Management
Most treatment information has been derived from small series gathered over several years, with variable definitions of disease used and, therefore, the best approaches to treatment are not clarified. Consensus guidelines have been proposed for management.63,64 An algorithm for the management of these cancers is shown in Figure 53.1.
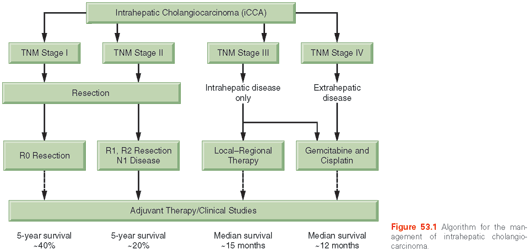
Surgery. Indications for resectability are not well described. The goal is to resect the tumor with an adequate margin of normal tissue, and to obtain microscopically free resection margins, while retaining enough liver tissue behind for the patient to have adequate liver function after surgery. The resection may vary from nonanatomic resections to segmental anatomic resections. Intrahepatic metastases tend to occur as multiple satellites, and although their presence impacts prognosis, it should not define resectability. However, for widespread hepatic metastases, curative resection is unlikely, and other forms of therapy should be considered. The presence of underlying advanced cirrhosis with portal hypertension may preclude surgical resection. Extrahepatic spread portends a poor prognosis, and carcinomatosis should be considered a contraindication to resection. A staging laparoscopy can help define respectability prior to a full laparotomy and is recommended.65
Outcomes after surgical resection for iCCAs have been reported in small series in the literature. The resectability rate ranges from 32% to 90%. The mortality of resection is slightly higher than series of hepatic resection for other indications, but is generally less than 10%. Lymph node metastases, positive margin status, and vascular invasion were reported as significant prognostic factors in an analysis of 449 patients.66 Other prognostic factors include satellite metastases, nodal metastases, tumor size, and a CA19-9 level greater than 1,000. Although rare, intrahepatic intraductal papillary tumors have an excellent prognosis if completely resected.67,68
The median survival after surgical resection is ~36 months. The 5-year survival rate with a curative R0 resection is ~60%, but curative resections are possible only in about 30% of patients. There is high risk of tumor recurrence both locally with intrahepatic metastases as well as with extrahepatic disease. However, there are no established guidelines for surveillance and follow-up after surgical resection for iCCAs. As a guide, surveillance could consist of laboratory tests of liver function and CA19-9 and radiologic evaluations every 3 months for the first 2 years after surgery and every 6 months thereafter for the first 5 years could be considered and modified based on the perceived risk. The role and utility of surveillance has not been formally established. Surgery is generally not indicated for recurrent CCAs.
Liver Transplantation. The outcomes from liver transplantation for iCCAs, unlike those for hilar CCAs have been disappointing, with a 5-year survival rate of 29% in data from the European Liver Transplant Registry.69 As a result of these poor outcomes, liver transplantation is not generally offered for this indication.
Local–Regional Therapies. Local–regional therapy for unresectable iCCAs include transarterial chemoembolization (TACE), transarterial radioembolization (TARE), radiofrequency ablation (RFA), or microwave ablation. They may be used for palliation and local control in persons with a good performance status. A median survival time of 20 months with TACE and 43.7 months with TARE have been reported in case series. For tumors smaller than 3 cm, RFA resulted in a median survival time of 38.5 months. Most tumors present with a large bulky tumor precluding complete ablation, and there is a potential risk of biliary complications arising from bile duct damage.
Chemotherapy. Based on pivotal data from the UK-ABC-02 study, gemcitabine in combination with cisplatin has been established as the standard of care for patients with biliary cancers.70 Gemcitabine was dosed at 1000 mg/m2 in combination with cisplatin at 25 mg/m2, with both agents given intravenously on days 1 and 8 every 21 days for a maximum of 6 months. The study was conducted using a phase II/III design and enrolled 410 patients across centers in the United Kingdom. Stratification was done based on the extent of disease (locally advanced versus metastatic), primary tumor site (intrahepatic/extrahepatic bile ducts, ampullary, gallbladder), performance status (Eastern Cooperative Oncology Group [ECOG] score 0, 1, or 2), prior therapy (yes/no), and recruiting center. The median overall survival in the gemcitabine/cisplatin group was 11.7 months compared to 8.1 months in the gemcitabine only group (p <0.001; 95% CI, 9.5 to 14.3) with a hazard ratio of 0.64 (95% CI, 0.52 to 0.80). Efficacy was evident across a range of endpoints, including progression-free survival (8 months versus 5 months; HR, 0.63; p <0.001) and response rate (26.1% versus 15.5%). Prespecified subgroups described earlier all derived clinical benefit. Only 17.6% (72 of 410) patients went on to receive second-line therapy. The role of subsequent lines of therapy with regard to survival benefit remains to be defined, but fluoropyrimidine-based regimens have demonstrated preliminary evidence of efficacy in studies with small numbers of patients with cholangiocarcinoma/gallbladder cancer,71,72 and should be investigated in larger, controlled studies. Of patients, 58.8% (241 of 410) had involvement of the bile ducts in this study; 19.5% (80 of 410) had intrahepatic disease; 17.8% (73 of 410) had intrahepatic involvement; and 13.9% (57 of 410) had hilar disease. Survival benefit was most prominent in the intrahepatic group on a subgroup analysis (HR, 0.57; 95% CI, 0.34 to 0.94). The subgroup analyses were not powered to demonstrate benefit within each subgroup and, as such, should be viewed as hypothesis generating. Response rate was 19% versus 11.7% in the subgroup comprising both biliary and ampullary cancers.
At a conceptual level, there is support for the consideration of combined modality chemoradiation therapy for patients with unresectable disease using gemcitabine- or fluoropyrimidine-based strategies, and efficacy has been observed in small studies.73,74 However, definitive data through controlled trials remains unavailable and, as such, this remains an area of active investigation, particularly from the standpoint of patient selection and advantages over chemotherapy alone.
The advent of molecular profiling has uncovered putative therapeutic targets, which include aberrations in the Akt–PI3K–mammalian target of rapamycin (mTOR), Fanconi, IDH1/2, ERBB–MEK, and fibroblast growth factor receptor (FGFR) pathways.43,75,76 Fusions in fused in glioblastoma–repressor of silencing 1 (FIG–ROS1) and various FGFR2 fusions such as FGFR2–BICC and others have been identified, and more such events will be identified with the increasing use of genomic sequencing studies. The pursuit of these targets may lead to an eventual individualized and heterogeneous approach to patients with both refractory and untreated disease in lieu of empiric approaches such as gemcitabine and cisplatin.
Perihilar Cholangiocarcinoma
pCCAs are cancers that arise from the extrahepatic biliary tract from the second order ducts to the origin of the cystic duct. These cancers are amongst the commonest malignancies of the biliary tract encountered in many parts of the world.
pCCAs should be distinguished from nonhilar, distal extracellular tumors because of their distinctive clinical presentations, natural history, staging, and management approaches. Although distinctive molecular and pathologic differences have yet to be determined, and the pathogenesis of these tumors may be similar, dCCAs are far less common than pCCAs and have better treatment outcomes. The reason for the predilection of pCCAs to predominantly arise at the liver hilum is not known.
Tumors that cause ductal obstruction at the hilar region are occasionally referred to as Klatskin tumors. However, the series reported in the classical paper by Klatskin included both intrahepatic and extrahepatic cancers, and was reported in an era in which the biliary tract was inaccessible to noninvasive preoperative imaging.77 Thus, use of the term Klatskin tumor to describe pCCAs is inaccurate, confusing, and best avoided.
Diagnosis
Patients with early-stage cancers are often asymptomatic. Most patients present with painless jaundice and its clinical sequelae of dark urine, light stool, and pruritus. Nonspecific gastrointestinal symptoms such as anorexia and nausea, as well as mild weight loss and fatigue are not unusual. Most patients will present with painless obstructive jaundice, associated with pruritus, abdominal pain, weight loss (30% to 50%), and fever in about 20%.78 The clinical presentation of pCCAs are indistinguishable from other malignancies causing large bile ductal obstruction such as dCCAs or pancreatic cancer. Obstruction of the bile duct and biliary stasis may lead to bacterial colonization and cholangitis, particularly in patients with biliary stones. Patients with cholangitis can present with high fever, pain, nausea, vomiting, and rigors.
Serum biochemical tests will reveal the evidence of cholestasis with elevations in bilirubin, alkaline phosphatase, and γ-glutamyltransferase. Serum aminotransferase levels may be normal or mildly elevated in the early stages. Serum CEA and CA19-9 levels are the most commonly elevated serum tumor markers. CA19-9 has limited value because levels can be elevated in benign biliary tract disease, cholangitis, or cholestasis. CA19-9 levels above 100 U/mL were found to be 89% sensitive and 86% specific for the diagnosis of malignancy in patients with PSC.79,80 CEA levels also have a low predictive value for cancer and are not helpful for a diagnosis.79,81 Both CA19-9 and CEA levels may be elevated in bile specimens in the presence of cancer.82 A combined index of CA19-9 and CEA has been proposed, with studies showing mixed results in predicting cancer. The presence of cholangitis or hepatolithiasis can cause elevations of tumor markers, and these tests should be repeated after symptoms have resolved. CA19-9 is a carbohydrate cell–surface antigen related to Lewis blood group antigens. Patients with a negative Lewis blood group antigen (representing 10% of the population) cannot synthesize CA19-9 and will not manifest an elevation in this marker. Additional potential markers for CCA include CA242, CA72-4, CA50, CA125, RCAS1, and serum MUC5AC. These have all been evaluated with mixed results.80,83–87 CA19-9 has also been defined as a poor prognostic factor in CCA.88
A diagnosis is usually based on history, cholangiography and cytology, or tissue analysis. pCCAs often arise and can progress to obstruction of one of the main bile ducts at the hilum before involving the other main duct. Unilateral obstruction of either the right or left bile duct alone may not lead to jaundice or an elevated bilirubin because of compensation from the normally draining lobe of the liver. However, the alkaline phosphatase and γ-glutamyltransferase may be elevated. Jaundice may occur when the tumor extends down the bile ducts to involve the confluence of the right and left ducts. Unilateral obstruction results in atrophy of the affected side of the liver and hypertrophy of the other side. This atrophy–hypertrophy phenomenon will also occur if the portal vein has also been blocked by the tumor. Because atrophy–hypertrophy results in an axial rotation of structures in the hepatoduodenal ligament, its effects need to be considered when interpreting imaging studies or in planning hepatic resections.89
Any patient who has a perihilar stricture, without evidence of ductal disease elsewhere in the biliary tree suggestive of PSC, and who has not had previous biliary surgery that might have resulted in stricture, is considered to have pCCA. The diagnosis and evaluation of these tumors depends on the available diagnostic technologies and expertise. The goals are to (1) ascertain the nature and extent of obstruction, (2) obtain tissue for diagnosis if possible, and (3) stage the tumor to determine spread and metastasis to guide therapy. An abdominal ultrasound will confirm the presence of a biliary obstruction. Additional testing with either a CT or MRI/magnetic resonance cholangiopancreatography (MRCP) is needed to identify the potential and for staging if a malignancy is suspected. The accuracy of CT and MRI/MRCP for the prediction of the extent of ductal involvement ranges from 84% to 91%; for hepatic arterial invasion, it ranges from 83% to 93%; for portal vein invasion, from 86% to 98%, and for lymph node metastasis, from 74% to 84%.90–92
Biliary tract imaging (cholangiography) with cytology is used to establish the diagnosis. Tissue biopsies can be obtained under fluoroscopic guidance, or using cholangioscopy during endoscopic retrograde cholangiopancreatography (ERCP). A cholangioscopy may allow for direct visualization, but often provides a lower amount of tissue for analysis.93–96 In one study, direct visualization for biliary strictures using a miniendoscope identified malignancy in 11 of 20 patients and resulted in modification of diagnosis of biliary stricture in 20 of 29 patients.97
Peroral cholangioscopy using the spyglass system may also be associated with a higher rate of cholangitis. Confocal laser endomicroscopy is an emerging technique that may be helpful. Specific criteria (Miami criteria) for the diagnosis of a malignancy within a stricture using this technique have been proposed, but need to be further validated, and the specificity needs to be improved.98 An endoscopic ultrasound (EUS) with fine-needle aspiration (FNA) may also be helpful for a diagnosis or to predict unresectability by detecting nodal spread. An intraductal ultrasound performed using an ultrasound probe passed into the common bile duct increased the accuracy of ERCP from 58% to 90%.99
pCCAs are less accessible than other distal CCAs for sampling. The highly desmoplastic nature of these tumors further limits the amount of cellular material that may be obtained for a cytologic analysis. As a result, establishing a tissue diagnosis of pCCA is extremely difficult. The diagnostic sensitivity of tissue or cytology examination remains poor. Indeed, benign disease has been noted in about 10% of surgical resections performed for presumed pCCAs.100–102 A positive diagnosis with brush cytology ranges from 44% to 80%,103–105 with pooled data from over 800 CCA patients reporting a sensitivity of 42%, a specificity of 98%, and a positive predictive value (PPV) of 98% among patients with confirmed cancer.103 A brush cytology is diagnostic and very useful when positive, but of little value when negative. In a study of 74 patients with pancreaticobiliary strictures, the sensitivity and specificity of brush cytology were 56% and 100%, respectively, and the positive predictive value was 100%.106 Intraductal tissue biopsies also have a low diagnostic yield with a pooled sensitivity of 56%, a specificity of 97%, and a PPV of 97%. The use of multiple sampling techniques should be considered to improve the diagnostic yield of sampling.
Mucobilia on ERCP is an uncommon finding that is highly suggestive of a papillary CCA. Papillary tumors could be either intrahepatic or extrahepatic. FNA is also useful if a mass can be seen on ultrasound examination or on CT scan.
Staging
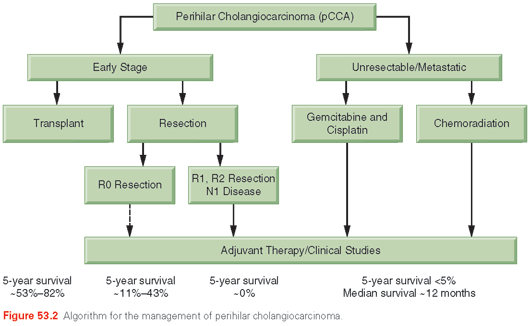
Disease staging in pCCA requires an assessment of the extent of ductal involvement as well as the extent of involvement of the liver parenchyma, lymph nodes, and vasculature and distant metastases.107 The TNM system (Table 53.2) does not help to define surgical resectability and, therefore, may not adequately predict outcome.51 A classification for hilar tumors was introduced and modified by Bismuth.108 This classification is based on the level of ductal involvement by the tumor, and provides a guide as to the extent of surgical resection that may be required for tumor eradication. However, it is not a true staging system and has low accuracy.109 A registry of pCCA has been initiated to collect data that may serve as a resource to guide the development of meaningful future staging classifications.110
Management
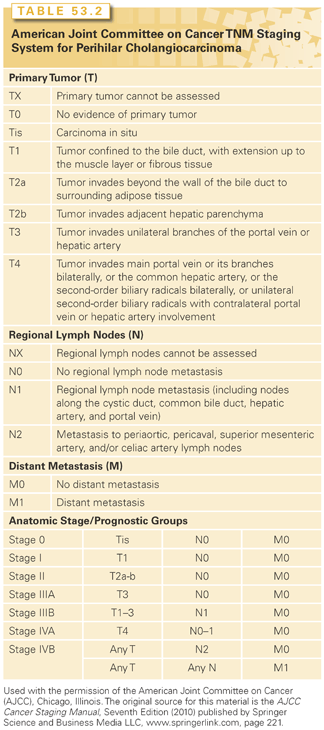
A suggested approach to the management of pCCA is presented in Figure 53.2. The local extent of the disease along the biliary tree can be determined by direct or imaging-defined cholangiography—either ERCP, magnetic resonance cholangiography MRCP or percutaneous transhepatic cholangiography (PTC). However, the extent of disease may not be appreciated because of tumor spread along the wall of the bile duct without lumenal compromise. PTC or MRCP may be more useful than ERCP in establishing the upper extent of disease. MRCP is less invasive, but may need to be supplemented with direct cholangiography at times. Vascular invasion has been assessed by MRI/MR angiography, angiography, or Doppler ultrasound. An MRI is useful to assess liver invasion or vascular involvement not clearly identified on CT scans. CT scans or MR angiography are replacing the need for an invasive angiography to assess vascular involvement. Color-flow Doppler ultrasound is very dependent on the operator, but can be effective at evaluating portal vein involvement and, in some cases, hepatic artery involvement.111 Positron-emission tomography (PET)/CT scans, intraductal ultrasound (US), and EUS have all been used for staging. EUS with FNA may also be helpful for a diagnosis by detecting distal lymph node involvement. A staging laparoscopy with or without ultrasound can identify tumor spread beyond that detected on cholangiography, vascular encasement, or lymph node involvement.
Liver Transplantation. Liver transplantation has emerged as a viable option for the treatment of early stage, unresectable pCCA in highly selected patients.112 For patients with tumors that are unresectable, a complete hepatectomy with liver transplantation may provide the only chance for a cure. The presence of extrahepatic nodal disease or metastases is a contraindication to transplant. In carefully selected patients, a multimodality approach combining preoperative chemoradiation, staging laparoscopy, and orthotopic liver transplantation has resulted in overall 5-year survival rates of up to 82%. A study of the combined experience of several centers showed an overall survival of 53% on an intention-to-treat analysis, with a 65% recurrence-free survival after 5 years.112 It should be noted that these data reflect results obtained with a highly selected group of patients.
The availability of adequate organs for transplantation has limited the use of liver transplantation as a treatment modality for pCCA. The use of living donor liver transplantation may overcome some of the limitations of organ availability for this indication because 5-year survival after living donor living transplantation (LDLT) was 69% compared with 63% after deceased donor transplantation. The outcomes are better in patients with pCCAs arising in the setting of PSC, with a 72% 5-year survival compared with 51% in non-PSC patients. Furthermore, patients with pCCAs undergoing neoadjuvant chemoradiation and liver transplantation have a quality of life that is similar to those for patients undergoing transplantation for other indications.113 If liver transplantation is being considered as a treatment option, FNA of the hilar lesion by EUS should be avoided because of the risk of tumor seeding.
In early reports, EBRT and bolus fluorouracil (5-FU), followed by brachytherapy, 5-FU, and liver transplantation, was used.114,115 Out of 28 patients, 11 were excluded because of metastatic disease found at the time of exploratory surgery. The rest underwent liver transplantation with an identifiable tumor noted on explant in 10 patients; 2 patients had recurrence after 40 months and 54 months, respectively; and 2 died of non–cancer-related causes. The median duration of follow-up was 41.8 months (range, 2.8 to 105.5 months); the 5-year actuarial survival rate for those transplanted was 87%. A follow-up protocol added a intrabiliary brachytherapy using iridium seeds after external beam radiation therapy (EBRT) and maintenance therapy with capecitabine until transplantation.115 Out of 56 enrolled patients, 28 received a transplant. The actuarial 1- and 5-year survivals were 88% and 82%, respectively, after transplantation. A similar protocol that combined neoadjuvant brachytherapy and infusional 5-FU followed by transplantation was reported, with 5 of 17 patients (29%) achieving long-term disease-free survival.93 Other small series have demonstrated 3-year survival rates from 0% to 53%.116
Earlier studies of liver transplantation for patients with all types of CCA in unselected patients showed very poor results. Meyer et al.117 reported the results of liver transplantation for cholangiocarcinoma in 207 patients collected by the Cincinnati Transplant Tumor Registry. Fifty-one percent had recurrence, with a median time of recurrence of 9.7 months, and the median time between recurrence and death being 2 months. In a series of 7 patients undergoing liver-related transplant for CCA, 6 were alive after a median follow-up of 20 months. Recurrences were noted in all patients in this series with iCCA.118
Surgery. Surgical resection is complex and associated with mortality and morbidity. The results of surgical resection for pCCA have been reported in many retrospective single-institution surgical series. The goals of surgical resection are to remove the tumor with negative resection margins. An en bloc resection of at least one lobe of the liver, the extrahepatic bile duct, and a complete periportal lymphadenectomy may be required.
The preoperative assessment serves to define the extent of resection that may be required. There is a role for preoperative biliary drainage in some, but not all patients. This could be performed either percutaneously or endoscopically with stenting or placement of a nasobiliary tube. Biliary drainage can alleviate symptoms in patients with severe obstructive jaundice, renal dysfunction, or pruritus.119 However, preoperative decompression can increase complications during or after surgery.120–123 Cholangitis may occur following bacterial colonization of bile and stenting may induce fibrosis, making it difficult to delineate the extent of tumor.120 Five randomized trials and a meta-analysis have not demonstrated a benefit, although retrospective studies suggest a disadvantage to preoperative stenting.124 It may be appropriate if a hemihepatectomy for CCA is planned in a jaundiced patient or if a pancreaticoduodenectomy is to be done in a patient with long-standing or severe jaundice. Other preoperative preparations include correcting a vitamin K deficiency and bowel preparation. The use of MRCP to guide decision making toward surgical resection may avoid the need for more invasive cholangiography and stenting.
Excision of the bile ducts may be possible up to the first order branches of the right and left bile ducts. If the tumor extends beyond this on one side, a partial hepatectomy may be needed, and a Roux-en-Y reconstruction performed. The contralateral preserved bile duct should be transected at the level of the first segmental branch to maximize the chance of a negative margin. If the resection is extended beyond the first order branches, a main drainage channel may need to be fashioned by suturing the individual segmental or sectoral ducts together. A caudate lobe resection is often routinely performed because invasion of the caudate ducts may occur. Several early branches of the left hepatic duct drain the caudate lobe and can be involved with the tumor involving the left main hepatic duct. Indeed, 46% of pCCAs microscopically involve the caudate lobe.125,126
Surgery is indicated in the absence of distant metastases where a preoperative workup suggests that an R0 resection is feasible. Bilateral biliary involvement to the point that all four sectional ducts are involved precludes curative resection.127 Other indicators of unresectability include bilateral intrahepatic bile duct spread, involvement of the main trunk of the portal vein, involvement of both branches of the portal vein or bilateral involvement of the hepatic artery and portal vein, or a combination of vascular involvement on one side of the liver with extensive bile duct involvement on the other side.89 With vascular replacement, it may be possible to resect some tumors previously considered unresectable.
A periportal lymphadenopathy is not a contraindication, and resection with microscopic positive margins (R1) determined after resection can provide significant palliation. A lymphadenectomy should include all soft tissue in the porta hepatis, excluding the portal vein and hepatic artery. The common hepatic artery nodes, the celiac artery nodes, the peripancreatic nodes, and the interaortocaval lymph nodes should be assessed because dissection may be indicated. Adequate staging may require sampling of at least seven nodes.128
Resectable disease is present in approximately one-third of patients with suspected pCCA. In a series from 2001 to 2008, of 118 patients referred for surgery, 51% were resectable and 41% underwent R0 resection.129 Operative mortality averaged about 8%130; 5-year survival rates after resection have ranged from 10% to 35%. The results of surgical resection highly depend on whether negative resection margins are achieved.107,131–135 Frozen sections are used to evaluate tumor margins at the time of surgery and to guide the extent of resection. However, the desmoplastic nature of these tumors and fibroinflammatory changes related to the presence of a biliary stent, often restricts an accurate determination of the presence of a tumor in frozen sections.
When negative margins are obtained, median survival of patients with a tumor-free margin is ~3.4 years with a 5-year survival rate from 11% to 43%. However, when margins are positive, median survival is 1 to 1.2 years and 5-year survival is almost zero.107,132,135,136 With positive microscopic margins, there were no 5-year survivors in one study.137 Other negative prognostic variables include tumor stage, nodal disease, tumor grade, bilirubin concentration, serum albumin level, postoperative sepsis, and absence of mucobilia.119,135,136,138,139 Recurrences most commonly occur locally at the resection bed or within the retroperitoneal lymph nodes. Distant metastases occur in one-third of cases, most commonly within the lung, mediastinum, liver, or peritoneum. Improved outcomes seen in more recent series may reflect increasing use of routine liver resections.
There are no established guidelines for surveillance and follow-up after surgical resection. There is high risk of recurrence, with peritoneal spread, hepatic metastases, local extrahepatic recurrence, and distant metastases (most commonly lung). Laboratory and radiologic evaluations every 3 months for the first 2 years after surgery and at longer 6-month intervals thereafter could be considered based on the perceived risk. The role of CA19-9 as a surveillance indicator is not established, but persistently rising levels may precede radiologic evidence of recurrence. The role of CT scans or MRI for surveillance and detection of tumor recurrence has not been evaluated in clinical trials. MRIs may be preferable to CT scans for surveillance because of the ability to concomitantly visualize the biliary tract. In a recent study, PET/CT scans demonstrated a higher positive predictive value compared to CT scans alone (94% versus 78%) for nodal metastases and a higher sensitivity (95% versus 63%) for distant metastases.140 Surgery is generally not indicated for recurrent CCA. Close surveillance and early diagnosis of recurrences may allow for eligibility for clinical trials.
Adjuvant Therapy. There is a lack of conclusive data regarding the efficacy of adjuvant radiation therapy or chemoradiation therapy for patients who have a gross residual tumor, a tumor involving the resection margins, or regional lymph nodes involved with a tumor after undergoing resection with curative intent. The reported series have been small with the potential for selection bias, and there are no randomized trials that support any particular adjuvant approaches as standard. There is a need to explore and effectively evaluate new regimens for adjuvant therapy in these patients. It is recommended that patients are enrolled in clinical trials to define the role of adjuvant therapy. Similarly, there is a paucity of data upon which to base decisions on the use of adjuvant chemotherapy. In a randomized study, mitomycin C and 5-FU were compared with observation alone in a study of 508 patients with resected pancreaticobiliary cancers that included 139 patients with bile duct cancers.141 No apparent differences in overall or 5-year disease-free survival were noted. A phase III study comparing adjuvant capecitabine versus observation alone in surgically resected patients has completed enrollment in the United Kingdom and outcomes data are eagerly awaited (NCT00363584). The primary endpoint of this study is 2-year overall survival. An analogous phase III study using gemcitabine in combination with oxaliplatin versus observation alone is underway in France with disease-free survival as the primary endpoint (NCT01313377). Data from these two pivotal studies will help define the role of adjuvant therapy more definitively. In the interim, the recommendation for patients is to participate in clinical trials whenever feasible.
Palliative Care. For unresectable tumors, palliation may be performed by percutaneous or endoscopic stent placement or by surgical bypass.
Stent placement. The goal is to drain the most functional lobe of the liver with a stent that traverses the malignant obstruction and allows for internal drainage. Percutaneous biliary drainage is more appropriate for the drainage of intrahepatic ducts and may be required for access to these ducts. Both internal and external biliary drainage are possible. Both plastic or metallic stents may be used, with one study reporting a longer survival and lower complication rates with the latter.142 Biliary catheters may exit the skin and remain capped. This allows for irrigation and provides easy access for cholangiography and stent changes as needed. However, percutaneous draining catheters may decrease quality of life. An attempt should be made to enable the drainage of greater than 50% of more of the liver volume, irrespective of whether one or more stents are used or one or more segments are drained. Imaging-based volumetric assessments may be useful to determine whether drainage will be adequate. A guided approach using MRCP may be beneficial to routine bilateral stenting. If bilateral stents are placed, they may be used side by side or by contralateral stenting through the mesh of the first stent (stent in stent). Plastic stents typically clog within 2 to 6 months, whereas metal stents last longer, up to 8 to 10 months. Endoscopic metallic stenting should be performed by an experienced biliary endoscopist. Catheter tract recurrence is a rare complication of PTC-placed stents, and was reported in 6 of 441 patients (2.6%) who underwent percutaneous biliary drainage for pCCAs.143 Patients with catheter tract recurrence had a lower survival than those without recurrence (17.5 months versus 23.0 months; p = 0.089).
Surgery. Surgical approaches have not been shown to be superior to percutaneous or endoscopic biliary drainage. A surgical bypass may avoid the need for long-term biliary tube placement, and its associated morbidity, such as cholangitis, occlusion, and need for frequent replacement. The disadvantage of surgical bypass for palliation is the morbidity associated with the procedure when there is limited overall life expectancy. If advanced unresectable disease is encountered at the time of a laparotomy for presumed resectable tumors, a bypass could be performed for palliation to avoid the need for another procedure. A surgical bypass for pCCA involving a bypass to intraparenchymal ducts using a defunctionalized limb of jejunum can be technically challenging but quite effective for palliation. A surgical biliary enteric bypass to segment III (Bismuth–Corlette cholangiojejunostomy), where the bile duct is accessed through the liver parenchyma anteriorly avoids the hilar region that may be involved with the tumor.144 A bypass to right-sided ducts may be challenging.145,146 The right lobe could be drained by a bypass to the anterior sectoral bile duct. Surgical implantation of large-bore tubes through the tumor has been used in the past but is rarely employed now.
Photodynamic Therapy. Photodynamic therapy with stenting has shown to improve survival, reduce cholestasis, and improve quality of life compared to stenting alone in a randomized study.147–149 In a small, multicentered, randomized controlled trial of 39 patients, patients who received photodynamic therapy with biliary stenting survived 493 days compared with 98 days for those treated with stenting alone.142 In a recently published meta-analysis of 6 studies, 170 patients received photodynamic therapy and were compared with 157 who had biliary stenting alone.150 There were statistical improvements in patient survival and performance status, and a trend in the decline of serum bilirubin was significantly improved and the risk of biliary sepsis was similar (15%). These data also suggest a possible role for photodynamic therapy in these patients.
Radiation Therapy. There are very few data regarding the efficacy of the use of radiation therapy either alone or in combination with other techniques for advanced stage disease, either unresectable or resected with gross residual tumor. Most of the reported series are small and no randomized comparisons exist. Long-term survivors have been rarely described.
External-beam irradiation was successful in clearing jaundice in 10 of 11 patients in a recent report; no other decompressive measures were used.151 Brachytherapy has been applied through percutaneous tubes, with a median survival of 23 months.152 The combination of surgery and radiotherapy was reported to provide a median survival of 14 months in unresectable or recurrent disease.153 However, other series have reported no benefit with radiation.154 Stereotactic body radiotherapy (SBRT) may have some efficacy but has the potential for severe toxicity. In a study of 27 patients (26 with pCCA and 1 with iCCA), of whom 18 were treated on a prospective phase II trial and received 45 Gy in three fractions over 5 to 8 days,155 the median overall survival was 10.6 months and the local control at 1 year was 84% with a median follow-up of 5.4 years. Six patients had severe duodenal or pyloric ulceration, and three patients developed duodenal stenosis. Interestingly, no such toxicity was observed in another group of 13 patients with Klatskin tumors.156 Eight of these received 48 Gy in four fractions and the others received a range of doses (32 to 56 Gy in 3 to 4 Gy per fraction), and median survival was 33.5 months.
No formal comparative studies have been performed, although the median survival of 1 year observed with radiation therapy appears to be superior to 3 months with chemotherapy or 6 months with best supportive care alone.
Other Approaches. Endobiliary radiofrequency ablation may potentially provide benefits that are similar to the use of photodynamic therapy for palliation of malignant ductal obstruction, but the experience with this has been limited.157 EUS-guided biliary drainage through a transgastric approach is technically feasible, but has high complication rates, such as bile leakage and peritonitis (20%) even in experienced hands.158–162
Systemic Therapy. As described earlier, based on the UK-ABC-02 data, gemcitabine and cisplatin should be regarded as the standard of care for patients with perihilar cholangiocarcinoma with unresectable disease. Although fluoropyrimidine-based therapies have shown evidence of preliminary efficacy, the role of subsequent lines of systemic chemotherapy remains to be definitively defined. Similarly, molecular profiling of these cancers may eventually result in a paradigm shift, allowing for the individualized treatment of patients based on single-agent/combination therapy based on perturbation of aberrant pathways.
Distal Cholangiocarcinoma
dCCAs are cancers arising from the extrahepatic common hepatic duct between the junction of the cystic duct and the papilla, but not involving either the cystic duct or the ampulla of Vater.
There is heterogeneity of cancers that arise from the extrahepatic bile duct. Other cancers that arise from the extrahepatic ducts but are considered separately from dCCAs include tumors at the liver hilum or cystic duct. Cancers arising at the hilum are considered separately as pCCAs, whereas those arising within the cystic duct are considered along with other gallbladder cancers.
Diagnosis
The typical presentation of dCCAs is with obstructive jaundice. In the case of tumors arising below the insertion of the cystic duct, the gallbladder may be palpable. The presentation is similar to that of pCCAs or cancers arising from the head of the pancreas. Patients may present with jaundice associated with pruritus, weight loss, fever, and occasionally, with abdominal pain. Cholangitis may occur, but is rare as a presenting symptom in the absence of prior interventions directed toward the biliary tract such as cannulation or stent placement. Bile is sterile, but can serve as a medium for bacterial growth and can become contaminated with instrumentation. Patients with cholangitis may present with fever, abdominal pain, nausea, vomiting, and rigors. Bacteremia with biliary tract flora such as Escherichia coli, Klebsiella, Proteus, Pseudomonas aeruginosa, Serratia, Streptococcus, and Enterobacter may be present.
The presence of obstructive jaundice is an indication for further diagnostic testing to evaluate for malignant obstruction resulting from tumors of the bile ducts.
Laboratory tests suggest extrahepatic biliary obstruction with elevations in serum bilirubin, alkaline phosphatase, and γ-glutamyltransferase levels. Transaminase levels may be elevated, but typically to a lesser level.
Tumor markers may not be very helpful. CA19-9 has low accuracy for diagnosis because the levels may be increased in the presence of pancreatic cancer, or in the presence of cholangitis or biliary obstruction from other causes. In patients with PSC, a cutoff of 100 IU has a sensitivity of 89% and specificity of 86% for CCA in PSC.163 The King’s College group index that incorporates both CEA and CA19-9 values has attained similar sensitivities for cancer arising in patients with PSC.164 Bile CEA levels are reportedly elevated in CCA, but not in benign diseases, other than intrahepatic stones.138
Evaluation involves abdominal US, and body imaging with CT scans or MRI, as well as biliary tract imaging with ERCP. US is cheap, noninvasive, and is the best initial test for the detection of biliary stones or for ductal dilation that can occur with long-standing obstruction. Abdominal imaging with either a CT scan or MRI should be obtained for the patient with painless jaundice in whom malignancy is suspected. The advantage of MRI is that an MRCP could also be performed at the same time. Although a CT scan can identify mass lesions, an ERCP or MRCP may be needed to evaluate for the site and nature of biliary obstruction if no mass lesions are noted.
In patients without PSC and with no visible mass lesions, the presence of a single stricture by ERCP indicates a malignancy. In patients with PSC, a malignancy may be associated with the deterioration of clinical status and liver function tests. However, dCCAs may also present in these patients without any change in liver biochemistries. There are no data to determine the efficacy, timing, or effectiveness of screening and surveillance for a malignancy in patients with PSC, although this is often done in clinical practice using CT scans or an MRI/MRCP, with CA19-9.164 The diagnosis of malignancy in patients who have a biliary tract stricture can be very difficult. Because of the dense associated stroma, well-differentiated cancers with little invasion are difficult to differentiate from the bile duct that has a fibrotic scar or stricture from PSC or other prior biliary injury. The presence of malignant-appearing cells within nerve sheaths (perineural invasion) is an important diagnostic criterion of malignancy that is not present in a benign stricturing disease such as PSC.
The differential diagnosis includes any cause of painless obstructive jaundice such as choledocholithiasis, pCCA, or pancreatic cancer. As with pCCA, dCCAs must be differentiated from benign fibroinflammatory strictures such as from immunoglobulin (Ig)G4 cholangiopathy or sclerosing cholangitis.165 The former can occur in the extrahepatic and intrahepatic ducts and is diagnosed by an elevated IgG4 in the serum, or by an increased number of IgG4-positive cells in tissue samples. The failure to consider these diagnoses may lead to inappropriate therapies, such as long-term stenting or hepatic resection, and these strictures may respond to corticosteroids.
Cancers of the lower bile ducts may not be readily distinguished from ampullary, duodenal, or pancreatic cancers. Although all of these cancers present in a similar manner to dCCA, establishing a diagnosis is helpful because dCCAs are less likely to metastasize widely and may have a more favorable outcome with aggressive treatment.
Staging
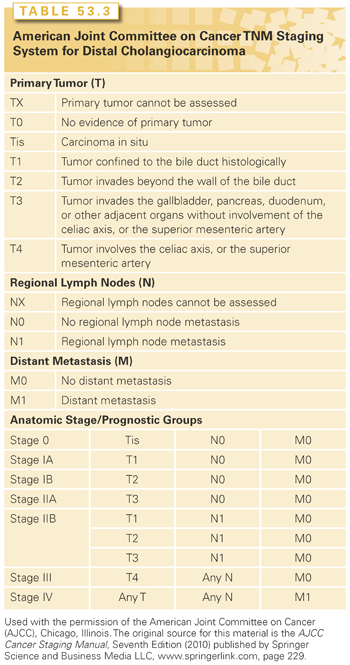
The AJCC’s TNM system may be used for staging dCCAs (Table 53.3).6 Prior TNM staging systems did not consider separate staging systems for extrahepatic bile duct tumors. However, the seventh edition (2010) separated the staging of dCCAs from that of pCCAs. In order to determine resectability of the tumor, staging is necessary to identify the extent of tumor spread and the relationship to portal vein and superior mesenteric artery. EUS with FNA may be useful in determining the extent of tumor spread and involvement of local lymph nodes.166 Although PET scanning for staging has been proposed, the benefit has not yet been shown.167 A staging laparoscopy with or without US may enable the direct visualization of the peritoneal surfaces for metastatic implants, as well as detect vascular or nodal invasion, any of which would preclude resection for cure.127,168,169
Management
An approach to the evaluation and management of the patient with suspected dCCA is presented in Figure 53.3.
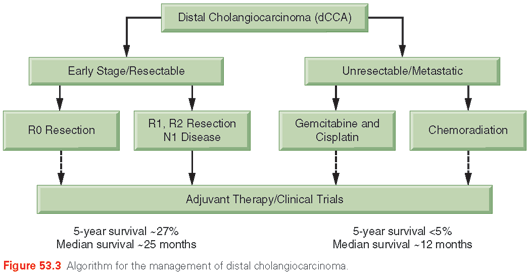
Biliary Decompression. If the distal ducts are dilated and extending into the lower common bile duct, therapeutic decompression by ERCP or percutaneous stenting could be performed at the time of the initial evaluation. We recommend obtaining brushings at ERCP even if no masses have been seen on abdominal imaging studies.
Surgery. Surgical resection can be considered for locally confined dCCA without major vascular involvement or distant metastases. An intraoperative assessment with the help of the pathologist must dictate the extent of resection. For localized dCCAs, a pancreaticoduodenectomy with resection of the extrahepatic bile duct to the level of the confluence may be required. Although dCCAs often involve the intrapancreatic portion of the common hepatic duct, on rare occasions the tumor may be confined to a small region of the duct and removed by an extrahepatic bile duct resection without a pancreaticoduodenectomy. The peripancreatic and periportal lymph nodes should be removed and examined, along with the interaortocaval lymph nodes, if necessary.
Although the incidence of dCCAs is lower than that of pCCAs, the resectability rate of dCCAs is much higher than that of pCCAs, which may contribute to improved outcomes. A pancreaticoduodenectomy with distal bile duct resection has a reasonable chance of providing a margin-negative resection for dCCAs. There is considerable morbidity and a mortality rate from 2% to 10%. Morbidity can arise from biliary fistulas in about 2% of patients or a fistula from the pancreatic–jejunal anastomosis in 5% to 10% of patients. Although many patients require pancreatic enzyme replacement after this procedure, few develop diabetes. Short-term outcomes and/or quality of life are similar between the pylorus-preserving and standard types of pancreaticoduodenectomy.170,171 Extensive en bloc–combined hepatic and pancreatic resections could be considered in the rare circumstance that there is extensive involvement of the entire bile duct without any evidence of distant spread. The morbidity of such extensive surgery is very high, and the overall prognosis is poor.126 The 5-year survival rates after an R0 resection is 27%, with a median survival time of 25 months. The expected 5-year survival is between 23% and 50%. Prognostic factors for poor survival include high p53 expression, nodal metastases, positive margins, pancreatic invasion, and perineural invasion.172,173
There are no established guidelines for surveillance and follow-up after surgical resection. Laboratory and radiologic surveillance modalities and intervals will be determined on perceived risk on an individual basis. Tumor recurrence may occur locally within the peritoneum or local nodes or with distant metastases.
Adjuvant Therapy. Postoperative adjuvant radiotherapy can be administered by intraoperative radiotherapy (IORT), EBRT, intrabiliary brachytherapy, or a combination of modalities. EBRT is widely available, noninvasive, and can deliver a homogeneous dose to a large volume. In most series, EBRT has been used to deliver a dose of 40 to 50 Gy (at 1.80 Gy per day) to the tumor bed and draining lymph node basin. In some series, a smaller volume (i.e., a boost) was treated with additional EBRT, intraluminal brachytherapy, or IORT to a total dose of 60 Gy or more. Most commonly, radiotherapy is administered in a continuous course during 5 to 6 weeks. However, the role of radiotherapy from an efficacy standpoint remains to be definitively ascertained. Similarly, as described earlier, the role of chemotherapy remains an area of active investigation in patients with biliary cancers.
Palliative Care. For unresectable dCCA, palliation by stenting for biliary decompression by itself, or in combination with chemotherapy may be considered. Plastic or metal stent placement can be performed at the time of ERCP or PTC, and an internal or external drainage. In general, replaceable plastic stents are used for those with a life expectancy of less than 6 months, and metal stents are used for those with a longer life expectancy, based on results of a randomized controlled trial.174 Plastic stents need to be replaced every 3 months for best results and to minimize cholangitis. This requires repeated endoscopy procedures. Metal expandable stents remain patent for a longer time and are associated with less cholangitis, but they cannot be readily removed. Tumor advancement may lead to a complete stent occlusion.
A randomized trial of surgical bypass versus endoscopic intubation favored the latter.175
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








