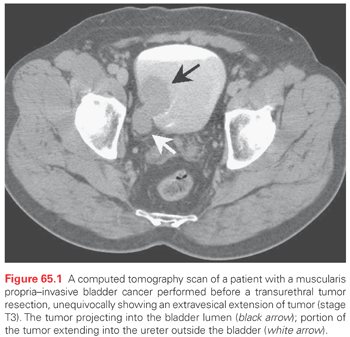
Patients who have documented muscularis propria–invasive bladder cancer require an additional set of studies: a chest x-ray or CT scan, liver function studies, creatinine and electrolytes level studies, and a CT evaluation of the pelvic and retroperitoneal lymph nodes. A bimanual examination is also performed at the time the tumor is transurethrally resected to evaluate for possible extravesical extension of the tumor and to determine mobility of the pelvic contents. An MRI lymphangiography, using a lymphotropic iron nanoparticle administered intravenously, shows potential.80 Nodes that appear to be enlarged on a CT may be differentiated by this technique as to whether they are inflammatory or malignant. The sensitivity and specificity of the test are quite high.
If there is a history of functional bowel abnormality, a barium study of the segment of bowel to be used for the diversion should be performed. It is the authors’ practice when using colon in the reconstruction of the urinary tract to obtain a barium enema or colonoscopy so that there are no surprises at the time of surgery. Finally, patients with muscularis propria–invasive bladder cancer must have a prostatic urethra and bulbous urethra biopsy to determine whether an orthotopic bladder may be placed or whether the procedure should encompass the urethra—that is, a cystoprostatourethrectomy in males or a cystourethrectomy and anterior exenteration in females.
Treatment of Non–Muscularis Propria–Invasive Bladder Cancer (Ta, Tis, T1)
Of patients with bladder cancer, 70% have disease that does not involve the muscularis propria at presentation. Approximately 15% to 20% of these patients will progress to stage T2 disease or greater over time. Of those presenting with Ta or T1 disease, 50% to 70% will have a recurrence following initial therapy. Low-grade tumors (G1 or G2) and low-stage (Ta) disease tend to have a lower recurrence rate at about 50% and a 5% progression rate, whereas high-risk disease (G3, T1 associated with CIS, and multifocal disease) has a 70% recurrence rate and a 30% to 50% progression rate to stage T2 disease or greater. Less than 5% of patients with non–muscularis propria–invasive bladder cancer will develop metastatic disease without developing evidence of muscularis propria invasion (stage T2 disease or greater) of the primary lesion.
Patients who are at significant risk for developing progressive or recurrent disease following TURBT are generally considered candidates for adjuvant intravesical drug therapy. This includes those with multifocal CIS, CIS associated with Ta or T1 tumors, any G3 tumor, multifocal tumors, and those whose tumors rapidly recur following TURBT of the initial bladder tumor. A number of drugs have been used intravesically, including bacillus Calmette-Guérin (BCG), interferon (IFN) and BCG, thioTEPA, mitomycin C, doxorubicin, and gemcitabine. Complications generally include frequency, dysuria, and irritative voiding symptoms. Over the long term, bladder contracture may occur with these agents. Other complications, which are specific for each drug, are as follows: BCG administration may result in fever, joint pain, granulomatous prostatitis, sinus formation, disseminated tuberculosis, and death; thioTEPA may cause myelosuppression; mitomycin C may cause skin desquamation and rash; and doxorubicin may cause gastrointestinal upset and allergic reactions. The proposed benefit of intravesical chemotherapy is to lessen the rate of recurrences and reduce the incidence of progression. Unfortunately, it cannot be clearly stated that any of these drugs accomplish these goals over the long term.
The use of electromotive installation as an adjunct to intravesical therapy remains controversial. A randomized trial sought to clarify the benefit of electromotive installation of mitomycin. Patients were randomized to TURBT alone (n = 124), immediate post-TURBT mitomycin (n = 126), or pre-TURBT electromotive mitomycin (n = 124). Trial results demonstrated that intravesical electromotive installation of mitomycin before TURBT reduced recurrence and improved the disease-free interval compared with intravesical mitomycin after TURBT and TURBT alone.81
Intravesical BCG therapy is typically initiated with an induction course of 6 weekly instillations, followed by a cystoscopic evaluation 1 month after induction. In cases in which CIS is present or suspected, only a biopsy can differentiate this from inflammatory change secondary to treatment. For those who respond to induction, maintenance BCG therapy for up to 3 years is a standard of care, although patients frequently discontinue therapy early due to bladder toxicity.82
A European Organisation for Research and Treatment of Cancer (EORTC) phase III trial in over 1,300 patients sought to evaluate whether a third of a dose versus a full dose and a 1-year treatment versus a 3-year treatment could suffice.83 The trial thus had four different doses and schedules of BCG maintenance therapy. No meaningful differences in toxicity, progression, or survival were observed across dose and schedules. However, the recurrence rate was lowest in high-risk patients treated with the full dose therapy for 3 years, supporting current treatment recommendations.
A number of studies have compared one intravesical chemotherapeutic agent with another. For the most part, BCG in these comparisons has a slight advantage in reducing recurrences.84 However, when the follow-up is more than 5 years, it appears that there is minimal overall effect at reducing the recurrence rate when compared with no treatment. BCG and epirubicin are the most commonly used agents in this setting and both are considered effective for the treatment of superficial bladder cancer. However, superiority of one over the other is unknown. A meta-analysis of over 1,100 patients treated with either drug reported that intravesical BCG was more efficacious, although also more toxic.85
BCG failure is a clinical concern and a treatment dilemma with limited truly effective nonsurgical options. The precise definitions of BCG failure are well outlined by the 2005 International Consensus Panel on T1 bladder cancer and include four subtypes of BCG failure.86 BCG refractory T1 disease should be of paramount concern and raises the concern for understaged diseases. Options for further intravesical treatment after BCG failure include BCG plus IFNα-2B, gemcitabine, valrubicin, docetaxel, and other novel agents.85–92 Unfortunately, however, no single agent has yet proven to be more reliably or durably effective than another, and a true consensus on continued intravesical treatment in this setting remains to be determined.
Approximately 70% of patients with high-grade disease will experience recurrence whether or not they are treated with intravesical therapy. Moreover, there is no well-documented evidence that the use of these agents prevents disease progression, for example, from stage Ta/T1 disease to stage T2 or greater disease. One-third of patients who are at high risk for disease progression (those with G3, T1 disease) will progress to stage T2 or greater disease whether or not they are treated with BCG.93 One-third of patients at 5 years who have disease progression and undergo a cystectomy die of metastatic disease. Thus, approximately 15% of patients with superficial disease at high risk for disease progression (CIS with associated Ta or T1 disease, rapidly recurrent disease, or G3 disease), irrespective of treatment modality, will die of their disease.94 If definitive therapy (cystectomy) is performed when the disease is found to progress into the muscularis propria (T2 or greater), there is no difference in cure rate when these patients are compared with those who present primarily with T2 or greater disease. These statistics have encouraged some to perform a preemptive cystectomy in those patients at high risk for progression before muscularis propria invasion is documented. Ten-year cancer-specific survivals of 80% are given as justification for this approach, as compared with 50% in patients in whom the cystectomy is performed when the disease progresses to involve the muscularis propria.95 Unfortunately, this approach subjects approximately two-thirds of these patients who are included in the 80% cancer-specific survival figure to a needless cystectomy, making it questionable as to whether there is in fact any survival advantage whatsoever. Although cystectomy remains the gold standard for recurrent BCG refractory T1 disease, there is an open protocol RTOG 0926 evaluating chemoradiation for such patients who opt for an attempt at bladder preservation or are otherwise not good cystectomy candidates.96
Treatment of Muscularis Propria–Invasive Disease
Surgical Approaches
The standard of care for squamous cell carcinoma, adenocarcinoma, TCC, and sarcomatoid or spindle cell carcinoma that invade the muscularis propria of the bladder is a bilateral pelvic lymph node dissection and a cystoprostatectomy, with or without a urethrectomy in the male. In the female, an anterior exenteration is performed, which includes the bladder and urethra (the urethra may be spared if uninvolved and an orthotopic bladder reconstruction is performed), the ventral vaginal wall, and the uterus. A radical cystectomy may be indicated in non–muscularis propria–invasive bladder cancers when G3 disease is multifocal or associated with CIS or when bladder tumors rapidly recur, particularly in multifocal areas following intravesical drug therapy. When the prostate stroma is involved with TCC or when there is concomitant CIS of the urethra, a cystoprostatourethrectomy is the treatment of choice.97 If the urethra needs to be removed, the type of urinary reconstruction is limited to an abdominal urinary diversion. In selected circumstances in the male, the neurovascular bundles coursing along the lateral side of the prostate caudally and adjacent to the rectum more cephalad may be preserved, sometimes preserving potency. Partial cystectomies may rarely be performed in selected patients, thus preserving bladder function and affording in the properly selected patient the same cure rate as a radical cystectomy.98 Patients who are candidates for such procedures must have focal disease located far enough away from the ureteral orifices and bladder neck to achieve at least a 2-cm margin around the tumor and a margin sufficient around the ureteral orifices and bladder neck to reconstruct the bladder. Practically, this limits partial cystectomies to those patients who have small tumors located in the dome of the bladder and in whom random bladder biopsies show no evidence of CIS or other bladder tumors.
Survival
The probability of survival from bladder cancer following a cystectomy is determined by the pathologic stage of the disease. Survival is markedly influenced by the presence or absence of positive lymph nodes. Some have argued that the number of positive nodes impacts survival in that, when resected, there is a potential for cure provided there are less than four to eight positive nodes.99,100 Positive perivesical nodes have a less ominous prognosis when compared with involvement of iliac or para-aortic nodes. Pathologic type may also impact outcome, but in most series, survival is more dependent on pathologic stage than on the cell type of the cancer. Most large series of survival statistics following treatment include all patients regardless of cell type. These series are generally constituted as to histologic type as follows: TCC, 85% to 90%; combination of TCC and either squamous cell or adenocarcinoma, 6%; pure squamous cell carcinoma, 3%; pure adenocarcinoma, 3%; small-cell and sarcomatoid or spindle cell carcinoma, 2% (Table 65.2).

Types of Urinary Diversion
Urinary diversions may be divided into continent and incontinent. Incontinent urinary diversions or conduits involve the use of a segment of ileum or colon and, less commonly, a segment of jejunum. The distal end is brought to the skin, and the ureters are implanted into the proximal end. The patient wears a urinary collection appliance. The advantages of a conduit (ileal or colonic) are its simplicity and the reduced number of immediate and long-term postoperative complications. In most series, 13% of patients who undergo a cystectomy and urinary diversion of this type will have a significant complication that impacts on hospital stay or recovery. Generally, the distal ileum is used for the urinary conduit or reservoir; however, if it has been irradiated or is otherwise involved, one may select the right colon or a short segment of jejunum. The latter is the least desirable choice because electrolyte problems may be significant. On occasion, during exenterative surgery when an end colostomy is created, a segment of distal bowel is used, thus obviating the need for an intestinal anastomosis.
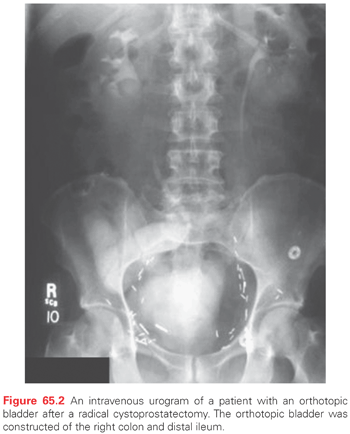
Continent diversions may be divided into two types: abdominal and orthotopic. Abdominal diversions require a continence valve, whereas an orthotopic neobladder depends on the urethral sphincter for continence. The reservoir is made of bowel that is fashioned into a globular configuration. In the abdominal type of continent diversion, the stoma is brought through the abdominal wall to the skin. The patient catheterizes the pouch every 4 hours. Orthotopic urinary diversions entail the use of bowel brought to the urethra, thus allowing the patient to void by Valsalva (Fig. 65.2). Patients must have the facility to catheterize themselves, because it is mandatory in the abdominal continent diversion and occasionally necessary in the orthotopic reconstruction. The advantage of continent diversions is the avoidance of a collection device. The advantage of an orthotopic bladder over all other types of continent diversions is that it rehabilitates the patient to normal voiding through the urethra, often without the need for intermittent catheterization or the need to wear a collection device. Postoperative and long-term complications of continent diversion are increased over the conduit types of diversions. Indeed, in some series, postoperative complications range from 13% to 30%. Long-term metabolic complications are also increased.
Complications of Cystectomy and Urinary Diversion
The complications of all types of urinary diversion may be divided into three groups: metabolic, neuromechanical, and surgical.
Metabolic Complications of Urinary Intestinal Diversion. When the intestine is interposed in the urinary tract, there is the potential for a number of metabolic complications.104 These may involve electrolyte abnormalities and altered drug metabolism, which may result in altered sensorium, infection, osteomalacia, growth retardation, calculi both within the reservoir as well as in the kidney, short bowel syndrome, cancer, and altered bile metabolism.
Depending on the segment used, different specific electrolyte abnormalities may occur. When the ileum and colon are used, hyperchloremic metabolic acidosis may result; when jejunum is used, hypochloremic or hyperkalemic metabolic acidosis may follow.
Hypokalemia is more common when the colon is used, whereas hypocalcemia is more common when the ileum and colon are used, and hypomagnesemia is more common when the ileum and the colon are used.
The most pervasive detrimental effect created by all urinary intestinal diversions is due to acidosis. Acidosis may result in electrolyte abnormalities, osteomalacia, growth retardation, altered sensorium, altered hepatic metabolism, renal calculi, and abnormal drug metabolism. In general, patients with normal renal function as well as normal hepatic function are less prone to acidosis and its complications.
Treatment for the metabolic acidosis is straightforward and can be accomplished with bicarbonate or with Bicitra solution, which is sodium citrate and citric acid. Polycitra, which is a combination of potassium citrate, sodium citrate, and citric acid, may also be employed. It has the advantage of supplying potassium, which, on occasion, is deficient. Chlorpromazine and nicotinic acid have been used to block the chloride bicarbonate exchanger, and thus lessen the potential for the acidosis.
Decreased renal function is seen in a majority of patients in the decade following a radical cystectomy, and choice of diversion does not predict the decline. Postoperative hydronephrosis, pyelonephritis, and uretero-enteric strictures represent factors that, if addressed, may mitigate the loss of function.105
Patients with conduits may have a 3% to 4% incidence of renal calculi over the long term. Those with reservoirs have up to a 20% incidence of calculi within the reservoir. The pathogenesis may be a metabolic alteration or infection, whereas reservoir stones are most commonly due to a surgical foreign body or mucus serving as a nidus.
There is a high incidence of bacteriuria in patients with either conduits or pouches, and the incidence of sepsis is 13%. There appears to be diminished antibacterial activity of the intestinal mucosa, with the immunoglobulins, which are normally secreted by the mucosa, being altered. In addition to this, when the bowel is distended, there can be a translocation of bacteria from the lumen into the bloodstream.
Because the intestine is interposed in the urinary tract, drugs that are eliminated unchanged from the body through the kidney and have the potential to be reabsorbed by the gut can in fact result in significant alterations in metabolism of that drug. Patients with a urinary diversion, when given systemic chemotherapy, have a higher incidence of complications and are more likely to have their chemotherapy limited when compared with patients without diversion who receive the same drugs and dose.106
The loss of the distal ileum may result in vitamin B12 malabsorption, which then manifests itself as anemia and neurologic abnormalities. Bile salt malabsorption may occur and result in diarrhea. Loss of the ileocecal valve may result in diarrhea with bacterial overgrowth of the ileum and malabsorption of vitamin B12 and fat-soluble vitamins A, D, E, and K. Loss of the colon may result in diarrhea and bicarbonate loss.
Neuromechanical Complications. Neuromechanical complications may be of two types: atonic, resulting in an atonic segment with urinary retention, and hyperperistaltic contractions. The latter is relevant in continent diversions, as this may result in incontinence and a low-capacity reservoir.
Surgical Complications. There are a number of complications that occur following any major surgical procedure, which include thrombophlebitis, pulmonary embolus, wound dehiscence, pneumonia, atelectasis, myocardial infarction, and death. Complications specific to cystectomy and urinary diversion are divided into short term and late. The short-term complications include acute acidosis (16%), urine leak (3% to 16%), bowel obstruction or fecal leak (10%), and pyelonephritis (5% to 15%). The longer term complications include ureteral or intestinal obstruction (15%), renal deterioration (15%), renal failure (5%), stoma problems (15%), and intestinal stricture (10% to 15%).107,108
The morbidity of salvage cystectomy for a recurrence following bladder sparing chemoradiation has also been described and appears acceptable when compared to primary cystectomy series.109
Selective Bladder-Preserving Approaches
The treatment options for muscularis propria–invasive bladder tumors can broadly be divided into those that remove the bladder and those that spare it. In the United States, a radical cystectomy with pelvic lymph node dissection remains the standard method used to treat patients with this tumor. Several reports from North America and Europe have described long-term results using multimodality treatment of muscularis propria–invading bladder cancer, with appropriate safeguards for early cystectomy should this treatment fail. For bladder-conserving therapy to be more widely accepted, this treatment approach must have a high likelihood of eradicating the primary tumor, must preserve good organ function, and must not result in compromised patient survival. It does appear that, for selected patients, bladder sparing therapy with salvage cystectomy reserved for tumor recurrence represents a safe and effective alternative to immediate radical cystectomy.110
Successful bladder-preserving approaches have evolved during the past 3 decades. They began with the use of radiation therapy but expanded when the National Bladder Cancer Group first demonstrated the safety and efficacy of cisplatin as a radiation sensitizer in patients with muscle-invasive bladder cancer that was unsuitable for cystectomy.111 The long-term survival with stage T2 tumors (64%) and stage T3 to T4 tumors (22%) was encouraging. This was validated by the National Cancer Institute–Canada randomized trial of radiation (either definitive or precystectomy) with or without concurrent cisplatin for patients with T3 bladder cancer, which showed a significant improvement in long-term survival with pelvic tumor control (67% versus 47%) in the patients who were assigned cisplatin.112 Additional single-institution studies showed that the combination of a visibly complete TURBT followed by radiation therapy or radiation therapy concurrent with chemotherapy safely improved local control.113,114 These findings led the RTOG to develop protocols for bladder preservation beginning with a TURBT of as much of the tumor as is safely possible, followed by the combination of radiation with concurrent radiosensitizing chemotherapy. One key to the success of such a program is the selection of patients for bladder preservation on the basis of the initial response of each individual patient’s tumor to therapy. Thus, bladder conservation is reserved for those patients who have a clinical CR to concurrent chemotherapy and radiation. A prompt cystectomy is recommended for those patients whose tumors respond only incompletely or who subsequently develop an invasive tumor (Fig. 65.3). Up to 30% of the patients entering a potential bladder-preserving protocol with trimodality therapy (initial TURBT followed by concurrent chemoradiation) will ultimately require a salvage radical cystectomy.

For over 2 decades, the Massachusetts General Hospital (MGH), the RTOG, and several centers in Europe have evaluated in phase II and III protocols concurrent chemoradiation plus neoadjuvant or adjuvant chemotherapy (Table 65.3). Radiosensitizing drugs studied in these series, either singly or in various combinations, include cisplatin, carboplatin, paclitaxel, 5-fluorouracil (5-FU), mitomycin C, and gemcitabine.113 The first RTOG study of patients treated with once-daily radiation treatment and concurrent cisplatin yielded a 5-year survival of 52% (42% with intact bladder).115 RTOG studies 8802 and 8903 used methotrexate, cisplatin, and vinblastine (MCV) chemotherapy as neoadjuvant treatment.116 In the latter study, the neoadjuvant therapy was tested in a randomized fashion.117 No improvement was seen in survival or in local tumor eradication as a result of neoadjuvant therapy, although the trial was closed early and underpowered to give a definitive answer. The toxicity of the MCV arm was considerable, with only 67% of patients able to complete the planned treatment. The use of contemporary neoadjuvant chemotherapy (dose-dense methotrexate, vinblastine, adriamycin, cisplatin [ddMVAC] or gemcitabine and cisplatin [GC]) regimens with appropriate supportive therapy in well-selected bladder-sparing patients may warrant further investigation.
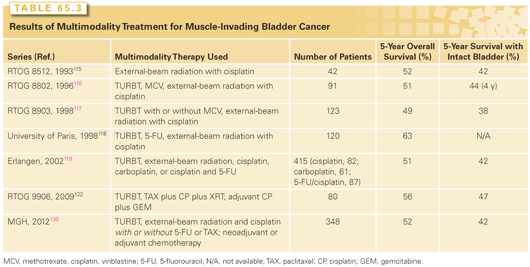
Other studies from Paris and Germany have reported their large experience with bladder sparing.118,119 The CR rate in the German study was 72%, and local control of the bladder tumor after the CR without a muscle-invasive relapse was maintained in 64% of the patients at 10 years. The 10-year disease-specific survival was 42%, and more than 80% of these survivors preserved their bladder. This series reported the sequential use of radiation with no chemotherapy (126 patients), followed by concurrent cisplatin (145 patients), then concurrent carboplatin (95 patients), and finally concurrent cisplatin with 5-FU (49 patients). The CR rates in these four protocols were 51%, 81%, 64%, and 87%, respectively.120,121 The 5-year actuarial survival with an intact bladder in these studies was 38%, 47%, 41%, and 54%, respectively. These results strongly suggest that radiochemotherapy, when given concurrently, is superior to radiation therapy alone; that carboplatin is less radiosensitizing than cisplatin; and that cisplatin plus 5-FU may be superior to cisplatin alone.
The RTOG protocols have subsequently explored both twice-daily radiation therapy and novel radiosensitization using cisplatin with or without 5-FU or paclitaxel.62,122,123,124 Complete response and bladder preservation rates are consistently high, with no one regimen clearly superior.62
Gemcitabine has been also tested in bladder-treatment protocols. In a phase I trial from the University of Michigan, 23 patients, mostly T2, were treated with gemcitabine and concurrent daily radiation. At a median follow-up of 5.6 years, an impressive 91% CR rate was observed, and the 5-year actuarial estimates of survival include a bladder-intact survival of 62%, an overall survival of 76%, and a disease-specific survival of 82%.125 A phase II study from the United Kingdom of 50 patients treated with concurrent weekly gemcitabine and hypofractionated radiation reported an 88% complete endoscopic response rate, a 3-year overall survival of 75%, and cancer-specific survival of 82%.126 Twice weekly low-dose gemcitabine was recently evaluated as a radiosensitizer with daily radiation in protocol RTOG 0712.
Cisplatin is not always an ideal drug for bladder cancer patients, because it may cause impaired renal function in many. A British group observed high response rates using the combination of 5-FU and mitomycin C with pelvic radiotherapy.127 These results led to the phase III Bladder Cancer 2001 (BC2001) trial, in which 360 patients with muscle-invasive bladder cancer were randomized to either radiotherapy alone or to radiotherapy with concomitant 5-FU and mitomycin C chemotherapy. Local–regional disease-free survival was superior for those patients receiving chemotherapy (67% versus 54% at 2 years; hazard ratio [HR] 0.68, p = 0.03 with median follow-up of 70 months). Survival at 5 years was higher with chemoradiotherapy (48% versus 35%), but did not reach statistical significance (HR 0.82; p = 0.16).128
Predictors of Outcome
A common feature of all the RTOG protocols was early bladder tumor response evaluation and the selection of patients for bladder conservation on the basis of their initial response to TURBT combined with chemotherapy and radiation.130 Bladder conservation was reserved for those who had a complete clinical response at the midpoint in therapy (after a radiation dosage of 40 Gy). Complete responders to induction therapy then received consolidation with additional chemotherapy and radiation to a total tumor dose of 64 to 65 Gy. Incomplete responders were advised to undergo a radical cystectomy, as were patients whose invasive tumors persisted or recurred after treatment. The current schema for trimodality treatment of muscle-invading bladder cancer is provided in Figure 65.3. Other tumor presentations associated with successful bladder-sparing therapy include: solitary T2 or early T3 tumors (typically <6 cm in size), no tumor-associated hydronephrosis, tumors allowing a visibly complete TURBT, invasive tumors not associated with extensive carcinoma in situ, and urothelial carcinoma histology (because alternative histologies have not been rigorously evaluated in bladder sparing protocols).
In the MGH series,130 the median follow-up for all surviving patients was 7.7 years. Of patients, 72% (78% with stage T2) had CR to induction therapy. The 10-year actuarial overall survival was 35% (T2, 43%; T3–T4a, 27%) and the 10-year disease-specific survival was 59% (T2, 67%; stage T3–T4a, 49%) (Figs. 65.4 and 65.5). The clinical stage and achieving a CR were significantly associated with both overall survival and disease-specific survival. A nomogram predicting response has been developed from this data.129 The use of neoadjuvant chemotherapy with MCV, however, was not associated with survival or incidence of metastases, although this may warrant further investigation in the modern era.


The 10-year disease-specific survival rate for the 102 patients (29%) undergoing a cystectomy was 44%, illustrating the very important contribution of prompt salvage cystectomy. The 10-year disease-specific survival with an intact bladder was 45% (T2, 52%; T3–T4a, 36%). No patient required a cystectomy due to bladder morbidity. The overall survival and disease-specific survival for all 348 patients and for some clinically important subgroups are shown in Table 65.4. The value of complete TURBT in bladder-sparing therapy is demonstrated in this report. Of the 348 patients followed, 227 underwent a complete TURBT and 116 had an incomplete TURBT. Patients who underwent a complete TURBT had improved CR, overall survival, disease-specific survival, and lower rates of cystectomy (22% versus 42%) compared to those with an incomplete TURBT. In a review of the patients who were complete responders after induction therapy, 55% developed no further bladder tumors, 29% subsequently developed a superficial occurrence, and 16% developed an invasive tumor.132 Most patients with superficial recurrence were treated successfully by TURBT and intravesical chemotherapy. For these individuals, the overall survival was comparable to those who had no failure. However, one-quarter of these patients ultimately required a salvage cystectomy.
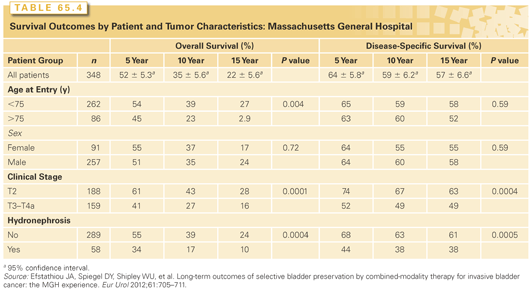
Notably, age is not a contraindication to successful bladder sparing therapy, and indeed, results are favorable in patients aged 75 years or older.130 This is an important consideration given that the elderly generally appear to be undertreated for invasive bladder cancer in the United States.131 Bladder-sparing chemoradiation remains a good option for those patients who are not cystectomy candidates and, often, such patients would be treated with daily radiation and appropriate concurrent chemotherapy without a break.
Radiation Treatment
The most common approach with external-beam irradiation reported from North America and Europe involves the treatment of the pelvis to include the bladder, the prostate (in men), and often, the low external and internal iliac lymph nodes for a total dose of 40 to 45 Gy in 1.8- to 2.0-Gy fractions during 4 to 5 weeks. Subsequently, the target volume is reduced to deliver a final boost dose of 20 to 25 Gy in 15 fractions to the primary bladder tumor. Some protocols call for partial bladder radiation as the boost volume if the location of the tumor in the bladder can be satisfactorily identified by the use of cystoscopic mapping, selected mucosal biopsies, and imaging information from CT or MRI. Figure 65.6 is an isodose color wash of a partial bladder boost in a three-dimensional–conformal plan. Plans using conventional fractionation that result in a whole bladder dose of 50 to 55 Gy and a bladder tumor volume dose of 65 Gy in combination with concurrent cisplatin-containing chemotherapy have been widely used. The available information suggests that higher doses per fraction may lead to a higher rate of significant late complications. Data looking at toxicity from urodynamic and quality-of-life studies indicate that lower dose per fraction irradiation given once or twice a day concurrent with chemotherapy results in excellent long-term bladder function and low rates of late pelvic toxicity.133,134

Because the bladder is not a fixed organ, its location and volume can vary considerably from day to day. This results in a number of logistic problems to ensure adequate coverage of the bladder. Studies have identified substantial movement of the bladder during the course of external-beam radiation therapy, and as a result of these findings, many have recommended that the bladder be emptied when simulated and prior to each treatment to maximize reproducibility and avoid a geographic miss. Forms of image-guided delivery (including daily cone-beam CT and fiducials) have also been employed for accurate localization. Another acceptable approach often employed in the United Kingdom, is for radiation to be delivered to 55 Gy in 20 fractions or 64 Gy in 32 fractions to the whole bladder without a tumor boost and without fields to specifically cover the pelvic lymph nodes.135
Brachytherapy is another technique to deliver a higher dose of radiation to a limited area of the bladder within a short period. This approach has been reported from institutions in the Netherlands, Belgium, and France. It is reserved for patients with solitary bladder tumors and as part of combined modality therapy with transurethral resection and external-beam radiation therapy as well as interstitial radiation therapy. External-beam doses of 30 Gy are used in combination with an implant tumor dose of 40 Gy. These groups report that for patients with solitary clinical stage T2 to T3a tumors less than 5 cm in diameter, local control rates at 5 to 10 years range from 72% to 84% with disease-specific survivals of approximately 75%.136
Comparison of Treatment Outcomes of Contemporary Cystectomy Series with Contemporary Selective Bladder-Preserving Series
Comparing the results of selective bladder-preserving approaches with those of radical cystectomy series is confounded by selection bias and the discordance between clinical (TURBT) staging and pathologic (cystectomy) staging. Clinical staging will understage the extent of disease 40% of the time with regard to penetration into the muscularis propria or beyond when compared to pathologic staging.137,138 The University of Southern California and Memorial Sloan-Kettering Cancer Center have reported their large cystectomy experience,102,136,139 and the national phase III protocol by Southwest Oncology Group (SWOG), Eastern Cooperative Oncology Group, and Cancer and Leukemia Group B (CALGB) has also reported valuable prospective data.103 The overall survival rates from these contemporary cystectomy series are comparable to those reported from single-institution and cooperative group results using contemporary selective bladder-preserving approaches with trimodality therapy and prompt salvage cystectomy for the minority of patients who recur (Table 65.5). An attempt to compare cystectomy to bladder-sparing therapy in a randomized fashion in the United Kingdom failed to accrue.

Bladder-Preservation Treatments with Less than Trimodality Therapy
It has been argued that trimodality therapy might represent excessive treatment for many patients with invasive bladder cancer and that comparable results could be obtained by TURBT, either alone or with chemotherapy. Herr140 reported the outcome of 432 patients initially evaluated by repeat TURBT for muscle-invasive bladder tumors. In that series, 99 patients (23% of the original 432 patients) initially treated conservatively without immediate cystectomy had a 34% rate of progression to a recurrent muscle-invading tumor at 20 years. In series combining TURBT and MVAC chemotherapy, only 50% of those found to have a clinical CR proved to be tumor-free at cystectomy.141 By comparison, one of the clearest examples of the improved success of trimodality treatment was reported in the study from the University of Paris.142 TURBT followed by concurrent cisplatin, 5-FU, and accelerated radiation was used by this group initially as a precystectomy regimen. In the first 18 patients, all of whom demonstrated no residual tumor on cystoscopic evaluation and rebiopsy (a CR) but who all underwent a cystectomy, none had any tumor in the cystectomy specimen (100% had a pathologic CR). Comparing approaches by TURBT plus MVAC chemotherapy alone with trimodality therapy, the 5-year survival rates are comparable (50%), but the preserved bladder rate for all patients studied ranged from 20% to 33% when radiation therapy was not used and from 41% to 45% when radiation therapy was used.142 Thus, trimodality therapy increases the probability of surviving with an intact bladder by 30% to 40% compared with the results reported with TURBT and chemotherapy alone.
Herr143 reported on 63 patients who had achieved a complete clinical response to neoadjuvant chemotherapy with a cisplatin-based regimen, who then refused to undergo a planned cystectomy. He reported that the most significant predictor of improved survival was complete resection of the tumor before starting chemotherapy. Over 90% of surviving patients had small low-stage invasive tumors that were completely resected. Thus, he concluded, selected patients with T2 bladder cancers may do well after a transurethral resection and chemotherapy.
Systemic Chemotherapy with Radical Therapy
Neoadjuvant Chemotherapy
The advantage of neoadjuvant chemotherapy is its potential to downsize and downstage tumors and to attack occult metastatic disease early, especially given the frequent postoperative complications and prolonged recovery that can delay or derail plans for adjuvant chemotherapy. Moreover, although trials described as follows suggest a survival advantage for neoadjuvant chemotherapy, there have been no contemporary studies supporting a benefit with adjuvant chemotherapy. The disadvantages of neoadjuvant therapy include the inherent difficulties in assessing response, the fact that clinical rather than pathologic criteria must be relied on, the debilitating effects of chemotherapy in some patients, increasing the risks of surgery and possibly complicating or delaying full recovery from surgery, and the possibility of the deleterious effects of the delay in cystectomy or radiation associated with neoadjuvant chemotherapy.144
Although downstaging of the primary tumor has been demonstrated, randomized studies using single-agent neoadjuvant chemotherapy have failed to demonstrate a survival benefit. Studies in patients with measurable metastatic disease clearly showed the superiority of MVAC over single-agent cisplatin on survival, inspiring further studies of multiagent neoadjuvant therapy.145
The study by Grossman et al.143 randomly assigned patients with muscularis propria–invasive bladder cancer (stage T2 to T4a) to radical cystectomy alone or three cycles of MVAC followed by radical cystectomy. During an 11-year period, 317 patients were enrolled. The authors reported that MVAC can be given before radical cystectomy, but the side effects are appreciable. One-third of patients had severe hematologic or gastrointestinal reactions, but, on the positive side, there were no drug-related deaths and the chemotherapy did not adversely affect the performance of surgery. The authors concluded:
1. The survival benefit associated with MVAC appeared to be strongly related to downstaging of the tumor to pT0. Of the chemotherapy-treated patients, 38% had no evidence of cancer at cystectomy, as compared with 15% of patients in the cystectomy-only group. In both groups, improved survival was associated with the absence of residual cancer in the cystectomy specimen.
2. The median survival was 77 months for the chemotherapy-treated patients compared with 46 months for the cystectomy-only group.
3. The 5-year actuarial survival was 43% in the cystectomy group, which was not significantly different from 57% in the chemotherapy-treated group.
Stratification by tumor stage indicated greater improvement in median survival with chemotherapy in subjects with T3–T4a disease (65 versus 24 months, chemotherapy versus observation) than in subjects with T2 disease (105 versus 75 months). The authors point out that their study is different from seven previous negative studies that used either single-agent cisplatin (demonstrated to be inferior to MVAC in measurable metastatic disease) or a two-drug combination. They also acknowledged problems of interpretation created by slow accrual and a lack of pathologic review.
The Medical Research Council and the EORTC performed a prospective randomized trial of neoadjuvant cisplatin, methotrexate, and vinblastine in patients undergoing cystectomy or full-dose external-beam radiotherapy for muscularis propria–invasive bladder cancer.146,147 In the initial report with a median follow-up of 7.4 years, the difference in 5-year survival between those who received chemotherapy (49%) and those who did not (43%) just reached clinical significance with a probability value of 0.048.147 However, the survival benefit did not reach the prespecified study goal. Long-term follow-up of the study with median follow-up of 8 years and more death events demonstrated that systemic chemotherapy plus local treatment improved overall 10-year survival by 6% and reduced the risk of bladder cancer death by 17% compared to local treatment alone. For patients whose local treatment included a cystectomy, the use of cisplatin, methotrexate, and vinblastine (CMV) resulted in a 26% reduction in the risk of death compared to surgery alone.148 Based on their interpretation of the data as presented, Sharma and Bajorin149 now recommend neoadjuvant chemotherapy, although others are concerned that the “number needed to treat” is very high.
A third randomized trial was the Nordic Cystectomy Trial 1.150 Patients were treated with two cycles of neoadjuvant doxorubicin and cisplatin. All patients received 5 days of radiation followed by cystectomy. A subgroup analysis was performed and showed a 20% difference in disease-specific survival at 5 years in patients with T3 and T4 disease, but there was no difference in stages T1 and T2, nor a difference when all entered patients were compared.
The Nordic Cystectomy Trial 2 included only stage T3 or T4a patients in an attempt to confirm the positive results in Nordic 1 in this subgroup of patients.151 This trial eliminated radiation therapy and substituted methotrexate for doxorubicin in order to lower toxicity. In 317 patients studied, no survival benefit was noted in the chemotherapy arm. The authors concluded that despite substantial downstaging, no survival benefit was seen with neoadjuvant chemotherapy after 5 years of follow-up, although the choice of chemotherapy was unconventional by contemporary standards.
Raghavan et al.152 published a meta-analysis of all completed randomized trials of neoadjuvant chemotherapy for invasive bladder cancer (2,688 patients). They concluded that single-agent neoadjuvant chemotherapy is ineffective and should not be used; current combination chemotherapy regimens improve the 5-year survival by 5%, which reduces the risk of death by 13% compared with the use of definitive local treatment alone (from 43% to 38%).
Additional meta-analyses have been published144,153–155 that showed a 4% to 6% absolute increase in 5-year survival. Many phase II studies are now investigating alternative neoadjuvant combinations including cisplatin/gemcitabine and dose-dense or accelerated MVAC, and time will tell whether any have superiority.156–162
In the 2014 National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Bladder Cancer, neoadjuvant chemotherapy is a category 1 recommendation for localized stage T2–T4a disease. According to the National Cancer Data Base in the United States, only 11.6% of patients underwent any perioperative chemotherapy, with most in the adjuvant setting.162 In the future, gene profiling may identify those most likely to respond to chemotherapy.163
Adjuvant Chemotherapy
The advantage of adjuvant, as opposed to neoadjuvant, chemotherapy is that pathologic staging allows for a more accurate selection of patients. This approach facilitates the separation of patients in stage pT2 from those in stages pT3 or pT4 or node-positive disease, all at a high risk for metastatic progression.
Adjuvant chemotherapy has been studied in two major clinical settings: (1) following bladder-sparing chemoirradiation and (2) following a radical cystectomy. In the former case, there is no guidance from pathologic staging, but experience has shown that up to 50% of those with invasive cancers have, in truth, a systemic disease.164 Respecting this, the RTOG studies have added adjuvant chemotherapy at first with MCV, later using cisplatin plus gemcitabine, and more recently adding paclitaxel.165 The results thus far do not indicate whether adjuvant chemotherapy is affecting survival.
The place of adjuvant chemotherapy after cystectomy has been studied more thoroughly, but again, the results are not clear. Investigators generally agree that in the face of positive nodes, and even with negative nodes and high pathologic stage of the primary tumor, adjuvant chemotherapy is likely to be important in improving survival. In reviewing existing reports of adjuvant trials in bladder cancer, there are five randomized trials using adjuvant chemotherapy.166–170 Three studies found no difference between adjuvant chemotherapy and cystectomy alone, but all three were seriously flawed in design or accrual.149 Two of the five studies169,170 showed a survival benefit for cystectomy and adjuvant chemotherapy over cystectomy alone, but both are subject to criticism for both method considerations and small accrual.
Nonetheless, in a follow-up report by Stockle et al.171 an analysis of 166 patients, including the 49 initially randomized patients, a difference was noted in the 80 patients who received adjuvant chemotherapy as compared with 86 patients who underwent cystectomy alone. The extent of nodal involvement proved important, and when patients were stratified by the number of nodes involved, adjuvant chemotherapy was most effective in patients with N1 disease.
In an important review of the current status of adjuvant chemotherapy in muscle-invasive bladder cancer, the Advanced Bladder Cancer Meta-Analysis Collaboration examined 491 patients from six trials, representing 90% of all patients randomized in cisplatin-based combination chemotherapy trials. They concluded that there is insufficient evidence on which to base reliable treatment decisions, and they recommended further research.172
More recent studies have used different adjuvant chemotherapy regimens or molecular stratification. A randomized trial performed in Italy randomized patients after cystectomy either to four courses of gemcitabine plus cisplatin (n = 102) or to the same treatment at time of relapse (n = 92). There was no difference in the 5-year overall survival across treatment arms. However, due to poor accrual, the study was insufficiently powered to detect a survival difference.173
As described earlier, p53 alteration status was hypothesized to be both prognostic for recurrence after cystectomy and predictive for a survival benefit conferred by adjuvant chemotherapy. A phase III trial separated patients based on p53 status, with all p53-negative patients followed with observation alone. Patients with p53 alteration (n = 114) were randomized postcystectomy to three cycles of adjuvant MVAC or observation. Neither p53 status nor MVAC adjuvant chemotherapy impacted risk of recurrence.57
Gallagher et al.174 studied adjuvant, sequential chemotherapy in a nonrandomized design, using as a basis the improvement in survival in breast cancer when sequential adjuvant chemotherapy was used. In this study and others similarly designed,175,176 adjuvant, sequential chemotherapy for patients with high-risk urothelial cancer did not appear to improve disease-specific survival over that observed with surgery alone.
Dreicer,161 in reviewing the published literature, made the case for adjuvant chemotherapy as the standard of care given the lethality of radical cystectomy alone in muscle-invasive bladder cancer, but he acknowledges that “suboptimal trial design, insufficient numbers of patients, and lack of standardization of the chemotherapy regimens used have plagued adjuvant studies.”
Combined Modality Treatment of Local–Regionally Advanced Disease
The place of combined modality therapy for advanced disease has not been settled. Several series have suggested an improvement in long-term survival in selected patients undergoing resection of persistent cancer deposits after MVAC or CMV.164,177
In our experience, carefully selected patients with locally advanced unresectable bladder cancer, including some patients with pelvic nodal masses, may experience long-term survival with the combination of chemotherapy and radiation. To be selected for this combined modality treatment, patients must have (1) an excellent performance status, (2) locally advanced measurable disease, (3) normal kidney function tests, and (4) no evidence of distant metastases beyond the common iliac lymph nodes. The initial treatment consists of four to six cycles of combination chemotherapy. If a significant regression of tumor is achieved, radiation treatment is administered in combination with radiosensitizing chemotherapy. These patients were carefully selected, but in the majority of patients so treated, excellent tumor shrinkage and long-term survival were achieved in patients who would otherwise have been expected to succumb rapidly if treatment had consisted of chemotherapy alone.
Quality of Life After Cystectomy or Bladder Preservation
Evaluating the quality of life in long-term survivors of bladder cancer has been difficult, and only recently have attempts been made to assess this in an objective and quantitative fashion.134,178–191 A number of problems arise in the interpretation of the published studies. Tools to assess quality-of-life variables were developed early for common prostate and gynecologic cancers, but until very recently no such instruments existed for bladder cancer. The instruments in use for bladder cancer have thus been adaptations of uncertain validity. The published studies are all cross-sectional and patients have follow-ups of varying lengths. This matters in a surgical series in which functional outcome improves with time and in a radiation series in which it may deteriorate. Despite these limitations, some conclusions can now be drawn.
A radical cystectomy causes changes in many areas of quality of life, including urinary, sexual, and social function, daily living activities, and satisfaction with body image.166–171,192 During the past decade, researchers have concentrated on the relative merits of continent and incontinent diversions. Available data have been mixed with some groups, surprisingly, reporting few differences between the quality of life of those with an ileal conduit and those with continent diversions. Hart et al.182 have compared outcome in cystectomy patients who have either ileal conduits, cutaneous Koch pouches, or urethral Koch pouches. Regardless of the type of urinary diversion, the majority of patients reported good overall quality of life, little emotional distress, and few problems with social, physical, or functional activities. Problems with their diversions and with sexual function were most commonly reported. After controlling for age, no significant differences were seen among urinary diversion subgroups in any quality-of-life area. It might be anticipated that those receiving the urethral Koch diversions would be the most satisfied, and the explanation why this is not so is unclear. It may be that the subgroups were too small to detect differences, but perhaps it is more likely that each group adapts in time to the specific difficulties presented by that type of diversion. Somani et al.184 reviewed 40 published studies that evaluated overall quality of life, reporting on 3,645 patients. Only two studies reported a better quality of life for those who had neobladder and only two reported a better body image.184 Another prospective study reported by Mansson et al.185 suggested that there may be a large cultural component to the response with big differences seen between Egyptian and Swedish men followed prospectively through trials of chemotherapy and cystectomy.
Porter and Penson183 attempted a systematic review of the literature, testing the premise that continent diversions result in improved health-related quality-of-life outcomes. They concluded that, whatever our assumptions, there is no literature to support the use of one urinary diversion over another. Reviews by Gerharz et al.187 and Somani et al.184 came to the same conclusion. It appears that women have more problems with continent diversions, particularly the need to catheterize, than do men.186
Zietman et al.188
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree