Prostate Cancer
Prostate cancer is the most commonly diagnosed noncutaneous malignancy in men in the United States. In 2007, over 218,000 cases will be diagnosed and over 27,000 men will die of the disease. The incidence of prostate cancer increased rapidly in the early 1990s because of the widespread use of the prostate-specific antigen (PSA) test, but subsequently leveled off in the late 1990s. Mortality from prostate cancer has also begun to decline recently, although the cause for this drop is not known.
The incidence of prostate cancer increases rapidly with age, particularly after the age of 50, although the presence of pathologic prostate cancer in men younger than 50 years of age has been demonstrated in autopsy series. Age, race, and family history are the most well established risk factors for prostate cancer. Scandinavians and Americans, and particularly African Americans, have a very high incidence of prostate cancer as compared with Asian men. The results of studies including men who migrate from areas of low incidence to areas of high incidence and acquire intermediate probabilities of developing prostate cancer suggest that environmental factors contribute to these differences. One such factor may be the high-fat diet of the Western developed world. Another potential risk factor may be serum hormone levels, particularly testosterone, although such data are controversial. Recent data from a clinical trial of 5α-reductase (the enzyme that converts testosterone to dihydrotestosterone) support the idea that alterations of the androgen milieu can alter the short-term risk of the development of prostate cancer.
A subset of patients who develop prostate cancer probably do so on the basis of genetic predisposition. Familial prostate cancer may be an important factor among patients who develop prostate cancer at a young age. First-degree relatives of men with prostate cancer have a two- to threefold increased risk of developing prostate cancer compared with the general population. Such data have led to a search for genetic loci that confer an increased risk of prostate cancer, including one on the long arm of chromosome 1 called HPC1 . Many studies are now evaluating potential candidate genes within this locus, including RNASEL , which encodes an enzyme that regulates cell proliferation.
HISTOLOGY
The vast majority of prostate cancers are adenocarcinomas. Most exhibit acinar-type differentiation, but some also have features of ductal differentiation. Pure large duct prostatic adenocarcinomas are uncommon. Typical prostatic adenocarcinomas may contain foci of mucinous differentiation or neuroendocrine differentiation, but the prognostic significance of these features remains uncertain. Small cell undifferentiated carcinoma of the prostate is rare but when present is often associated with areas of adenocarcinoma. Whether presenting in pure form or intermixed with adenocarcinoma, small cell undifferentiated carcinoma in the prostate carries a grave prognosis. Other tumors occurring in the prostate include transitional cell carcinoma (most often by invasion from the urethra), sarcomas of stromal origin, and metastases from other organs.
The putative precursor of invasive adenocarcinoma is prostatic intraepithelial neoplasia (PIN), in which cytologically dysplastic cells are found lining normal ducts and acini. PIN is divided into low and high grades; however, only the latter is geographically associated with invasive adenocarcinoma. Although the natural history of PIN is unknown, many foci of high-grade PIN demonstrate partial loss of the basal cell layer, and a transition to small invasive glands is occasionally observed. A diagnosis of high-grade PIN on needle biopsy should prompt additional studies to rule out invasive tumor.
Small foci of adenocarcinoma are found incidentally at autopsy in more than 30% of men over the age of 50 who die of unrelated causes. Thus, there is a large pool of these so-called latent or “autopsy” prostate cancers present in the older male population. Whether clinical cancers arise from latent tumors or develop by an independent pathway is unknown.
Although a variety of grading schemes for prostate cancer have been developed, the most widely used in the United States is the Gleason grading system, which is based strictly upon architectural rather than cytologic features of the cancer. According to this scheme, the pattern of infiltrating tumor glands is assigned a grade from 1 (well differentiated) to 5 (poorly differentiated). Because many adenocarcinomas show more than one pattern, the grades for the two most common patterns present in a tumor are added together to give a Gleason sum or Gleason score. The prognostic utility of the Gleason grading system has been validated in numerous studies wherein patients diagnosed with low Gleason score cancers have an excellent prognosis, whereas those with high Gleason score cancers have a poor prognosis. The main shortcoming of the Gleason grading system is that the majority of cancers are intermediate in grade. Less than 30% of cancers in most studies are within the Gleason score 2–4 or 8–10 groups. As a result, the Gleason score provides little prognostic information in most cases.
Tissue staining for PSA has become an important adjunct to confirming the diagnosis of prostate cancer. This is particularly useful in poorly differentiated cancers or those cancers that manifest themselves initially at metastatic sites as poorly differentiated carcinoma.
DIAGNOSIS AND STAGING OF PROSTATE CANCER
The detection of prostate cancer has been greatly enhanced by the introduction of the PSA test. Optimal detection of prostate cancer is now achieved through the combination of the digital rectal examination (DRE) and PSA. Biopsies are facilitated by transrectal ultrasound, which allows the physician to locate the areas of abnormality. The morbidity from biopsy is now minimal with the use of spring-loaded biopsy guns. Optimal information is acquired when multiple specially coordinated biopsies are performed. With such biopsies, the grade of the cancer, the number of cores positive for cancer, and the percentage of cancer per core should be determined.
A bone scan should be performed after the diagnosis of prostate cancer is made, particularly in men with a PSA in excess of 20 ng/mL or high Gleason grade disease (Gleason scores of 8–10). Although the incidence of radiographically detected regional lymph nodes is quite low, scanning by computed tomography (CT) or magnetic resonance imaging (MRI) should be considered, particularly in patients with high-grade, high-stage cancers or in those with high PSA serum levels. Recent techniques with newer MRI contrast agents (lymphotrophic nanoparticle-enhanced MRI) may help identify regional lymph node involvement. Endorectal coil MRI should be performed at experienced centers in patients being considered for radical prostatectomy in whom it is necessary to assess the extent of local disease. With the widespread use of PSA, the proportion of localized prostate cancers has increased.
Approximately 80% of cancers arising in the prostate gland arise in the peripheral zones, whereas 20% arise in the periurethral, or transition, zone. Cancers arising in the transition zone traditionally had been diagnosed by transurethral resection of the prostate. However, with increasing use of PSA and of medical therapies for benign prostatic hyperplasia, the frequency of cancers diagnosed in this manner has diminished.
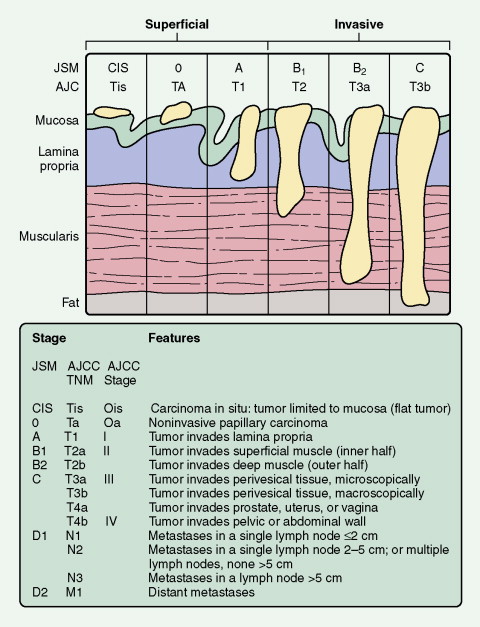
Two systems are commonly used for staging of prostate cancer: the Whitmore-Jewett system (stages A through D) and the American Joint Committee on Cancer (AJCC) tumor/node/metastases (TNM) system last modified in 2002 (see Fig. 8.11 ). The AJCC TNM system is used more commonly now.

The majority of cancers that are currently diagnosed are detected as a result of an abnormal PSA or DRE, or both. Organ-confined, palpable cancers are classified as T2 (see Fig. 8.11 ). Cancers diagnosed strictly on the basis of an abnormal PSA with no associated palpable abnormality are currently classified as T1c. Cancers that on physical examination extend into the seminal vesicles or palpably extend beyond the prostate are categorized as T3 cancers. Stage IV cancers are those that have metastasized either to regional lymph nodes (N1) or distant lymph nodes, bone, or viscera (M1) (see Fig. 8.11 ).
Careful examination of the prostate following its removal at the time of radical prostatectomy provides critical prognostic information. There is good correlation between the grade of cancer found at the time of biopsy and that found at the time of radical prostatectomy; when discrepancy occurs the biopsy results most frequently undergrade the cancer. With careful examination of the prostate, the volume of cancer can be ascertained, as can the degree of local extension. Cancers that are confined within the capsule are less likely to recur than those that invade through the capsule or into the seminal vesicle or demonstrate positive margins. Clinically localized tumors are frequently upstaged into T3 cancers pathologically. The frequency of lymph node involvement at the time of radical prostatectomy has apparently decreased in recent years, perhaps in part because of the more careful selection of surgical patients afforded by the use of the PSA.
CLINICAL MANIFESTATIONS
Most patients diagnosed with prostate cancer are asymptomatic, and the diagnosis is made as a result of an abnormal PSA or DRE. Because the prevalence of benign prostatic hyperplasia in the population of men susceptible to prostate cancer is high, many men will manifest mild degrees of prostatism. With locally advanced prostate cancer, urinary obstruction may occur, as may hematospermia. Carcinoma should be considered when obstructive urinary symptomatology develops over a short period of time. Some patients initially present with symptoms of metastatic disease either from painful bony metastasis or from lymphadenopathy. In such patients, immunostaining for PSA may be of particular value in distinguishing a carcinoma of prostatic origin.
Bladder Cancer
Over 60,000 new cases of bladder cancer are diagnosed in the United States each year, with over 13,000 deaths attributable to this disease ( ). Bladder cancer generally arises as a result of exposure to environmental carcinogens. In the United States the most important carcinogen is cigarette smoke, contributing to at least 50% of cases. On a worldwide basis, environmental toxins and Schistosoma haematobium have a more significant role. Specific genetic abnormalities have been delineated in association with bladder cancer. Monosomy 9 is frequently associated with superficial papillary transitional cell carcinomas. Tumors associated with a higher malignant potential frequently contain abnormalities on chromosome 17p, including TP53 abnormalities. On the other hand, tumors with fibroblast growth factor receptor 3 ( FGFR3 ) mutations have a much better prognosis and are unlikely to progress to muscle invasive disease ( ).
HISTOLOGY
Ninety percent of bladder cancers are transitional cell carcinomas, also known as urothelial carcinomas. These are designated grade 1 (well differentiated) to grade 3 (poorly differentiated), although in recent staging, tumors are designated simply as low grade or high grade. They often show a papillary architecture when presenting as superficial lesions. High-grade tumors may be localized within the bladder but are sometimes associated with widespread transitional cell carcinoma in situ. Alternatively, patients may present with carcinoma in situ in the absence of grossly recognizable tumor. Both high-grade papillary lesions and carcinoma in situ are associated with a substantial risk for developing muscularis invasion. Some transitional cell carcinomas show areas of squamous or adenocarcinomatous differentiation, the significance of which is uncertain. Pure squamous cell carcinomas and adenocarcinomas of the bladder are much less common than transitional cell carcinomas but are less responsive to therapy.
STAGING OF BLADDER CANCER
Because bladder cancer can metastasize rapidly, sometimes even before symptoms become apparent, staging studies in a patient with this diagnosis are imperative. Chest films, radionuclide bone scanning, tests of liver and renal function, and CT scans are all valuable.
Standard staging systems have been based on intravenous urography, bimanual examination, and transurethral resection. At present, two systems are used: the Jewett-Strong-Marshall (JSM) system and the AJCC system (see Fig. 8.30 ). Both classify bladder cancers as superficial, invasive, or metastatic. Superficial disease is limited to the mucosa or submucosa (JSM stages O and A or AJCC Ta and T1); this is the most common form of bladder cancer. Lesions that extend through the submucosa and into the muscularis are classified as invasive bladder cancer. These include lesions that involve superficial or deep muscles (JSM stages B1 and B2 or AJCC T2a and T2b). Although it was formerly believed that the depth of muscle invasion (superficial vs deep) was most important, it now seems that the presence of any muscle involvement is prognostically significant. Almost 50% of patients with muscle invasion eventually die of disease, usually associated with distant metastases. Stages III (T3, T4) and IV (N+) denote lesions invading the perivesicular fat and extending beyond it, respectively.
CLINICAL MANIFESTATIONS
The most common symptom of bladder cancer is painless hematuria. Carcinoma in situ can cause symptoms of urinary tract irritation. Endoscopically the bladder mucosa may appear normal; a definitive diagnosis can be established only after a urinary cytologic specimen or a mucosal biopsy specimen is obtained and examined. Urinary obstruction caused by urethral blockage and pain secondary to metastatic disease occur occasionally but are relatively uncommon initial manifestations. Sites of metastases include the liver, lung, lymphatic system, and bones.
Kidney Cancer
In 2007, over 50,000 new cases of kidney cancer will be diagnosed in the United States, resulting in 12,900 deaths ( ). Renal cell carcinomas (RCCs), which originate within the renal cortex, constitute 85% to 90% of primary renal neoplasms. Transitional cell carcinomas of the renal pelvis are the next most common kidney cancer subtype (∼︀8%). In the past, RCC was frequently diagnosed when symptoms arose from local extension of disease, metastases, or a variety of paraneoplastic phenomena. However, an increasing proportion of patients are currently diagnosed as a result of an incidental finding on noninvasive radiologic examination performed for other reasons. This change in the pattern of presentation may have contributed to better outcomes in RCC ( ). In the last 5 years, significant progress has been made in understanding the biology of clear cell kidney cancer (the most common histologic subtype), which has led to many treatment advances, particularly the use of anti-angiogenic drugs ( ; ).
HISTOLOGY
RCCs constitute a group of heterogeneous tumors (adenocarcinomas) arising from the epithelium of the renal tubules. These tumors have unique pathologic, cytogenetic, and molecular characteristics. They also have distinct biologic behavior, clinical manifestations, and therapeutic response. The current classification, published by the World Health Organization in 2004 ( Table 8.1 ), is based on histomorphology, presumptive histogenic origin, and cytogenetic and molecular characteristics of the renal tumors.
| Malignant |
|
| Benign |
|
Clear cell carcinoma is by far the most common type of RCC and arises from the proximal tubules. It typically has a deletion in the short arm of chromosome 3, often in the area of the von Hippel-Lindau (VHL) gene. The product of this gene, the VHL protein, plays a crucial role in the “hypoxia-inducible pathway.” It forms with several proteins a complex that has ubiquitin ligase activity and targets cellular proteins for ubiquitin-mediated degradation by proteasomes. One of the substrates is hypoxia-inducible factor (HIF), a heterodimeric transcription factor critical for the expression of hypoxia-inducible genes. Under normal conditions, the two isoforms of HIF, HIF1α and HIF2α, are hydroxylated on one of the proline residues. VHL proteins can then bind to and target the hydroxylated HIF for polyubiquitination and eventual degradation. Under hypoxic conditions, or when VHL is mutated or absent, HIF cannot bind to VHL and therefore is not directed for degradation. As the result, HIF accumulates within the cell and activates many hypoxia-inducible genes, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor, and others ( ), leading to tumor growth and invasion. Microscopically, tumor cells have “clear” cytoplasm due to the loss of cytoplasmic lipid and glycogen during the processing of the tissue and preparation of slides, although high-grade clear cell RCC often has more eosinophilic and granular cytoplasm. Indicative of a worse prognosis, sarcomatoid differentiation is found in about 5% of the cases.
Papillary RCC (PRCC) is the second most common subtype of RCC. Two types of PRCC are recognized based on the histomorphology. Accounting for about two thirds of PRCCs, type I contains papillae that are lined with a single layer of tumor cells with scant pale cytoplasm and low-grade nuclei. In contrast, type II tumor cells have abundant eosinophilic cytoplasm and large pseudostratified nuclei with prominent nucleoli. Type I PRCC has a better prognosis than type II PRCC. Chromosomal gain, including tri- or tetrasomy 7 and 17, and loss of Y chromosome are the most common cytogenetic changes observed in PRCC ( ). On the other hand, papillary adenomas are benign epithelial neoplasms of papillary or tubular architecture, with increased incidence with age, and in patients on long-term dialysis or acquired renal cystic disease.
Chromophobe RCC accounts for approximately 5% of RCCs. It is a malignant tumor characterized by large pale cells with prominent cell membranes and irregular nuclei. The prognosis is significantly better than in clear cell RCC with mortality less than 10%. This subtype shows extensive chromosomal loss, most commonly involving chromosomes 1, 2, 6, 10, 13, 17, and 21 ( ).
Other rare type of RCC include collecting duct and medullary carcinoma (both with very aggressive behavior), oncocytomas (benign tumors), and others ( Table 8.1 ).
Although the majority of RCCs are sporadic in nature, less than 5% of the cases present as part of the inherited cancer syndromes, including VHL syndrome (clear cell RCC), hereditary papillary renal cell carcinoma syndrome, hereditary leiomyomatosis, renal cell cancer (papillary RCC), and Birt-Hogg-Dube syndrome (chromophobe RCC). In general, hereditary cases present at a younger age and are much more likely to be multifocal and bilateral. A comparison of genetic defects between sporadic and hereditary RCC based on histologic appearance is provided in Table 8.2 .
| Sporadic Renal Cell Carcinomas | Renal Cell Carcinomas in an Inherited Syndrome | |||
|---|---|---|---|---|
| Histologic Appearance | Incidence (%) | Gene and Frequency (%) | Rare Syndrome * | Gene |
| Conventional | 75 | VHL (60) | VHL diseaseFCRCHereditary paraganglioma | VHL Chromosome 3p translocation SDHB |
| Papillary | 12 | MET (13) | HPRC | MET |
| TFE3 (<1) | HLRCC | FH | ||
| Chromophobe | 4 | Birt-Hogg-Dubé syndrome | BHD | |
| Oncocytoma | 4 | Birt-Hogg-Dubé syndrome | BHD | |
| Collecting duct | <1 | |||
| Unclassified | 3–5 | |||
* Additional rare syndromes or infrequent associations are not included.
STAGING OF KIDNEY CANCER
The most widely used system to assess the extent of invasion and dissemination of RCC is the AJCC staging method that is based upon the TNM classification ( Table 8.3 ). These criteria clearly define the anatomic extent of disease and stage and have been shown to correlate with survival.
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor |
| T1 | Tumor less than 7 cm in diameter and limited to the kidney |
| T1a | Tumor 4 cm or less in greatest dimension and limited to kidney |
| T1b | Tumor more than 4 cm but less than 7 cm, and limited to kidney |
| T2 | Tumor more than 7 cm in greatest dimension limited to the kidney |
| T3 | Tumor extends into major veins or invades the adrenal gland or perinephric tissues, but not beyond Gerota’s fascia. |
| T3a | Tumor directly invades the adrenal gland or perinephric tissues but not beyond Gerota’s fascia. |
| T3b | Tumor grossly extends into the renal vein or its segmental (muscle-containing) branches, or vena cava below the diaphragm. |
| T3c | Tumor grossly extends into the vena cava above the diaphragm or invades the wall of the vena cava. |
| T4 | Tumor invades beyond Gerota’s fascia. |
| Regional Lymph Nodes (N) | |
| NX | Regional lymph nodes cannot be assessed. |
| N0 | No regional lymph node metastases |
| N1 | Metastasis in a single regional lymph node |
| N2 | Metastases in more than one regional lymph node |
| Distant Metastasis (M) | |
| MX | Distant metastasis cannot be assessed. |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
The 2002 version of the AJCC staging system separates T1 lesions into T1a (tumors limited to the kidney and <4 cm) and T1b (intrarenal tumors >4 cm and <7 cm) ( ). This division of T1 reflects the higher rate of survival in patients with T1 tumors smaller than 4 cm and the increasing tendency to use nephron-sparing surgery (such as partial nephrectomy, radiofrequency ablation, cryoablation, and others) in these patients.
CLINICAL MANIFESTATIONS
The classic triad of symptoms—hematuria, flank pain, and abdominal mass—is observed in only about 10% of patients. However, individual symptoms occur in almost half of patients with kidney cancer. A variety of paraneoplastic phenomena can be associated with renal adenocarcinoma, including hypertension and hepatosplenomegaly not caused by metastases.
NOVEL TARGETED AGENTS IN THE TREATMENT OF ADVANCED RENAL CELL CARCINOMA
Although surgery is the mainstay of therapy for RCC in its localized form, advanced disease has been classically treated with immunotherapy such as interferon-α and interleukin-2. Responses were modest at best, and long-term disease control was rare. However, over the last few years RCC has become a model disease for targeted therapeutics based on the growing understanding of the underlying molecular pathways in this disease. Clear cell RCC is characterized by the inactivation of the VHL tumor suppressor gene, which results in the dysregulation of hypoxia response genes and subsequent promotion of tumor angiogenesis, growth, and metastasis. In advanced RCC substantial clinical activity has been reported with VEGF blockade using a variety of approaches including antibodies (bevacizumab) and small-molecule VEGF receptor inhibitors (sunitinib and sorafenib). These novel agents are replacing immunotherapy as a new standard of care. Several clinical trials are still in progress with the goal of defining the optimal efficacy of these agents as monotherapy or in combination ( ).
Testicular Cancer
Testicular cancer, which in the United States is diagnosed in over 7000 patients each year, serves as a model of a curable neoplasm. Most patients are young, in their third or fourth decade of life. In patients over the age of 50, testicular neoplasms are usually due to a malignant lymphoma.
HISTOLOGY
Germ cell cancers of the testis can be divided into seminomas and nonseminomas. This distinction is based on the extreme radioresponsiveness of seminomas and their tendency toward localized tissue invasion, as opposed to the tendency of nonseminomas to be more radioresistant and to metastasize via hematogenous routes to the lung or liver. Histologic variants of nonseminoma include embryonal carcinoma, teratoma, choriocarcinoma, and yolk sac tumor (also known as endodermal sinus tumor). Germ cell tumors can also arise from extragonadal sites, including the retroperitoneum and the mediastinum. Mediastinal nonseminomas have a particularly poor prognosis, whereas mediastinal seminomas have a similar prognosis to testicular seminomas.
Fewer than 5% of testicular malignancies originate from the gonadal stroma; these are mainly Leydig cell tumors, which can occur at any age and secrete both testosterone and estradiol, causing sexual precocity in prepubertal boys and gynecomastia in adults. About 10% of Leydig cell tumors metastasize; these demonstrate histologic features of aggressive behavior, such as blood vessel invasion and poor cell differentiation. Sertoli cell tumors are rare (only about 100 cases have been reported) and are also found in all age groups. They may secrete estrogens and thus cause gynecomastia. Metastases occur in 10% to 20% of cases.
Lymphomas, usually of the diffuse large cell type, represent 5% or less of testicular malignancies. They tend to be bilateral and often disseminate, particularly to the central nervous system. Involvement of the testes may occur in systemic lymphomas (about 10% of cases) and in some patients may herald an underlying malignant lymphoma.
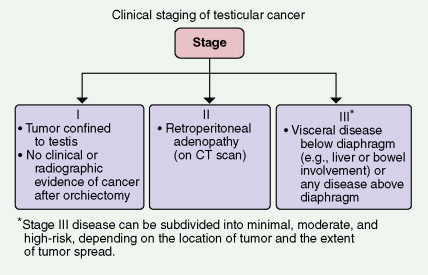
STAGING OF TESTICULAR CANCER
The staging evaluation of testicular cancers usually includes an abdominopelvic CT scan and chest radiography; sometimes suspicious abnormalities on a chest film prompt CT scanning of the chest as well. In certain high-risk patients, a bone scan and brain MRI are performed. Stage I nonseminomas are often surgically staged by retroperitoneal lymph node dissection. Circulating biologic markers, such as human chorionic gonadotrophin (β-hCG), lactate dehydrogenase, and α-fetoprotein (AFP), are prognostically important and are useful in following the clinical course.
Cancer confined to the testis, with no clinical, laboratory, or radiologic evidence of distant metastases, constitutes stage I disease (see Fig. 8.69 ). If there is no disease above the diaphragm but evidence of retroperitoneal adenopathy is demonstrated on CT scan or lymphangiogram, the cancer is classified as stage II disease. Stage III disease is marked by persistence of positive biologic markers after orchidectomy or by subdiaphragmatic visceral involvement (e.g., of the liver, spleen, or inferior vena cava) or supradiaphragmatic metastases to the lung parenchyma or central nervous system. Prognosis can be determined by criteria established by the International Germ Cell Consensus Criteria ( Table 8.4 ).
| Nonseminoma | Seminoma |
|---|---|
| Good Prognosis | |
| Testis/retroperitoneal primary | Any primary site |
| and | and |
| No nonpulmonary visceral metastases | No nonpulmonary visceral metastases |
| and | and |
Good markers—all of
| Normal AFP, any hCG, any LDH |
| 58% of nonseminomas | 90% of seminomas |
| 5-year PFS 89% | 5-year PFS 82% |
| 5-year survival 92% | 5-year survival 86% |
| Intermediate Prognosis | |
| Testis/retroperitoneal primary | Any primary site |
| and | and |
| No nonpulmonary visceral metastases | Nonpulmonary visceral metastases |
| and | and |
Intermediate markers—any of
| Normal AFP, any hCG, any LDH |
| 28% of nonseminomas | 10% of seminomas |
| 5-year PFS 75% | 5-year PFS 67% |
| 5-year survival 80% | 5-year survival 72% |
| Poor Prognosis | |
| Mediastinal primary | No patients classified as |
| or | poor prognosis |
| Nonpulmonary visceral metastases | |
| or | |
Poor markers—any of
| |
| 16% of nonseminomas | |
| 5-year PFS 41% | |
| 5-year survival 48% | |
CLINICAL MANIFESTATIONS
The manifestations of testicular cancer are protean, ranging from detection of an asymptomatic nodule or swelling on self-examination of the testes to the development of symptoms secondary to metastatic disease. Patients who present with a painful lesion in the scrotum are often initially diagnosed and treated for epididymitis before the true diagnosis of cancer is established. Epididymitis often occurs concomitantly in patients with testicular cancer and may explain the associated pain. The sudden, acute appearance of a rapidly enlarging testis, particularly characteristic of choriocarcinoma, is usually associated with hemorrhage into neoplastic tissue. Back or abdominal pain secondary to retroperitoneal adenopathy, dyspnea caused by pulmonary metastases, weight loss, gynecomastia, supraclavicular lymphadenopathy, and urinary obstruction may also be evident at the time of presentation.
Penile Cancer
Cancer of the penis, which occurs almost exclusively in uncircumcised men, is exceedingly uncommon in the United States, representing less than 2% of malignancies of the male genitourinary tract. The highest rate of incidence is seen in men over 45 years of age, with a predominance of African Americans over whites in a ratio of 3:1. A higher incidence of penile cancer is observed in those Eastern nations where circumcision is not routinely practiced.
Although the precise etiology of penile cancer is unknown, a clear link to poor hygiene in the uncircumcised male has been established, implicating the growth of possibly carcinogenic micro-organisms in smegma retained beneath the prepuce. Approximately 20% of patients have a history of past or present venereal disease. An etiologic role for herpes simplex infection has been postulated but not proven.
HISTOLOGY
The vast majority of penile cancers are squamous cell carcinomas. Rare cases of adenocarcinoma, melanocarcinoma, and sarcoma have been reported, the latter being in some instances associated with Kaposi’s sarcoma. Metastatic disease of the penis originating from primary tumors of the rectum, prostate, or bladder also occurs but is quite uncommon.
Approximately 50% of penile cancers metastasize via lymphatic vessels to the deep and superficial inguinal nodes. Most of these malignancies are relatively unresponsive to radiation therapy, and a consistently effective chemotherapeutic protocol has yet to be developed. Treatment of penile cancer is therefore heavily reliant on surgery. Depending on the extent of disease, this may involve partial, complete, or radical penectomy, with inguinal lymphadenectomy when necessary to remove affected nodes. In cases of penile cancer detected at an early stage, cure is sometimes achieved by a partial penectomy that preserves a penile stump adequate for sexual activity and urination. The overall 5-year survival for cancer of the penis is 80% for men without nodal involvement, but less than 50% in men with nodal metastases.
CLINICAL MANIFESTATIONS
At the time of presentation, the penile lesion, invariably located in the preputial area, may appear either as a flat ulcer with raised edges, usually extending into the underlying tissues, or as a clustered papillomatous growth resembling the much more common, sexually transmitted condyloma acuminatum. All such penile lesions are suspect and should be biopsied to confirm the histologic diagnosis. Because of overlying psychological factors associated with any abnormality of the penis, patients may delay seeking medical attention until the malignancy is well established. However, most of these lesions are slow to grow and metastasize, and therefore the prognosis even for fairly well advanced cases remains relatively good unless extensive involvement of the inguinal lymph nodes is already present.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree