State, building upon potential synergy between cisplatin and 5-fluororuacil, reported a major response rate of 70% with a complete response rate of 27% using a regimen of cisplatin 100 mg/m2 intravenously and a 5-fluorouracil 1,000 mg/m2 per day continuous infusion over 96 hours recycled every 3 weeks in patients with recurrent or disseminated disease.95 Other investigators confirmed the significant activity of the regimen, albeit with a somewhat lower major and complete response rate on average (50% and 16%, respectively),96 establishing it as the standard regimen to which new therapies are compared.
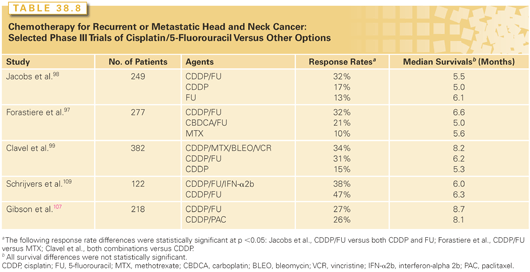
Despite an improvement in response rates associated with the use of combination therapies like cisplatin and 5-flurouracil, demonstrating a statistically or clinically significant improvement in survival compared to single-agent therapy has proven elusive. Table 38.8 summarizes the results of three randomized trials that compared treatment with cisplatin and infusion 5-fluorouracil to that with different single agents.97–99 Treatment with combination chemotherapy led to a significant increase in response rate, albeit at the cost of greater toxicity. Overall survival did not significantly improve. The meta-analysis reported by Browman et al.100 yielded similar conclusions. These data do not support the routine use of cisplatin-based combinations for patients with recurrent or metastatic SCC. Combination therapy seems most appropriate for patients with a good performance who have significant symptoms (e.g., pain) for which the higher anticipated response rate will translate into better palliation.
The activity of paclitaxel and docetaxel in head and neck cancer has fostered the development and evaluation of taxane and cisplatin combinations. Docetaxel with cisplatin is associated with a major response rate of 40% to 53%, with complete response rates approximating 6% to 18%101,102; a weekly schedule of paclitaxel (80 mg/m2) and carboplatin (area under concentration [AUC] versus time curve, 2) appeared more efficacious than an every 3-week dosing (paclitaxel 175 to 200 mg/m2 intravenously over 3 hours followed by carboplatin AUC 6) in two separate phase II studies.103–105 ECOG compared a high-dose (200 mg/m2) and moderate-dose (135 mg/m2) paclitaxel, both by 24-hour infusion and followed by the same dose of cisplatin (75 mg/m2) in a randomized study (E1393). No significant difference in response rate or survival was found between the arms.106 Another randomized trial done under the auspices of ECOG compared standard cisplatin and 5-fluorouracil with paclitaxel 175 mg/m2 intravenously over 3 hours and cisplatin 75 mg/m2 (E1395).107 Objective major response rates (27% versus 26%) and median survivals (8.7 versus 8.1 months) were no different between the arms. The reported quality of life was better on the paclitaxel arm over the first 16 weeks of treatment.108
Attempts have been made to improve the efficacy of combination chemotherapy through the development of a variety of triplets. The addition of interferon-alpha2b (IFN-α2b) to cisplatin and 5-fluorouracil failed to significantly improve response or survival in a randomized trial.109 Phase II studies of a taxane with cisplatin or carboplatin and a third drug (e.g., 5-fluorouracil, ifosfamide), have yielded major response rates of 55% to 86%110–113; whether these regimens translate into better survival outcomes compared to a cisplatin-based doublet that may be less toxic await further evaluations.
There is great interest in the combination of standard chemotherapy with newer targeted agents. One ECOG study compared cisplatin versus cisplatin and cetuximab as first-line treatment in 123 patients. The arm including the cetuximab had a significantly higher response rate (10% versus 26%, p = 0.03), but no significant difference was found in the primary endpoint of progression-free survival (2.7 versus 4.2 months, p = 0.09) or in overall survival (8.0 versus 9.2 months, p = 0.21), although the trends favored the combination arm.82 In a larger trial (EXTREME),114 442 patients were randomized to cisplatin or carboplatin and 5-fluorouracil with or without cetuximab for six cycles. Subsequent maintenance with cetuximab alone was allowed on the investigational arm, but there was no crossover to cetuximab on the standard arm. Both median progression-free (5.6 versus 3.3 months) and overall (10.1 versus 7.4 months) survivals were significantly improved on the triplet arm, at the cost of more sepsis (nine patients versus one patient; p = 0.02), grade 3 skin reactions (9%), and grade ≥3 infusion reactions. Quality of life outcomes were reported to not be significantly different between the treatment arms.115 Tumor EGFR copy number and degree of EFGR expression were not predictive of benefit with cetuximab.116,117 Of interest, data from Vermorken and colleagues118 suggests that the therapeutic effect of cetuximab, when combined with chemotherapy, is mainly additive rather than synergistic. Whether allowing patients to crossover to cetuximab on the doublet arm at progression would have decreased or eliminated the observed survival difference is of interest for future research. A similar but not identically designed randomized trial evaluated cisplatin and 5-flourouracil with or without the EGFR antibody panitumumab in 657 patients. Response rate (36% versus 25%, p = 0.0065) and median progression-free survival (5.8 versus 4.6 months, p = 0.0036) were significantly improved with the incorporation of the panitumumab, but not the primary endpoint of overall survival (11.1 versus 9.0 months, p = 0.1403). Overall survival was improved in the p16-negative subgroup (p = 0.0115).119
With advances in RT techniques that facilitate reirradiation with acceptable morbidity, this approach has been increasingly explored in patients with unresectable local or regional recurrence, often with integrated chemotherapy. The observed median survivals in these series are similar to those obtained in phase II trials of chemotherapy alone, but more durable responses occur in selected patients and there is a clearer plateau on the survival curve. In two larger series involving 169 and 115 patients, respectively, among patients treated with a variety of RT fractionation schedules and concurrent chemotherapy regimens, 2-year survival rates exceeded 20%.120,121 In two sequential RTOG studies, a regimen of daily paclitaxel (20 mg/m2) and cisplatin (15 mg/m2) added concurrently to split-course RT (total dose 60 Gy, 1.5 Gy twice-daily fractions; granulocyte colony-stimulating factor support during off weeks) (RTOG 96-11) yielded a better 2-year survival rate than concurrent 5-fluororuacil and hydroxyurea added to the same RT schedule (RTOG 96-10) (24.9% versus 16.9%, p = 0.44).122,123 The reported 2-year survival rates exceed the rate of 10.5%, which was observed in a subgroup of 124 patients with local disease only who had previously received RT and participated in E1393 or E1395.124 Randomized trials comparing chemotherapy alone to reirradiation and chemotherapy are needed.
Nasopharynx Cancer
Many of the same drugs and regimens used in the treatment of head and neck SCC are also active in NPC. There are reports of a small proportion of patients with recurrent or metastatic disease being controlled long term with chemotherapy alone.125 Available data support the use of cisplatin-based combination chemotherapy (e.g., cisplatin/5-fluorouracil; cisplatin/bleomycin/5-flurouracil +/− epirubicin), although there is a lack of randomized studies to clarify the relative efficacies and toxicities of different options. Site-specific phase II studies report major response rates of 70% or higher with regimens containing cisplatin.126–128 In a review of the Princess Margaret Hospital experience, single-agent or noncisplatin-based combination chemotherapy was associated with a major and complete response rates of 25% and 8%, respectively, in 40 patients, whereas cisplatin-based combination therapy produced major and complete response rates of 70% and 23%, respectively, in 30 patients.126 The substitution of carboplatin may be associated with less activity.127
With regard to other agents, paclitaxel as a 175 mg/m2 3-hour infusion is active with a response rate of 22% in a series of 24 patients with undifferentiated NPCs.128 The combination of it with carboplatin has yielded response rates consistently greater than 50%.129–131 Gemcitabine is active in NPCs,132,133 and combinations including it appear promising, with response rates exceeding 70%.134,135 Capecitabine, prolonged 5-fluorouracil infusion, and cetuximab all have modest activity in the refractory setting, and no major responses were seen in one study with gefitinib.136–139 There is keen interest in looking to exploit the association NPCs have with EBV for therapeutic purposes. Potential gene therapy approaches are discussed elsewhere.140,141
GENERAL PRINCIPLES OF COMBINING MODALITIES
Surgery Plus Radiation Therapy
RT may be administered preoperatively or postoperatively. An analysis of available data suggests there is no compelling difference in survival rates comparing the two sequences42; local–regional control may be improved with postoperative treatment.142
Combined modality therapy should be avoided for lesions with a high cure rate (70% or greater) by either surgery or RT alone. The increased morbidity from combined treatment is not associated with a significantly improved control rate, and many patients with local or regional failure can be salvaged by secondary procedures.
The advantages of postoperative compared with preoperative RT include less operative morbidity, more meaningful margin checks at the time of the surgery, a knowledge of tumor spread for RT planning, safe use of a higher RT dose, and no chance the patient will refuse surgery. The disadvantages of postoperative RT include the larger treatment volume necessary to cover surgical dissections, a delay in the start of RT with possible progression, and the higher dose required to accomplish the same rates of local–regional control.
Preoperative Radiation Therapy
Preoperative RT should be considered for the following situations: (1) fixed-neck nodes, (2) delayed initiation of postoperative RT by >8 weeks, (3) use of the gastric pull-up for reconstruction, and (4) open biopsy of a positive neck node.
Postoperative Radiation Therapy
Postoperative RT is considered when the risk of recurrence above the clavicles exceeds 20%. The operative procedure should be one stage and of such magnitude that RT is started no later than 6 to 8 weeks after surgery. The operation should be undertaken only if it is believed to be highly likely that all gross disease will be removed and margins will be negative.
Although no definitive randomized trials have addressed the efficacy of postoperative RT in the treatment of head and neck cancer, excellent data that has bearing on this issue is available from the Medical College of Virginia. Two groups of surgeons operated on patients with head and neck cancer: general surgical oncologists who used surgery alone and reserved RT for treatment of recurrent disease, and otolaryngologists who routinely sent patients with locally advanced disease for postoperative RT.143 Of 441 patients, 125 were treated surgically between 1982 and 1988 and had ECE and/or positive margins, 71 were treated with surgery alone, and 54 received postoperative RT. Local control rates at 3 years after surgery alone compared with surgery and RT were: for ECE, 31% and 66% (p = 0.03); positive margins, 41% and 49% (p = 0.04); and ECE and positive margins, 0% and 68% (p = 0.001). A multivariate analysis of local control revealed that the use of postoperative RT (p = 0.0001), macroscopic ECE (p = 0.0001), and margin status (p = 0.09) were of independent significance. Cause-specific survival rates at 3 years were 41% for surgery alone and 72% for surgery and postoperative RT (p = 0.0003). A multivariate analysis of cause-specific survival showed that postoperative RT (p = 0.0001) and the number of nodes with ECE (p = 0.0001) significantly influenced this endpoint.
In another series, Lundahl et al.144 reported on 95 patients with node-positive SCC who were treated with a neck dissection and postoperative RT at the Mayo Clinic. A matched-pair analysis was performed using a series of patients treated with surgery alone; 56 matched pairs of patients were identified. The recurrence rates in the dissected neck (relative risk [RR] = 5.82, p = 0.0002), recurrence in either side of the neck (RR = 2.21, p = 0.0052), and death from any cause (RR = 1.67, p = 0.0182) were significantly higher for patients treated with surgery alone.
Thus, it appears that postoperative RT may significantly improve both local–regional disease control and survival for patients who are at high risk for failure after surgery.
Indications for postoperative RT include close (<5 mm) or positive margins, ECE, multiple positive nodes, invasion of the soft tissues of the neck, endothelial-lined space invasion, PNI, and more than 5 mm of subglottic invasion.42 The authors currently recommend 60 Gy in 6 weeks to 66 Gy in 6.5 weeks for patients with negative margins and fewer than three indications for RT. For patients with close (<5 mm) or positive margins, we recommend 70 Gy in 7 weeks or 74.4 Gy at 1.2 Gy twice a day. Concomitant cisplatin chemotherapy should be considered for patients with positive margins and/or ECE.43–45
Given the appreciation that HPV-related cancers have a better prognosis than HPV-unrelated disease, there is interest in potential deescalation of therapy with the intent of decreasing toxicity without compromise in survival.145 Although this is an important area of future research, modification in standards of care based on the HPV status of the tumor is not recommended outside of a clinical trial at present.146
CHEMOTHERAPY AS PART OF CURATIVE TREATMENT
Systematically designed and randomized studies have established a role for drug therapy as part of the standard combined modality management of head and neck SCC in several settings. These include the therapy of unresectable disease, for organ preservation, and for patients with poor risk pathologic features after surgery. Chemotherapy has been shown to improve the likelihood of disease control compared to RT alone in patients with advanced disease, albeit with increased acute toxicity. In certain circumstances, response to chemotherapy has been used to triage patients to different local–regional treatments.
Chemotherapy has been integrated with surgery or RT in a variety of ways including induction, concurrent with RT, and/or maintenance. Unlike outcome studies of surgery and/or RT in which site-specific results are reported, albeit typically using a retrospective methodology, many of the trials evaluating the role of chemotherapy enrolled patients with a variety of head and neck SCCs. Even when site specific, although prospective, subsites are combined. This is less of an issue for studies evaluating therapy for NPC.147,148 Nonetheless, important lessons have been learned from these studies, further enhanced by use of a random assignment methodology. In this section, general principles for the integration chemotherapy with local–regional treatment will be discussed with a focus on the results of randomized trials.
The Meta-Analysis of Chemotherapy on Head and Neck Cancer (MACH-NC) included 63 randomized trials published from 1965 through 1993, all of which compared local–regional treatment with or without chemotherapy.149 Individual patient data was available on 10,741 patients. The absolute improvement in 5-year survival overall was 4% (p <0.001). However, the significant improvement appeared limited to those patients who received concomitant treatment (absolute difference of 8% at 5 years, p <0.001). Neither the difference seen at 5 years with induction (2%, p = 0.10), nor maintenance (1%, p = 0.74) chemotherapy was statistically significant.149 In an update of this analysis, now including trials through 2000 and totaling 17,346 patients,149 the superior efficacy of concurrent therapy was confirmed, and was greater than that seen with induction chemotherapy. Survival benefit diminished with patient age and, on subset analysis, was not significant in patients over 70 years of age.
Tumor HPV status has emerged as an important predictor of favorable treatment response and survival, particularly for patients with oropharynx cancer.7 In ECOG 2399, the response to chemotherapy to all protocol treatment, progression free-survival, and overall survival were all improved in the HPV-positive group.7 Subsequent analysis of RTOG 0129 demonstrated that tobacco use (>10 pack-years) and the extent of nodal disease (N2b-N3) both adversely affect the prognosis associated with HPV-positive tumors.8
Induction Chemotherapy
In untreated patients with local or regionally advanced M0 head and neck SCC, treatment with cisplatin-based combination chemotherapy will yield major response rates approximating 90%, with clinical complete response rates in the 30% range.150 Enthusiasm that response rates of this magnitude should translate into survival benefit when induction chemotherapy was combined with surgery or RT is understandable. Yet, in the original report of the MACH-NC analysis, which included 31 induction studies, all but 2 suggested no survival benefit.149
However, a more careful look at these and other data do provide grounds for continued interest in this approach. Many of the included studies had significant methodologic limitations by more contemporary trial standards. A subset analysis, limited to the 15 trials that used cisplatin and infusional 5-fluorouracil, suggested survival benefit (hazard ratio [HR], 0.88; 95% confidence interval [CI], 0.79 to 0.97).149 Even in the absence of survival improvement, there seemed to be a correlation between response to chemotherapy and subsequent response to RT, which provided a basis for subsequent organ preservation initiatives.151,152 Finally, patterns of failure were affected with less distant metastases in certain studies when induction chemotherapy was incorporated. As local–regional control improves, the rate of clinically apparent distant metastases is increasing,153 and induction chemotherapy is, on average, better tolerated than maintenance therapy as a way to give additional systemic therapy.
The study reported originally by Paccagnella et al.154 is illustrative of these types of trials, and provides further insights.155 Two hundred and thirty-seven patients with stage III or IV head and neck cancer were randomized to four cycles of induction cisplatin and infusional 5-fluorouracil followed by standard local–regional treatment (i.e., surgery plus RT if resectable, RT alone if unresectable). Resectability was assessed pretreatment, not after chemotherapy, and was a stratification criteria. Overall, there was no significant difference between the arms with regard to overall survival or local–regional control, although the incidence of distant metastases was lower among patients treated with chemotherapy. On a subset analysis, however, patients with unresectable disease benefitted from the incorporation of induction chemotherapy for all outcomes, including local–regional control, distant control, and overall survival (3-year survival 24% versus 10%, p = 0.04). Among resectable patients, improvement in distant control was offset by a decrement in local–regional control with the integration of induction chemotherapy, and reported survival rates in this subgroup were similar on both treatment arms.
Historically, then, there was no established role for induction chemotherapy prior to planned surgery and postoperative RT, and a limited role only in selected settings prior to RT. However, with the incorporation of taxanes into induction regimens containing cisplatin and 5-fluororuacil, newer data suggest that the indications for induction chemotherapy may further evolve.
Three randomized trials have compared the relative efficacies of induction chemotherapy with standard cisplatin and 5-fluorouracil versus a triplet including a taxane and these same two drugs with one or both being dose adjusted.156–158 All three studies randomized patients with advanced M0 head and neck cancer to either cisplatin and 5-fluororuacil or a triplet, followed by the same RT-based treatment. In one study, this was RT alone, whereas, in the other two, concurrent therapy with carboplatin and cisplatin, respectively, were employed. In general, the taxane-containing triplet was associated with a higher response rate to induction chemotherapy, and improved both progression-free and overall survival. More neutropenia was observed with triplet therapy but, overall, it was as well-tolerated as standard cisplatin and 5-fluorouracil.
These studies were designed to determine which induction chemotherapy was more efficacious, and provide convincing evidence that the triplet of a taxane with cisplatin and 5-flurouracil is superior to standard cisplatin and 5-fluorouracil alone as induction therapy. However, an alternative design is necessary to define the role of induction with such triplets in standard practice. For this population, as discussed in the next section, concurrent chemotherapy and RT alone without induction chemotherapy is the more established standard therapy. Randomized studies are necessary to determine whether a sequential approach using induction with a triplet followed by RT-based treatment (typically with concurrent chemotherapy), is superior to concurrent chemotherapy and RT alone such that the added duration of treatment and potential toxicity is justified.
To date, available randomized trials have failed to demonstrate a clear overall survival benefit with the incorporation of induction chemotherapy. The combination of docetaxel, cisplatin, and 5-fluorouracil has been the focus of these investigations. One study available only in abstract form was confounded by the lack of an intention to treat an analysis with unequal exclusions among treatment arms. Even with those methodologic limitations, it failed to demonstrate a significant improvement in overall survival with the incorporation of induction docetaxel, cisplatin, and 5-fluorouracil or cisplatin, and 5-fluorouracil.159 In the PARADIGM study, 145 patients with local or regionally advanced SCC were randomized to induction docetaxel, cisplatin, and 5-fluorouracil followed by carboplatin or docetaxel concurrent with RT versus concurrent cisplatin with concomitant boost radiation. Patients could have unresectable disease or be resectable, with the intent of therapy being organ preservation. The study was closed early because of slower than expected accrual, so it was somewhat underpowered. There was no difference in overall or progression-free survival between the arms with a median follow-up of 49 months; the 3-year overall survival rates were 73% on the induction arm and 78% on the concurrent arm (p = 0.77); and the 3-year progression-free survival rates were 67% and 69%, respectively (p = 0.82). A subset analysis of the group with advanced neck disease (N2b/N2c,N3), felt to be at increased risk of distant metastases, demonstrated no advantage with the incorporation of induction chemotherapy.160 The DECIDE trial used a similar design, but only patients with N2/N3 were eligible and, for the concurrent therapy, hydroxyurea and 5-fluorouracil was used. Among 280 patients accrued with minimum 24-months follow-up, there was no significant difference between the sequential and concurrent arms with regard to overall survival (75% versus 73%, p = 0.70) or disease-free survival (69% versus 64%, p = 0.39); the cumulative incidence of distant failure, however, was lower in the induction arm (10% versus 19%, p = 0.025).161 Finally, in 256 enrolled patients with stage III or IVA oral cavity cancer, induction with docetaxel, cisplatin, and 5-fluorouracil prior to surgery and postoperative radiation failed to improve overall survival (p = 0.918) or disease-free survival (p = 0.897) compared to proceeding directly to surgery and postoperative radiation alone.162
The optimal role of induction chemotherapy is currently controversial. A review of the National Comprehensive Cancer Network (NCCN) guidelines highlights this reality, because concurrent chemoRT alone and induction followed by RT-based therapy are both listed as treatment options for certain disease scenarios.146 Although concurrent chemoRT alone remains the standard to which new treatments are compared for local or regionally advanced disease and generally receives the higher category rating in these guidelines, induction is well-suited for certain settings in patients who are medically fit. Examples include when immediate therapy is needed in the hope of avoiding a tracheostomy or PEG, in organ preservation settings where the degree of response affects the decision to proceed with surgery versus RT-based therapy, or in patients with advanced neck disease at higher risk for distant metastases.
After induction chemotherapy, there is some controversy as to whether to proceed with RT alone or concurrent chemoRT, and if the latter, which drug to use.156–158 Concern exists regarding the tolerance to high-dose cisplatin after cisplatin-based induction treatment. In a randomized phase II trial, concurrent cetuximab and RT was compared with high-dose cisplatin and RT after induction docetaxel, cisplatin, and 5-fluorouracil in 116 patients with advanced hypopharynx or larynx cancer. Toxicity was substantial on both arms, but treatment compliance was better with cetuximab therapy. There was no significant difference in larynx function preservation and overall survival between the arms; more local failures occurred on the cetuximab arm.163
Concurrent Chemotherapy and Radiation for Gross Disease
Although there are a number of ways to integrate chemotherapy with RT, available data most strongly support concurrent chemotherapy. Given proven efficacy in patients with poor prognostic and unresectable disease, more recent investigations have applied the approach in better prognostic, organ preservation, and adjuvant settings.
Concurrent chemoRT programs vary in many ways, of which the type of chemotherapy (i.e., specific agents, single, combination) and RT schedule (i.e., dose, fractionation) are the most apparent variables. In general, three main approaches can be discerned: single-agent or combination chemotherapy with continuous-course RT; combination chemotherapy with split-course RT, often with altered fractionation; and chemotherapy alternating with RT.164 Although continuous course RT may be desirable and more attractive from a radiobiologic perspective, local toxicities may preclude it depending on the concurrent agents used. The first two approaches are the most common.
A variety of drugs and combinations have been utilized concurrently with RT. When only one drug is used, the MACH-NC indicates that the impact is largest with a platin, of which cisplatin is the predominant one studied, a conclusion shared in another meta-analysis reported by Browman and colleagues.165 Of interest, platin plus 5-fluorouracil (HR, 0.75) offered no clear advantage compared to platin alone (HR, 0.74).166 The results of a three-arm randomized study comparing concurrent cisplatin and RT, concurrent cisplatin, 5-fluorouracil and split-course RT (with possible resection depending on response), and definitive RT alone in patients with unresectable disease reported by Adelstein et al.,167 in the E1392 study, are consistent with this assessment. Although daily,168 weekly,46,169 and every 3 week schedules of cisplatin intravenously concurrent with RT have been applied, the last schedule is the one most studied and is a widely accepted standard. If weekly dosing is used, 20 mg/m2 weekly appears too low because it did not significantly improve overall survival or failure-free survival in one randomized study.169 Attempts to improve the efficacy of concurrent cisplatin through intra-arterial administration170,171 did not prove more efficacious in a randomized trial when compared to intravenously delivered cisplatin, although toxicity profiles differed.172 In absence of a proven efficacy advantage with intra-arterial delivery, intravenous cisplatin is preferred because it is logistically easier to administer.
Most randomized trials to date have compared chemoRT to RT alone. As such, studies evaluating the efficacy of different chemoRT programs are limited. For example, for purposes of the MACH-NC analysis, “platin” included both cisplatin and carboplatin. Yet the relative efficacy of these agents, when given concurrently, is not well-studied. In one three-arm randomized study by Jeremic et al.168 using a daily schedule for each drug with RT and a control arm of RT alone, both the cisplatin (6 mg/m2 per day) and carboplatin (25 mg/m2 per day) arms appeared comparable, and superior in efficacy to RT alone. However, in a randomized study reported by the Hellenic Cooperative Oncology Group using an every 3 week schedule for each drug (cisplatin 100 mg/m2, carboplatin AUC, 6), their equivalence seemed less clear.173 The RTOG reported a randomized phase II study comparing three different chemotherapy regimens, all delivered concurrently with 70 Gy in 2 Gy fractions: ARM 1, cisplatin 10 mg/m2 per day and 5-fluorouracil 400 mg/m2 per day continuous infusion for the final 10 days of treatment; ARM 2, hydroxyurea 1 g every 12 hours and 5-fluorouracil 800 mg/m2 per day continuous infusion every other week; or ARM 3, weekly paclitaxel 30 mg/m2 and cisplatin 20 mg/m2. Among 231 analyzable patients, 2-year disease-free and overall survival rates were for ARM 1, 38.2% and 57.4%; for ARM 2, 48.6% and 69.4%; and for ARM 3, 51.3% and 66.6%, respectively.174
Because anemia may adversely affect the efficacy of RT, the integration of an appropriate hematopoietic growth factor has been investigated. In a multicenter, double-blind, randomized, placebo-controlled trial, the addition of erythropoietin 300 IU/kg three times weekly during postoperative RT was evaluated in 351 patients with head and neck SCC.175 Although target hemoglobulin levels were reached in 82% of patients receiving erythropoietin compared to 15% receiving placebo, local–regional progression-free survival (adjusted RR, 1.62 [95% CI, 1.22 to 2.14]; p = 0.0008), local–regional progression (RR, 1.69 [CI, 1.16 to 2.47]; p = 0.007), and survival (RR, 1.39 [CI, 1.05 to 1.84]; p = 0.02) were all inferior on the erythropoietin arm. Consistent with the current FDA alert, an erythropoietin-stimulation agent is contraindicated during curative-intent RT-based therapy.176 Transfusion then, is the preferred approach to address potential radiation resistance attributed to anemia, but a recent analysis of two randomized trials failed to demonstrate that prophylactic transfusion improved overall survival or other disease control endpoints.177 Use of a hypoxic radiosensitizer represents another strategy to potentially address tumor hypoxia. The results of one meta-analysis were consistent with potential benefit178; recent randomized trials, however, have not been convincing in terms of improved disease control with such a strategy.179,180
An important question is whether the use of newer, more efficacious, and altered fractionated RT programs181 obviates the benefits accrued with the addition of chemotherapy. A single institution study reported by Brizel et al.182 compared a more aggressive RT schedule with or without concomitant 5-fluorouracil and cisplatin. Patients who underwent RT alone received 75 Gy in 60 twice-daily fractions; those who underwent concomitant chemotherapy received 70 Gy in 56 twice-daily fractions with a 7-day split. Chemotherapy consisted of two cycles of concomitant cisplatin 12 mg/m2 per day and 5-fluorouracil 600 mg/m2 per day each for 5 days, followed by two cycles of maintenance chemotherapy. Among the 116 patients included, chemoRT was associated with improved 3-year rates of local–regional control (70% versus 40%, p = 0.01), relapse-free survival (61% versus 41%, p = 0.08), and overall survival (55% versus 34%, p = 0.07). In another randomized study reported by Jeremic et al.,183 the addition of daily cisplatin to hyperfractionated RT also leads to incremental benefits. The results of these studies are consistent with the MACH-NC analysis, which demonstrated significant HRs, that are consistent with benefits among patients receiving postoperative RT (HR, 0.79), conventional RT (HR, 0.83), or altered fractionated RT (HR, 0.73), suggesting a benefit for adding concomitant chemotherapy regardless of the type of RT schedule.166
Of note, the converse—once concurrent chemotherapy is added, does an altered fractionation RT schedule further improve outcome compared to that seen with standard fractionation—has not been established in randomized trials. Neither RTOG 0129 (standard versus concomitant boost RT both with concurrent high-dose cisplatin)8 nor GORTEC 99-02 (accelerated RT with or without concurrent carboplatin and 5-flourouracil, standard fractionated RT with concurrent carboplatin and 5-flourouracil)184 demonstrated improved overall survival with the incorporation of altered fractionated RT with concurrent chemotherapy versus standard fractionation with concurrent chemotherapy to justify the added logistical complexity and potential added toxicity.
For patients who are not cisplatin candidates, using a carboplatin-based program (e.g., carboplatin/5-fluorouracil)147 or other concurrent programs that have different side effect profiles and that withstood the scrutiny of a randomized trial is recommended. There has been great interest in cetuximab and concurrent RT in this regard.185 In a randomized study reported by Bonner et al.,186 patients with local–regionally advanced head and neck cancer were randomized to RT alone (213 patients) or combined with weekly cetuximab dosed in a standard fashion (211 patients); median follow-up was 54 months. The median duration of survival was 49 months after combined therapy compared with 29 months after RT alone (p = 0.03). Other than an acneiform rash and infusion reactions, grade 3 or greater complications were similar in the two groups of patients. The results of this trial have been confirmed with longer follow-ups.186 Patients with oropharynx cancer appeared to derive the largest benefit with the integration of cetuximab, suggesting that HPV-related disease may have an improved outcome with the use of this agent; other studies, however, suggest greater activity of EGFR-directed antibody therapy in HPV-negative disease.114,119 Randomized data comparing cetuximab and RT to other chemoRT programs are not available. One RTOG study evaluating concurrent cisplatin and RT versus cetuximab and RT in patients with HPV-related cancers is currently in progress. Investigators at Memorial-Sloan Kettering reported a phase II trial of cisplatin and cetuximab concurrent with RT in patients with local–regionally advanced head and neck SCC. Efficacy was impressive, although there were toxicity concerns.187 The RTOG completed accrual to a large randomized trial (RTOG 0522, n = 895 evaluable patients) intended to assess the efficacy and safety of this regimen compared to concurrent cisplatin and RT. With a median follow-up of 2.4 years among surviving patients, the incorporation of cetuximab failed to significantly improve 2-year progression-free survival (63% versus 64%, p = 0.66) or overall survival (83% versus 80%, p = 0.17), but mucosal and skin toxicity were increased.188
Choosing among the numerous concurrent programs can be difficult. In the NCCN guidelines,146 concurrent cisplatin with RT is the preferred choice, although several other options are listed. It is important to emphasize that concurrent chemoRT may be associated with significant toxicity; treatment-related mortality, albeit infrequent (<5% in the cooperative group setting), may occur. Morbidity from chemotherapy (dependent on the agent chosen) and RT are possible, and there are both acute (e.g., mucositis, blood count suppression) and chronic (e.g., dry mouth, swallowing dysfunction, fibrosis) toxicities. Selected studies have begun to report long-term, not just acute, toxicities.189 Appropriate infrastructure, an experienced multidisciplinary team, and a cooperative patient are necessary to optimize both efficacy and safety.
Nasopharynx Cancer
Current practice has been particularly affected by the Intergroup Study 0099 (Table 38.9).190 In it, 147 patients with stage III or IV NPC were randomized to definitive RT (70 Gy, 35 fractions over 7 weeks) versus cisplatin 100 mg/m2 intravenously on days 1, 22, and 43 concurrent with the same dose of RT followed by three planned cycles of cisplatin and infusional 5-fluorouracil. Although only 63% and 53% of patients received all the planned concurrent and maintenance treatments, respectively, local–regional control, distant control, progression-free, and overall survivals were all significantly improved with chemoRT.
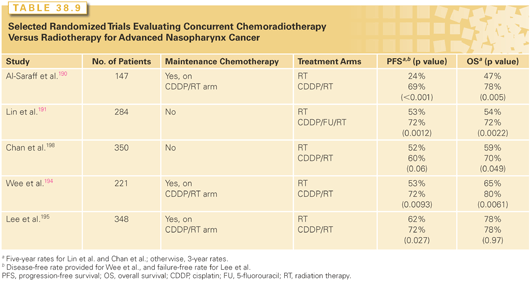
One of the potential limitations of the Intergroup Study was how generalizable its results would be to endemic NPCs, because 24% of patients entered in the trial had World Health Organization (WHO) type I histology. However, subsequent reports of randomized trials in which WHO types II and III predominated have similarly shown a survival advantage with concurrent cisplatin-based concurrent chemotherapy without191–193 or with maintenance chemotherapy.194,195
One relatively small randomized trial (n = 206) with a median follow-up of 26.3 months, suggested that weekly carboplatin dosed at 100 mg/m2 concurrent with RT when compared to standard high-dose cisplatin, did not yield inferior disease-free or overall survival. Both arms received adjuvant platin and 5-fluorouracil.196 However, another randomized study involving 408 patients who received concurrent carboplatin at AUC 6 every 3 weeks after induction chemotherapy failed to demonstrate an improvement in overall survival at 5 years or other disease outcomes compared to patients receiving radiation alone after the same induction regimen.197
Another limitation of the Intergroup Study was that it was not designed to delineate the proportional benefits of concurrent and maintenance chemotherapy. Although current NCCN guidelines recommend concurrent and maintenance chemotherapy in M0 patients with a more advanced disease based on the Intergroup experience,146 in reviewing available data, the benefits of maintenance chemotherapy appear more controversial. As noted, other randomized studies have demonstrated a survival improvement with concurrent therapy alone.191,198 Earlier randomized trials, summarized elsewhere, failed to demonstrate a survival benefit when either maintenance or induction chemotherapy was added to definitive RT.199,200 Furthermore, a meta-analysis of updated individual patient data on 1,753 patients enrolled in eight randomized trials, besides confirming an absolute survival benefit of 6% at 5 years with incorporation of chemotherapy with RT (HR, 0.82, 95% CI, 0.71 to 0.91; p = 0.006), also reported a significant association between the timing of chemotherapy and overall survival (p = 0.005), with the largest benefit being attributed to concomitant therapy.201 A recently reported randomized trial involving 508 patients with nonmetastatic, stage III to IV nasopharyngeal cancer failed to demonstrate a significant difference in 2-year failure-free survival, overall survival, local–regional failure-free survival, or distant failure-free survival after a median follow-up of 37.8 months. Of note, the direction of each of the previous endpoint comparisons favored the adjuvant arm, albeit not significantly so, with associated p values of 0.13, 0.32, 0.10, and 0.12, respectively.202 A longer term follow-up of this trial will be of interest. Currently, the NCCN guidelines list both concurrent chemoRT followed by maintenance chemotherapy and concurrent chemoRT alone as treatment options for advanced disease, with the former having the higher category rating.146
Selected randomized studies have demonstrated evidence of a positive biologic effect with the use of induction chemotherapy, but no survival benefit has been documented.200–205 Such promising results have engendered interest in the potential for enhanced efficacy with newer drugs and combinations. A randomized phase II trial evaluating induction with docetaxel and cisplatin prior to concurrent cisplatin and RT was consistent with a benefit compared with concurrent cisplatin and RT alone.200 Programs incorporating newer taxane- and cisplatin-based triplet induction regimens warrant further study.206 There is also interest in the role of plasma EBV–DNA assays as a way to assess disease and monitor response.199
Organ Preservation
Organ preservation therapy is intended to control disease without compromise in survival while optimizing function or cosmesis.207 The term implies that the tumor is potentially resectable for cure, and that the morbidity from surgery is significant. Although conservation surgical procedures can achieve the same goals, the label of organ preservation is more commonly applied to nonsurgical approaches. In that regard, the role of chemotherapy integrated with RT is best established for more advanced primary tumors. In this setting, conservation surgical procedures become less feasible, and local control rates with RT alone are lower than seen with earlier stage disease.
Total laryngectomy is one of the surgical procedures most feared by patients.208 Thus, larynx preservation has been a central focus of many organ preservation studies, including those that established integrated chemotherapy and RT as a standard organ preservation treatment option. Studies commonly focused on patients with advanced tumors of the larynx, hypopharynx, and oropharynx (particularly the base of tongue), in whom primary surgical management would jeopardize the voice box.209
Initial chemoRT approaches to larynx preservation utilized induction chemotherapy. The response to initial chemotherapy was used to triage patients to either definitive RT (a partial response or better at the primary site; surgery to the primary site was reserved for salvage) or primary surgical management (lower than a partial response). The randomized and landmark Veterans Administration (VA) Larynx Preservation Study demonstrated that such an approach could be pursued in patients with advanced laryngeal cancer without compromise in survival when compared to primary treatment with surgery and RT.151 Over 60% of patients on the chemoRT arm avoided total laryngectomy. Among long-term survivors, patients treated on the chemoRT arm had better emotional well-being, were less depressed, and also reported less pain.210
A similarly designed randomized trial in patients with pyriform sinus and aryepiglottic fold tumors reported by the European Organization for Research and Treatment of Cancer (EORTC) confirmed these findings.152 However, a small randomized study (n = 68) limited to patients with T3 disease with a fixed cord done by the Groupe d’Etude des Tumeurs de la Tete et du Cou (GETTEC) reported that survival was superior on the primary surgery arm (84% versus 69% at 2 years, p = 0.006).211 When the MACH-NC performed a collective analysis of the VA, EORTC, and GETTEC studies, the rate of larynx preservation among survivors was 58%. A nonsignificant (6%) decrement in survival at 5 years was seen in the chemoRT group (39% versus 45%; pooled HR, 1.19; 95% CI, 0.97 to 1.46; p = 0.10).149
The data reviewed in the prior section highlighting the therapeutic benefits of a concurrent chemoRT relative to an induction or RT alone approach have obvious implications for the larynx preservation setting. RTOG 91-11 was designed to assess the impacts of adding chemotherapy to RT and its timing (concurrent versus induction) with regard to achieving larynx preservation. Four hundred and ninety-seven patients with larynx cancer were randomized to one of three arms: primary RT, 70 Gy to the primary site, 50 to 70 Gy to nodes; induction chemotherapy with cisplatin and infusional 5-fluorouracil for three cycles followed by RT in responders, surgery in nonresponders; and cisplatin 100 mg/m2 on days 1, 22, and 43 concurrent with RT. Surgical salvage was an option on all three arms. The recently updated 10-year results are summarized in Table 38.10.148,212 As anticipated, the rate of grade 3 or 4 mucosal toxicity was highest on the concurrent arm; however, this did not translate into more significant speech or swallowing impairment at 2 years compared to the other treatment arms. Noteworthy is that, although the larynx preservation rate and local–regional control was highest and statistically superior with concurrent treatment, there was no significant difference in overall survival rates among the arms. Deaths not attributed to larynx cancer were highest in the concurrent arm (30.8%) versus 20.8% on the induction arm and 16.9% in the RT alone group, raising concern regarding the long-term morbidity of concurrent therapy. However, late effects were similar among the groups, and there were no substantial differences in speech or swallowing function reported.212
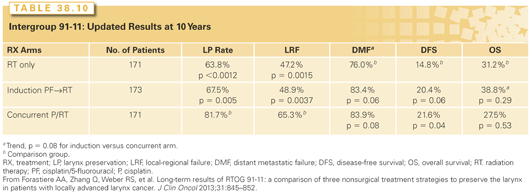
In another randomized larynx-preservation study, induction chemotherapy followed by RT and alternating chemotherapy and RT approaches were compared in 450 patients with advanced larynx or hypopharynx cancer. Both treatment arms used cisplatin and 5-flourouracil and allowed surgical salvage. Overall and progression-free survival rates were similar on both arms. Survival with a functional larynx in place was higher with alternating chemoRT, although the difference was not statistically significant (median, 2.3 years versus 1.6 years; HR, 0.85; 95% CI, 0.68 to 1.06).213
Available randomized phase III data support a concurrent chemotherapy–RT strategy administered with organ preservation intent for patients with advanced oropharynx cancer. A phase III study from the Groupe d’Oncologie Radiothérapie Tête et Cou (GORTEC) demonstrated improved local–regional control (66% versus 42% at 3 years, p = 0.03) in patients with advanced oropharynx cancer who received concurrent chemotherapy (carboplatin and 5-fluorouracil) and RT (70 Gy) compared to RT alone.147 A non–site-specific trial from the Cleveland Clinic, which included a high proportion of patients with advanced oropharynx cancer, yielded similar results.214
Although concurrent chemotherapy is the current cornerstone of organ preservation treatment of advanced disease, other paradigms deserve mention. In an Italian study, 195 patients with T2 to T4 oral cavity cancer were randomized to either primary surgical management or induction chemotherapy with cisplatin and 5-fluorouracil followed by a surgical procedure, which could be modified based on response. Overall survival was similar on both arms, but less postoperative RT was necessary (33% versus 46%) and fewer mandible resections were performed (31% versus 52%) on the chemotherapy arm.215 As a further extension of this concept, Laccourreye and colleagues216 have pioneered the selective observation without local–regional treatment of patients with laryngeal cancer who have a complete response to induction chemotherapy. Durable tumor control without the addition of surgery or RT has been reported in a small subset of patients with early stage tumors.216 The University of Michigan has developed a larynx preservation program whereby the triage to RT-based treatment or surgery occurs after only one cycle of chemotherapy.217 The intent is to improve survival and minimize morbidity through the timely selection of appropriate therapy, including referral to surgery if indicated. The implication is that induction chemotherapy has little other therapeutic benefit, and some patients who are slow to respond may be triaged unnecessarily to total laryngectomy.218 Conversely, newer sequential strategies of induction chemotherapy followed by planned concurrent chemotherapy are looking to optimize both local–regional and distant control. Induction with more efficacious triplet chemotherapy, including a taxane combined with cisplatin and 5-fluorouracil, is already being incorporated into larynx preservation strategies with evidence of improved larynx preservation rates.219
Adjuvant Therapy after Surgery
The use of maintenance chemotherapy after the completion of local–regional treatment has been evaluated in several randomized trials, but with disappointing results. Suboptimal compliance with maintenance treatment may in part explain the lack of benefit, because tolerance of chemotherapy can be poor after surgery and RT.220 Despite these limitations and the lack of convincing survival benefit, patterns of failure were affected in selected studies, with a decrease in distant metastases, consistent with a biologic effect of chemotherapy.220,221
The results of Intergroup 0034 highlight these points. In this trial, 442 analyzable patients were randomized after definitive surgical therapy to either postoperative RT alone (50 to 60 Gy) or three cycles of standard dose cisplatin and 5-fluorouracil by infusion followed by the same RT-dosing scheme. The randomization was stratified by risk, with surgical margins less than 5 mm, cancer in situ (CIS) at the margins, and ECE being considered high-risk features. Overall, there was no significant difference in overall survival, disease-free survival, or local–regional control between the treatment arms, although there was a significant decrease in incidence of distant metastases on the investigational arm (p = 0.03). Interestingly, on a subset analysis, adjuvant chemotherapy had no significant impact in the low-risk group, but a more dramatic impact on survival and tumor control was seen among high-risk patients. Given the success of concurrent chemoRT as definitive treatment, its application in the adjuvant setting was a logical extension. A pilot study done by the RTOG demonstrated that concurrent high-dose cisplatin every 3 weeks with RT was feasible in the adjuvant setting.222 Early randomized studies demonstrated an improvement in local–regional control with the incorporation of concurrent mitomycin.223,224 A study comparing weekly cisplatin concurrent with postoperative RT versus RT alone with ECE, yielded a significant improvement in both local–regional control and survival with combined modality treatment.46
Two randomized studies, both published in 2004, have further clarified the indications for postoperative chemoRT in the poor risk adjuvant setting. RTOG 9501 and EORTC 22931 had very similar designs.43–45,209 Patients were randomized after surgery if they had poor risk features to either standard postoperative RT alone (60 to 66 Gy, over 6 to 6.5 weeks, standard fractionation) or the same RT with three planned cycles of concurrent cisplatin at 100 mg/m2 every 3 weeks. What constituted poor risk differed somewhat between the studies: the RTOG required the presence of two or more positive lymph nodes, ECE, or positive margins; the EORTC required ECE, positive margins, pT3 or pT4 with any N, N2, or N3 disease, level IV nodes or stage IV disease in patients with oral cavity or oropharynx primaries, PNI, or vascular embolism.43–45 Both studies demonstrated a significant improvement in local–regional control and disease-free or progression-free survival with combined modality therapy. These improvements translated into a significant advantage in overall survival in the EORTC study (p = 0.02), but only a trend (p = 0.19) in the RTOG study. Neither study showed a significant impact on distant control with the addition of chemotherapy. Acute toxicity was greater with the addition of the cisplatin, but there was no difference in late toxicity.
A subsequent analysis of the EORTC and RTOG studies was performed to better understand which pathologic subgroups may benefit the most from the concurrent addition of cisplatin to RT.203 Patients having evidence of ECE or a positive margin derived the largest benefit from combined modality adjuvant therapy. Conversely, patients in whom their only poor risk factor was two or more positive lymph nodes without ECE seemed to do just as well with RT alone. Long-term follow-up of RTOG 9501 yielded results consistent with this conclusion.45
The EORTC and RTOG studies focused on patients who were previously untreated with the exception of prior surgery. Janot et al.,225 on behalf of the GETTEC and GORTEC groups, addressed the potential role of concurrent chemotherapy and reirradiation after salvage surgery. In this randomized study enrolling 130 patients, the standard arm was salvage surgery alone. The combined modality treatment significantly improved disease-free survival (HR, 1.68 [95% CI, 1.13 to 2.5], p = 0.01), although overall survival was not significantly improved and both acute and chronic toxicities were worse.
Some investigators have questioned whether extracapsular nodal spread has the same adverse prognostic implications in patients with HPV-related disease.226 The related therapeutic question is whether this pathologic risk factor should drive the addition of concurrent chemotherapy to radiation in the postoperative setting for patients with HPV-related disease. These issues are the focus of ongoing investigations. For now, the NCCN does not recommend different approaches to treatment in the poor-risk adjuvant setting based on tumor HPV status.146
Toxicity Reduction
Xerostomia is one of the most troubling side effects of RT-based treatment. Extra oral fluids, artificial saliva, other topical measures, and humidity are commonly utilized. Available data indicates that IMRT applied with salivary-sparing intent decreases this symptom.34,227,228 Historically, the emphasis has been on parotid sparing. Sparing other salivary glands when possible further improves salivary outcomes.229,230 Pharmacotherapy may also help. The cholinomimetic and muscarinic agent, pilocarpine, at a dose of 5 mg three times a day was shown in a randomized trial to improve the production of saliva as well as symptoms of dry mouth compared to placebo in patients treated with at least 40 Gy to the head and neck.231 Excessive sweating was the most common side effect. Cevimeline, a similar agent with a more selective mechanism of action, was associated with a significant increase in unstimulated salivary flow at dosing of 30 to 45 mg three times a day.232 Amifostine is a thiol with chemo- and radioprotectant properties. Objective and subjective measures of salivary function were improved in an open-label, randomized study among patients who received 200 mg/m2 of amifostine intravenous daily 15 to 30 minutes before RT.233 Grade 3 toxicities were infrequent, but nausea, vomiting, hypotension, and allergic reactions were more common among patients treated with amifostine. The benefits when given with chemoRT are more controversial.234 There have been concerns regarding the potential of tumor protection by amifostine, but a recent meta-analysis did not demonstrate any adverse impact on progression-free or overall survival in patients treated with RT or chemoRT.234 Finally, acupuncture may be of benefit in the management of xerostomia in selected patients.38,235
Mucositis is a troubling symptom typically exacerbated by aggressive altered fractionated RT programs and the use of concurrent chemoRT. A variety of rinses (e.g., topical anesthetics, antifungals) and systemic pain medications are used for symptomatic relief. Concurrent amifostine with RT has not been clearly shown to decrease mucositis.233,234 Iseganan hydrochloride, a synthetic peptide with broad spectrum antibacterial activity, was evaluated in a multinational, double-blind, placebo-controlled trial among patients receiving definitive or postoperative RT-based therapy. No improvement in oral mucositis was found compared to placebo.236 Recombinant human keratinocyte growth factor has been shown to decrease the incidence and duration of mucositis in the transplant setting.237 Two randomized studies demonstrated improvement in observer-assessed mucositis with palifermin use, but no clear difference in patient-reported pain, opioid analgesic use, or treatment breaks when compared to placebo.238,239
How to optimally follow-up with patients after treatment for head and neck cancer is less well studied than how to treat it. Approaches are informed by patterns of failure. Most relapses occur within the first 3 years and are front loaded, and relapses above the clavicles are potentially curable. The lung is the most common site of distant spread.
The schedule proposed in the NCCN practice guidelines is reasonable.146 Patients are seen for follow-up head and neck examinations every 1 to 3 months during year 1, every 2 to 6 months during year 2, every 4 to 8 months during years 3 to 5, and every 12 months thereafter. Thyroid function tests are obtained every 6 to 12 months if the neck was irradiated. Many practitioners obtain annual chest x-rays or other chest imaging to monitor for a second primary lung cancer and to document distant metastases. The impact of this imaging on outcomes is not well established, which is consistent with a vague recommendation from the NCCN of chest imaging “as clinically indicated.” Tobacco history should be considered for purposes of lung cancer screening.146 Posttreatment baseline imaging within 6 months of therapy is recommended. Additional studies such as CT, MRI, and PET scans may subsequently be necessary to determine whether there is a recurrence or a complication, but otherwise are not routinely performed in surveillance. Speech and swallowing evaluations and rehabilitation are obtained as indicated. Counseling is indicated for patients in whom tobacco or alcohol contributed as a risk factor for tumor development.
The oral cavity consists of the lips, the floor of mouth, the anterior two-thirds of the tongue, the buccal mucosa, the upper and lower alveolar ridges, the hard palate, and the retromolar trigone.
The AJCC staging system is used.26
The ratio between men and women with lip cancer is approximately 15:1.240 Persons with light-colored skin and/or prolonged exposure to sunlight are most prone to develop lip carcinoma.
Anatomy
The lips are composed of the orbicularis oris muscle with skin on the external surface and mucous membrane on the internal surface. The transition from skin to mucous membrane is the lip vermilion. The blood supply is from the labial artery, a branch of the facial artery. The motor nerves are branches of cranial nerve (CN) VII. The sensory nerve to the upper lip is the infraorbital branch of CN V (V 2), and the mental nerve (V 3) supplies the lower lip.
Pathology
The most common neoplasms are SCCs. Basal cell carcinomas arise on the skin of the lip and may secondarily invade the vermilion. Keratoacanthoma occurs on the skin of the lips and may be mistaken grossly and histologically for SCC.
Leukoplakia and CIS are common problems on the lower lip and may precede the appearance of carcinoma by many years. Primary lesions arising from the moist mucosa of the lip are considered under the section Buccal Mucosa.
Patterns of Spread
SCC can originate from the skin of the lip or the vermilion, which may invade the adjacent skin and orbicularis muscle. Advanced lesions invade the adjacent commissures of the lip, the buccal mucosa, the skin and wet mucosa of the lip, the adjacent mandible, and eventually the mental nerve. PNI occurred in 2% of the cases reported by Byers and coworkers241 and was related to recurrent lesions, large tumor size, mandibular invasion, and poorly differentiated histology. Lymphatic spread is to the submental (IA) and submandibular (IB) lymph nodes and then to the jugular chain. The risk for lymph node metastases is approximately 5% at diagnosis and is increased by high-grade histology, large lesions, invasion of the mucosa of the lip, and for patients with recurrent disease.
Clinical Picture
The vermilion of the lower lip is the most common site of origin. SCC may present as an enlarging discrete lesion that is not tender until it ulcerates. Some lesions develop slowly on a background of leukoplakia or CIS and present as superficially ulcerated lesions with little or no bulk. Erythema of the adjacent skin suggests dermal lymphatic invasion. Palpation of the lip will reveal the extent of induration. Paresthesia of the skin of the lip indicates PNI.
Treatment
Selection of Treatment Modality
Early lesions may be cured equally well with surgery or RT. Surgical excision is preferred for the majority of lower lip lesions up to 2 cm in diameter that do not involve the commissure; the treatment is simple and the cosmetic result is satisfactory. Removal of more of the lip with simple closure usually results in a poor cosmetic and functional result and, therefore, requires reconstructive procedures. RT is often preferred for lesions involving the commissure, for lesions over 2 cm in length, and for upper lip carcinomas. Advanced lesions with bone, nerve, or node involvement frequently require a combined modality approach.
The regional lymphatics are not treated electively for early cases. Advanced lesions, high-grade lesions, and recurrent lesions should be considered for elective neck treatment. Clinically positive nodes are managed as previously discussed in Clinically Positive Neck Lymph Nodes.
Surgical Treatment
Surgical treatment for early lesions (0.5 to 1.5 cm) utilizes a V- or W-shaped excision, depending on the size of the defect, which facilitates cosmetic primary closure. If the vermilion is diffusely involved with little or no involvement of the muscle, a vermilionectomy may be performed and the mucosa from the labial vestibule of the oral cavity advanced to cover the defect.
Irradiation Technique
Lip cancer may be successfully treated by EBRT, interstitial brachytherapy, or a combination of both. Interstitial brachytherapy may be accomplished with removable sources such as iridium-192 (192Ir). EBRT techniques use orthovoltage (55.8 Gy at 1.8 Gy per fraction) or electrons (60 to 66 Gy at 2 Gy per fraction) with lead shields behind the lip to limit exit EBRT. IMRT is not indicated except for the occasional patient with advanced neck disease and/or clinical PNI where it is necessary to extend the dose distribution to the skull base and reduce the dose to the contralated parotid. For more advanced lesions, combining chemotherapy with EBRT is appropriately considered.146
Results of Treatment
MacKay and Sellers242 reviewed 2,864 patients with all stages of lip cancer, of whom 92% were managed initially by RT. The primary lesion was controlled by the initial treatment in 84% of cases; an additional 8% were salvaged by later treatment for an overall local control rate of 92%. Of those who presented with clinically involved nodes, 58% had control of disease, but only 35% had control of disease when neck nodes appeared later. The 5-year, cause-specific survival rate was 89%; the 5-year absolute survival rate was 65%.
Mohs and Snow243 reported the results for 1,448 patients treated with microscopically controlled surgery for SCCs of the lower lip between 1936 and 1976. Eighty-three percent had cancers less than 3 cm in diameter, with a 5-year cure rate of 96.6%. For 192 patients with cancers that measured 2 cm or more, the cure rate dropped to 60%.
Complications of Treatment
Oral competence, which permits patients to control oral secretions and effectively suck, speak, and swallow, requires the sphincteric function of an intact orbicularis oris muscle. Hence, disruption of the sphincteric function resulting from division of the orbicularis oris should be restored. Microstomia and drooling secondary to oral incompetence may occur after a large flap reconstruction. If the oral opening is too small, the patient may not be able to inset a denture.
There will be some atrophy of the irradiated tissues; this progresses with time. Soft tissue necrosis may occur, but this problem is reduced by plans that prolong the treatment.
Anatomy
The floor of the mouth is a U-shaped area bounded by the lower gum and the oral tongue; it terminates posteriorly at the anterior tonsillar pillar. The paired sublingual glands lie immediately below the mucous membrane; the paired genioglossus and geniohyoid muscles separate them. Bony protuberances, the genial tubercles, occur at the point of insertion of these two muscle groups at the symphysis. The mylohyoid muscle arises from the mylohyoid ridge of the mandible and is the muscular floor for the oral cavity; it ends posteriorly at about the level of the third molars. The submandibular gland rests on the external surface of the mylohyoid muscle between the mandible and the insertion of the mylohyoid. The submandibular duct (the Wharton duct) is about 5 cm long. It courses between the sublingual gland and the genioglossus muscle and exits in the anterior floor of the mouth near the midline.
Pathology
Most neoplasms are SCC, usually of moderate grade. Adenoid cystic and mucoepidermoid carcinomas account for about 5% of malignant tumors in this area.
Patterns of Spread
Primary
Approximately 90% of neoplasms originate within 2 cm of the anterior midline floor of the mouth, penetrating early beneath the mucosa into the sublingual gland and eventually into the genioglossus and geniohyoid muscles. The mylohyoid muscle acts as an effective barrier until the lesion becomes advanced. Extension toward the gingiva and periosteum of the mandible occurs early. When the tumor reaches the periosteum, the tumor usually spreads along the periosteum rather than through it. Mandible invasion is a late manifestation. The skin of the lower lip may be involved in advanced cases. Posterior extension occurs in the muscles of the root of the tongue. One or both submandibular ducts are frequently obstructed by the tumor or after the biopsy; it may be difficult to distinguish between tumor extension and infection in an obstructed duct. The submandibular gland frequently enlarges, becoming firm and occasionally painful when the duct is obstructed. Extensive lesions may follow the anatomic plane of the mylohyoid muscle to its posterior extremity and emerge in the submandibular space of the neck.
Lymphatic
Approximately 30% of patients will have clinically positive nodes on presentation; 4% will have bilateral nodes. The reported incidence of conversion from N0 to N+ with no neck treatment varies from 20% to 35%.39,244 For T1 or superficial T2 lesions, the risk for occult metastasis is probably 10% to 15%.39,244
The first nodes involved are in levels IB and II; the risk for bilateral spread is fairly high.
Clinical Picture
On physical examination, the earliest lesions appear as a red area, slightly elevated, with ill-defined borders, and very little induration. As the lesion enlarges, the edges of the tumor become distinct, elevated, and “rolled,” with a central ulceration and induration. Some lesions start with a background of leukoplakia. Bimanual palpation will determine the extent of the induration and the degree of fixation to the periosteum. Large lesions bulge into the submental space and rarely grow through the mylohyoid muscle into the soft tissues of the neck. Gross invasion of the mandible may be detected, especially when the anterior teeth have been removed. A tumor may grow through the mandible to involve the gingivolabial sulcus and lip. The submandibular duct and gland are evaluated by bimanual palpation.
Treatment
Selection of Treatment Modality
Early Lesions. Surgery or RT are equally effective treatments for T1 or T2 lesions. Most patients are treated surgically because of the risk of soft tissue or bone necrosis after RT.
A few patients are seen after excisional biopsy of a tiny lesion, and the only finding is a surgical scar with varying degrees of induration under the scar (TX). The margins are often equivocal. These patients are treated with reexcision or brachytherapy.
Moderately Advanced Lesions. The usual recommendation for moderately advanced anterior midline lesions is rim resection or segmental mandibulectomy and osteomyocutaneous free flap reconstruction; postoperative RT or chemoRT is added depending on the pathologic findings. The clinically N0 neck is usually managed by a bilateral functional neck dissection for midline lesions.
Advanced Lesions. Massive lesions have a poor prognosis with combined surgery and postoperative chemoRT. Only palliation can be offered in some cases.
Surgical Treatment
Wide Local Excision.Small lesions (5 mm or less in size) may be excised transorally with a 1-cm margin with primary closure or a skin graft. If the duct is involved, the submandibular gland and duct are removed in continuity.
Rim Resection. Rim resection of the mandible in continuity with excision of the primary lesion preserves the arch and may be combined with postoperative RT. Periosteal invasion is often an indication for this procedure. Patients who have been edentulous for a long time may have an atrophic mandible and are not suitable because the mandible is likely to fracture.
Segmental Mandibulectomy.A partial segmental mandibulectomy with resection of the floor of the mouth is done for lesions invading bone. An osteomyocutaneous flap is usually used to repair the defect.
Irradiation Technique
Superficial T1 cancers are treated with either brachytherapy or intraoral cone RT to approximately 65 Gy, and the neck is observed. Larger lesions are treated with EBRT to 45 to 50 Gy over 5 weeks followed by an interstitial implant for an additional 20 to 30 Gy. Lesions that are suitable for intraoral cone RT may be boosted with this technique prior to EBRT of the primary lesion and upper neck. Use of EBRT alone results in suboptimal cure rates and is discouraged.245
External-Beam Irradiation. Opposed lateral EBRT portals are used to treat anterior floor of the mouth carcinomas. The entire width of the mandibular arch is included and the superior border is shaped to spare part of the parotid gland. The level I and level II nodes are included to the level of the thyroid notch if the neck is clinically negative; the lower neck may be electively irradiated. If the neck is clinically positive, the portals are enlarged to include all of the upper neck nodes, and an en face low neck field is added. IMRT may be useful to reduce the dose to the contralateral parotid in patients with positive nodes.
Interstitial Irradiation. The implantation of T1 to T2 lesions confined to the floor of the mouth with minimal extension to the mucosa of the tongue can be accomplished with iridium using the plastic tube technique.
Intraoral Cone Irradiation. Intraoral orthovoltage or electron cone RT requires daily positioning by the physician and is preferable to interstitial RT because there is little or no irradiation of the mandible.246 An intraoral cone can be used for well-circumscribed anterior superficial lesions and is easiest to perform in the edentulous patient.
Combined Treatment Policies
Postoperative RT is preferred, because the risk of bone complications and fistulae is higher with preoperative RT. Concurrent chemotherapy may be necessary based on pathologic findings.
Management of Recurrence
RT failures are treated by an operation. The salvage rate is good for patients with T1 to T2 lesions and poor for those with more advanced lesions.
Surgical treatment failures may be treated by a repeat operation and postoperative RT.
Results of Treatment
Rodgers et al.247 reported on 194 patients treated with surgery and/or RT at the University of Florida between 1964 and 1987. The local control rates after RT versus surgery alone or combined with RT were: T1, 32 out of 37 (86%) versus 10 out of 11 (91%); T2, 25 out of 36 (69%) versus 16 out of 19 (84%); T3, 11 out of 20 (55%) versus 9 out of 9 (100%); and T4, 2 out of 5 (40%) versus 6 out of 10 (60%).247 The 5-year cause-specific survival rates were comparable for the treatment groups.247 Mild-to-moderate and severe complications were observed as follows: RT alone, 49 out of 117 (42%) and 6 out of 117 (5%); surgery alone, 3 out of 36 (8%) and 6 out of 36 (17%); and surgery and RT, 8 out of 41 (20%) and 6 out of 41 (15%), respectively.247
Two hundred seven patients treated with RT alone at the Centre Alexis Vautin between 1976 and 1992 were reviewed by Pernot and colleagues.248 Local control and cause-specific survival rates at 5 years were as follows: T1, 97% and 88%; T2, 72% and 47%; and T3, 51% and 36%, respectively. Six percent of patients developed complications necessitating surgical intervention and one patient experienced a fatal complication.
Follow-Up
There are two major difficulties in follow-up after RT: soft tissue ulcers and enlarged submandibular glands. An ulcer in the floor of the mouth within 2 years of treatment can be either a recurrence or necrosis. If the lesion appears to be soft tissue necrosis, a trial of conservative therapy is adequate. Failure to stabilize or resolve is an indication for biopsy. A negative biopsy does not rule out recurrence, and repeat deep biopsies may be necessary. An enlarged submandibular gland(s) may be a sequel to obstruction of the submandibular duct; a contrast-enhanced CT is useful to distinguish between an enlarged submandibular gland and a tumor in a lymph node.
The follow-up for surgical cases may be difficult if skin grafts or flaps have been used because of the associated induration and thickness of the flaps. If the submandibular ducts have been reimplanted, stenosis may occur with subsequent enlargement of the submandibular glands.
Complications of Treatment
Radiation Therapy. A small soft-tissue necrosis may develop, usually in the site of the original lesion where the dose is highest. These are moderately painful and respond to local anesthetics, antibiotics, and the tincture of time. Treatment with pentoxifylline 400 mg three times daily may be beneficial.
If the ulceration develops on the adjacent gingiva, the underlying mandible is exposed. These areas are mildly painful. They are managed by discontinuing dentures, local anesthetics, antibiotics, and smoothing of the bone by filing, if needed. These small bone exposures do not often progress to osteoradionecrosis (ORN) and either sequestrate a small piece of bone or are simply recovered by the mucous membrane. Severe ORN may require daily hyperbaric oxygen (HBO) treatments for 4 to 6 weeks, either alone or in conjunction with surgical intervention.
Surgical. These include bone exposure, orocutaneous fistula, and failure of osteomyocutaneous flaps. Salvage procedures after RT are associated with an increased risk of complications.
Anatomy
The circumvallate papillae locate the division between the oral tongue and the base of the tongue. The arterial supply is mainly by way of paired lingual arteries that are branches of the external carotid. The sensory pathway is by way of the lingual nerve to the gasserian ganglion.
Pathology
More than 95% of oral tongue lesions are SCCs. Coexisting leukoplakia is common. Verrucous carcinoma and minor salivary gland tumors are uncommon. Granular cell myoblastoma is a benign tumor of uncertain origin that occurs on the dorsum of the tongue and may be confused histologically with carcinoma.
Patterns of Spread
Primary
Nearly all SCCs occur on the lateral and ventral middle and posterior thirds of the oral tongue. They tend to remain in the tongue until large unless they originate near the junction with the floor of the mouth. PNI and vascular space invasion may occur.
Anterior third lesions usually are diagnosed early. Advanced lesions invade the floor of the mouth and root of the tongue, producing ulceration and fixation. Posterior third lesions grow into the anterior tonsillar pillar and base of tongue.
Lymphatics
The first-echelon nodes are the level Ib and II nodes.20 The submental and level V lymph nodes are seldom involved. Rouvière13 describes lymphatic trunks that bypass the level I to II nodes and terminate in the level III lymph nodes. Byers et al.249 evaluated nodal spread pattern in 277 patients treated surgically at the M.D. Anderson Cancer Center and observed skip metastases to the level III or IV nodes without involvement of levels I and II in 16% of patients. Of patients with oral tongue cancer, 35% have clinically positive nodes at diagnosis and 5% are bilateral. The incidence of occult disease is approximately 30%. The incidence of positive nodes increases with T stage. Patients with N1 or N2 ipsilateral nodes have a significant risk of developing node metastasis in the opposite neck.
Clinical Picture
Mild irritation of the tongue is the most frequent complaint. As ulceration develops, the pain worsens and is referred to the external ear canal. Extensive infiltration of the muscles of the tongue affects speech and deglutition and is associated with a foul odor.
The extent of disease is determined by visual examination and palpation. The tongue protrudes incompletely and toward the side of the lesion as fixation develops. Posterior oral tongue lesions may grow behind the mylohyoid and present as a mass in the neck at the angle of the mandible. Invasion of the hypoglossal nerve is rare.
Differential Diagnosis
The differential diagnosis includes granular cell myoblastomas, which are usually slow growing, nontender masses and 0.5 cm to 2.0 cm in size. The lesions are well circumscribed, firm, and slightly raised; they may be multiple. Aggressive behavior is rare, and wide local excision is preferred. Pyogenic granulomas mimic small exophytic carcinomas. Tuberculous ulcer and syphilitic chancre are rare.
Treatment
Selection of Treatment Modality
Both surgery and RT result in cure rates that are similar for similar stages. The disadvantages of surgery include removal of part of the tongue and the decision of whether to do a neck dissection for the N0 neck. The disadvantage of RT is the risk of necrosis.
Excisional Biopsy (TX). An excisional biopsy of a small lesion may show inadequate or equivocal margins. An interstitial implant or reexcision will produce a high rate of local control.
Early Lesions (T1 or T2). A partial glossectomy with primary closure or a skin graft may be done transorally and is usually the preferred therapy. Depending on the depth of invasion, an elective neck dissection may be indicated. Postoperative RT would only be added for indications previously discussed.
Moderately Advanced Lesions (T2 or T3). The preferred treatment for the majority of these patients is partial glossectomy, neck dissection, and postoperative RT-based treatment.
Advanced Lesions (T4). Bi- or trimodality treatment will cure a minority of these patients. Some patients are best treated with palliative intent.
Surgical Treatment
Early Lesions (T1 or T2). A partial glossectomy and primary closure is performed.
Moderately Advanced Lesions (T2 or T3). A partial glossectomy with primary closure, skin graft, or flap reconstruction is performed. Frozen section control is essential. Positive margins are an indication for excision of additional tissue.
Advanced Lesions (T4). Near total or total glossectomy and sometimes a laryngectomy is performed.
Irradiation Technique
The ability to control the primary lesion is enhanced by giving all or part of the treatment with an interstitial RT or by intraoral cone.250–252 Superficial T1 tumors may be treated with 192Ir brachytherapy alone using the plastic tube technique. Larger lesions that have an increased risk for subclinical neck disease may be treated with EBRT and a brachytherapy boost or with brachytherapy combined with an elective neck dissection. The time factor is critical for oral tongue cancer, and the EBRT part of the treatment is shortened (30 Gy in 10 once-daily fractions, or 38.4 Gy in 1.6 Gy twice-daily fractions) in order to increase the proportion of the RT given by either interstitial or intraoral cone therapy. The interstitial therapy is given after the EBRT; the intraoral cone therapy should be done prior to the EBRT. Elective neck RT is indicated for nearly all lesions.
Combined Treatment Policies
Postoperative RT or chemoRT is administered to the primary site and neck for indications previously outlined. IMRT may be useful to reduce the dose to one or both parotids.
Management of Recurrence
Local recurrence after RT or surgery is heralded by ulceration, pain, or increased induration. Recurrences have a slightly elevated or rolled border, whereas necroses do not. A biopsy should be done as soon as ulceration appears if it is within the original tumor site. Ulcers that appear on adjacent normal tissues are likely due to RT and not cancer.
RT failure is managed by surgery. Surgical failure occasionally is salvaged by re-resection and postoperative RT-based treatment. Recurrence in the soft tissues of the neck is rarely eradicated by any procedure.
Nodes appearing in a previously untreated neck are managed by neck dissection with or without postoperative RT or chemoRT.
Results of Treatment
The local control rates for 170 patients treated with RT alone versus surgery alone or with RT between 1964 and 1990 at the University of Florida included: for T1, 79% versus 76% (p = 0.76); for T2, 72% versus 76% (p = 0.86); for T3, 45% versus 82% (p = 0.03); and for T4, 0% versus 67% (p = 0.08).253 The differences in 5-year survival between the two treatment groups were not statistically significant.
The results of brachytherapy alone or combined with EBRT for 448 patients treated at the Centre Alexis Vautin were reported by Pernot et al.254 and revealed the following 5-year local control and survival rates: T1, 93% and 69%; T2, 65% and 41%; and T3, 49% and 25%, respectively. Shorter time intervals between brachytherapy and EBRT were associated with significantly improved local control and survival for those who received both modalities.
Complications of Treatment
Surgical. Orocutaneous fistula, flap necrosis, and dysphagia are the most common complications after surgery. Damage to the lingual nerve or the hypoglossal nerve is rare. Fistula and flap necrosis may result in carotid artery hemorrhage. Enunciation difficulties occur whenever the tongue is bound down by scarring. The incidence of complications increases for surgical salvage attempts after RT failure. Of 65 patients, 13 (20%) treated with surgery alone or combined with RT at the University of Florida developed significant complications.253
Radiation Therapy. A minor soft-tissue necrosis is fairly common and is treated with broad-spectrum antibiotics, local anesthetics such as viscous lidocaine, and analgesics. Pentoxifylline 400 mg three times daily may be beneficial. Hyperbaric oxygen treatment may be tried in difficult cases. If the necrosis is persistent and the pain is uncontrollable, it must be resected.
The edentulous person is less likely to develop bone complications compared with those who are dentulous.255 The most frequent problem involving the mandible is bone exposure. If the patient has dentures, they should be discontinued or altered to relieve the pressure over the exposed bone. If sharp bony edges appear, they are filed and the bone edge is lowered to speed healing. Healing may require months or even years.
If ORN develops, HBO has been used with some success. If conservative measures are unsuccessful, segmental mandibulectomy and an osteomyocutaneous flap reconstruction is performed.
Severe complications were observed in 9 of 105 patients (9%) treated with RT at the University of Florida.253 Pernot and colleagues254 observed the following soft tissue and/or bone complications in a series of 448 patients: Grade 1, 19%; Grade 2, 6%; and Grade 3, 3%.
Epidemiology
SCC is relatively uncommon in the United States. In Southern India, it is common and is related to chewing a combination of tobacco mixed with betel leaves, areca nut, and lime shell.256
Anatomy
The buccal mucosa is the mucous membrane covering the inner surface of the cheeks and lips, ending above and below with a transition to the gingiva. It ends posteriorly at the retromolar trigone. The parotid duct opens into the buccal mucosa opposite the second upper molar. The buccal mucosa is innervated by a branch of the mandibular nerve.
Pathology
Most malignant tumors are low-grade SCCs that frequently appear on a background of leukoplakia or lichen planus. Verrucous carcinoma occurs. Minor salivary gland tumors and melanomas are rare.
Patterns of Spread
Early lesions are usually discrete and exophytic. As they enlarge, they penetrate the underlying muscles and eventually extend to the skin. Peripheral growth occurs into the gingivobuccal sulci and eventually onto the gingiva and into bone.
The lymphatic spread is first to the level I and level II nodes. The incidence of positive nodes on admission is 9% to 31%, and the risk of occult disease is 16%.20,244
Clinical Picture
Small lesions produce the sensation of a lump that is felt with the tongue. Pain is minimal, unless there is posterior extension to involve the lingual and dental nerves. Pain may be referred to the ear. Obstruction of the Stensen duct will produce parotid enlargement. Extension posteriorly, behind the pterygomandibular raphe or into the buccinator and masseter muscles, causes trismus.
Differential Diagnosis
The differential diagnosis includes lues and tuberculosis; both are rare. If the first biopsy reveals chronic inflammation or pseudoepitheliomatous hyperplasia, a repeat biopsy may be necessary.
Treatment
Selection of Treatment Modality
Small lesions (≤1 cm) may be excised with primary closure; small lesions that involve the lip commissure are sometimes treated by RT. Lesions 2 cm to 3 cm in size can be treated with surgery or by RT (usually the former). Larger lesions are usually treated with surgery, and postoperative RT or chemoRT.
Surgical Treatment. Lesions that invade the mandible or maxilla require bone resection along with the soft tissues. Repair may require a maxillary prosthesis. A myocutaneous flap repairs full-thickness removal of the cheek.
Irradiation Technique. Buccal mucosa lesions are suited for treatment with electrons, an intraoral cone, and interstitial techniques to spare the contralateral normal tissues. When tumors extend into one of the gingivobuccal gutters or onto bone, treatment must be entirely by EBRT.
Results of Treatment
Diaz et al.257
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree