The most important procoagulant expressed by tumor cells is tissue factor (TF), a transmembrane glycoprotein and the physiologic initiator of coagulation. TF is present on neoplastic cells as well as tumor-associated endothelial cells in a variety of cancers. TF expression occurs early in neoplastic transformation, driven by oncogenic mutations in KRAS and TP53 genes.9 TF may contribute to tumor growth, metastasis, and angiogenesis through a variety of mechanisms including the formation of the TF/VIIa complex and activation of protease-activated receptor 2.10 TF expression is associated with increased angiogenesis, tumor invasiveness, and worsened prognosis in various malignancies.11 Secretion of TF-containing microvesicles into the circulation may also account for the systemic coagulopathy of cancer. Emerging reports suggest that the degree of TF expression by tumor cells or elevated levels of circulating systemic TF may be predictive of VTE and mortality in some cancers, particularly pancreatic.12–14
Tumor cells also express plasminogen activator inhibitor-1, a potent inhibitor of the fibrinolytic system that has prothrombotic properties and also promotes tumor growth and angiogenesis. Proinflammatory cytokines such as tumor necrosis factor, interleukins-1 and -6, and interferons are elevated in malignancy. Their enhanced expression results from activation of monocytes or direct release from tumor cells, and can in turn activate coagulation. Finally, interactions mediated by platelet P-selectin between circulating carcinoma mucins and platelets lead to platelet aggregation and platelet-rich thrombus formation without accompanying thrombin generation, and this may also contribute to the prothrombotic state in cancer.15
Antineoplastic therapy further exacerbates the prothrombotic state in cancer. Several studies have documented changes in the markers of thrombin generation within hours of chemotherapy administration.16 Chemotherapy can induce endothelial cell activation leading to increased TF expression, elevated levels of plasma von Willebrand factor and factor VIII coagulant protein, and decreased levels of natural anticoagulants antithrombin and proteins C and S.17 Chemotherapy can induce release of cell-free DNA, with neutrophils as a predominant source and this in turn acts as a trigger for the intrinsic pathway.18 DNA and RNA from platelets and neutrophils generate neutrophil extracellular traps (NET). These form a specialized extracellular matrix that can activate coagulation. In animal models, cancers can induce an increase in peripheral blood neutrophils that are sensitized toward NET formation and spontaneous thrombosis is associated with NET generation in vivo.19 Thus, extracellular chromatin released through NET formation is a potential novel cause for cancer-associated thrombosis.
Most recently, scientists are exploring the complex crosstalk between the coagulation cascade, complement pathway, and the immune system in their synergistic roles in tumor growth.20–22 It is possible that cooperation among these pathways also contributes to the hypercoagulability in malignancy and may explain the high frequency of anticoagulant failure in patients with cancer-associated thrombosis.
EPIDEMIOLOGY OF CANCER-ASSOCIATED THROMBOSIS
Incidence and Prevalence
The incidence of VTE is increased several-fold in patients with cancer when compared with patients without cancer.1,23–25 In general, the risk among patients with cancer is estimated to be 13 per 1,000 person-years (95% confidence interval [CI] = 7 to 23) in a recent systematic review.26 However, rates vary significantly depending on specific cancer subgroups, settings, and over time, with more contemporary studies demonstrating higher incidence. Among ambulatory patients, the reported incidence of VTE varies from 7.8% during 26 months (0.3% per month) to 1.93% during a median follow-up of 2.4 months (0.8% per month) to 12% during 8 months (1.5% per month).27,28 The cause of this increase in the rate of VTE is likely related to improved diagnostic technologies as well as a true increased incidence related to newer antineoplastic drugs and regimens. The advent of highly sensitive multidetector-row computed tomography scans for routine staging has also led to an increased diagnosis of “incidental” PE.29 The term incidental does not, however, indicate a more benign natural history. Retrospective studies show that the consequences of incidentally discovered PE are no different than those following symptomatic VTE including risk of recurrent VTE and mortality.30,31
Rates of arterial thromboembolism (ATE) are less well studied. The use of bevacizumab-containing regimens is clearly associated with a high risk of arterial events.32 In a pooled analysis of five randomized studies, 4.4% of patients receiving bevacizumab and chemotherapy developed arterial events, compared with 1.9% of patients receiving chemotherapy alone. Rates were particularly high in older patients. In a large meta-analysis of clinical trials with sunitinib and sorafenib, both oral vascular endothelial growth factor receptor inhibitors, the incidence of ATE was 1.4% (95% CI = 1.2% to 1.6%).33
Risk Factors
Patients with cancer comprise a heterogeneous group and include patients undergoing surgery, hospitalization, receiving antineoplastic therapy, or end-of-life care. The risk of VTE differs across these various cancer subpopulations as well as over the natural history of the disease. A recent update by the American Society of Clinical Oncology VTE Guidelines Panel recommends that patients with cancer be assessed for VTE risk at the time of chemotherapy initiation and periodically thereafter (recommendation 6.1).34 Table 134.1 provides a comprehensive list of cancer-related, treatment-related, and patient-related risk factors and biomarkers for cancer-associated VTE.
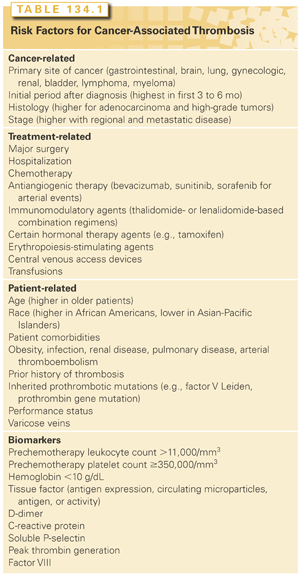
Cancer-related risk factors include the primary site, stage, and time after initial diagnosis. The primary site of cancer is historically the best-known and commonly used risk factor. Cancers of the pancreas, stomach, brain, ovary, kidney, and lung have long been associated with VTE; hematologic malignancies, particularly lymphomas, are also strongly associated with VTE.23,27,28,35 Patients with metastatic disease have a 2- to 20-fold increased risk of VTE.5,23 VTE is more likely to occur in the initial period after diagnosis. In a population-based study, the adjusted odds ratio for developing VTE in the first 3 months was 53.5 (95% CI = 8.6 to 334.3), declining to 14.3 (95% CI = 5.8 to 35.2) and 3.6 (95% CI = 2.0 to 6.5) in the 3-month to 1-year and 1- to 3-year intervals, respectively.23
Treatment-related risk factors include surgical interventions, central venous access devices, hospitalization, and systemic therapy. Patients with cancer undergoing surgery have a two-fold increased risk of postoperative DVT and a three-fold increased risk of fatal PE compared with patients without cancer. The risk of VTE increases significantly when patients with cancer are hospitalized. Patients with cancer on active therapy are at greater risk for VTE. Specific chemotherapeutic agents are associated with higher rates of VTE. In a prospective study, platinum-based regimens were significantly associated with VTE.36 Even within this class of agents, rates were higher in patients receiving cisplatin as compared with oxaliplatin.37 Studies of newer cancer regimens that include drugs such as thalidomide, lenalidomide, and bevacizumab have reported very high rates of VTE and ATE events. For thalidomide-containing regimens, rates of VTE up to 34% in patients with myeloma have been reported.38,39 Bevacizumab, an antivascular endothelial growth factor antibody, has been clearly associated with an increased risk of ATE; data on its association with VTE are conflicting.1,32,40,41 Even supportive therapy drugs have been associated with increased VTE. In a large meta-analysis, erythropoiesis-stimulating agents were associated with a 1.6-fold increased risk of thromboembolism.42
Finally, patient-related risk factors further add to risk. In particular, comorbid conditions including infection, ATE, renal disease, pulmonary disease, and anemia can strongly affect risk.
Biomarkers range from tests as simple as the complete blood count to novel assays that may be predictive of VTE in cancer.43 Prechemotherapy platelet counts of ≥350,000/mm3 (odds ratio = 1.8; 95% CI = 1.1 to 3.2) and leukocyte counts >11,000/mm3 (odds ratio = 2.2; 95% CI = 1.2 to 4.0) have been independently associated with VTE.44 Other candidate biomarkers predictive of VTE include D-dimer, tissue factor, thrombin generation assays, soluble P-selectin, factor VIII, and C-reactive protein.43 These are not recommended for clinical use until validation studies have been completed.
Risk factors for arterial events are less well investigated. In hospitalized patients receiving chemotherapy, variables associated with ATE included age 65 years or older; male gender; African American ethnicity; sites of cancer including leukemia, colon, prostate, and lung; and presence of comorbid illnesses.35 For patients receiving bevacizumab-containing regimens, age over 65 years and prior history of ATE were significant risk factors in a pooled analysis of clinical trials.40 The relative risk of arterial events associated with sorafenib and sunitinib is 3.03 (95% CI = 1.25 to 7.37; p = 0.015) compared with control patients.33
Risk Assessment Score
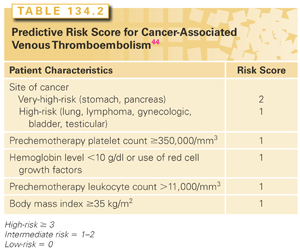
Multiple randomized studies have attempted to evaluate the benefit of thromboprophylaxis in patients with cancer, utilizing one or two risk factors to select a high-risk group.45,46 Low event rates in the control arms of these studies indicate that a high-risk population was not selected. Hence the updated American Society of Clinical Oncology VTE Guidelines Panel recommends against the use of single risk factors or biomarkers to identify high-risk patients.34 Instead, the panel recommends the use of a validated risk score that incorporates five variables to identify high-risk patients (Table 134.2). This risk score was originally developed and validated in a US national cohort study.34,44 Observed rates of VTE over median follow-up of 2.5 months in the development and validation cohorts were 0.8% and 0.3% for low-risk, 1.8% and 2% for intermediate-risk, and 7.1% and 6.7% for high-risk patients, respectively. This model was subsequently externally validated in a prospective European cohort study, with rates of 17.7% in the high-risk cohort as compared to 1.5% in low-risk patients, over a 6-month follow-up period.47 Multiple other cohort studies have further validated this score, although absolute rates vary between studies based on heterogeneous populations and follow-up periods.48
PREVENTION OF CANCER-ASSOCIATED THROMBOSIS
Prophylaxis in Major Surgery
Few clinical trials have been conducted to study the efficacy and safety of thromboprophylaxis specifically in patients with cancer having major surgery. Randomized controlled trials of in-hospital prophylaxis in patients having major abdominal surgery for cancer have shown no difference in the incidence of DVT or major bleeding between unfractionated heparin (UFH) administered three times daily and a low-molecular-weight heparin (LMWH) enoxaparin given once daily.49,50 Overall, up to 15% of patients with cancer will develop DVT following surgery, despite standard prophylaxis with UFH or LMWH. In addition to LMWH and UFH, fondaparinux is approved for prophylaxis following major abdominal surgery. This selective inhibitor of activated factor X is given once daily subcutaneously and is associated with a lower risk of heparin-induced thrombocytopenia compared with UFH and LMWH. In a phase 3 randomized trial, fondaparinux was found to be comparable to LMWH dalteparin in patients undergoing high-risk abdominal surgery.51 A post hoc analysis of patients with cancer suggested that fondaparinux was associated with a statistically significant reduction in VTE (4.7% versus 7.7%; p = 0.02).
Mechanical methods of prophylaxis, such as graduated compression stockings or intermittent pneumatic calf compression devices, can lower the risk of VTE but are less effective than anticoagulants.52 Their use should be limited to patients in whom anticoagulation is contraindicated. There is weak evidence that combined mechanical and pharmacologic prophylaxis may further reduce VTE, especially in the patients who are at highest risk.
Extending prophylaxis beyond hospitalization can further reduce the risk of VTE in patients with cancer. In a multicenter, placebo-controlled trial, patients undergoing elective, curative abdominal surgery for cancer received LMWH enoxaparin for the first 6 to 10 days after surgery and then were randomized to continue with enoxaparin or placebo injections for 4 weeks.53 Extended prophylaxis with enoxaparin significantly reduced the rate of VTE by 60% (12.0% versus 4.8%; p = 0.02) at 1 month, and this benefit was maintained at 3 months. The absolute risk reduction of 7% means that 14 patients must be treated to avoid one case of DVT. Overall, there was no difference in bleeding during the treatment period and no difference in mortality up to 1 year of follow-up. Similar results were also reported in a subgroup analysis of an open-label randomized trial in which patients having abdominal surgery were randomized to receive LMWH dalteparin once daily and compression stockings, or to stockings alone for 21 days after hospital discharge.54 Dalteparin significantly reduced the incidence of DVT from 19.6% to 8.8% (p = 0.03) as well as that of proximal DVT from 10.4% to 2.2% (p = 0.02). Accordingly, 9 patients must be treated to avoid one episode of DVT while 12 must be treated to avoid one episode of proximal DVT.
The increased risk of postdischarge symptomatic VTE has been shown to peak at 3 weeks after cancer surgery in two large prospective studies.55,56 One study found that 46% of the deaths occurring within the first month after cancer surgery were due to fatal PE, and the other study reported that 1 in 85 women will develop VTE within the first 12 weeks after cancer surgery.55 The American Society of Clinical Oncology and the American College of Chest Physicians (ACCP) recommend that extended prophylaxis in patients undergoing cancer surgery should be considered, especially in patients with high-risk features.34,57 These include previous history of VTE, anesthesia lasting ≥2 hours, bed rest for ≥4 days, advanced malignancy, and older age.55
Prophylaxis for Central Venous Catheters
Contemporary trials have provided evidence that neither warfarin (dose fixed at 1 mg daily or adjusted to an international normalized ratio [INR] between 1.5 and 1.9) nor prophylactic doses of LMWH is effective in reducing symptomatic catheter-related thrombosis.58–60 With or without prophylaxis, symptomatic catheter-related thrombosis rates of approximately 4% were reported in these studies, and bleeding may be increased in those who received an anticoagulant. Based on these data, the ACCP guidelines have recommended against routine prophylaxis using LMWH and fixed-dose warfarin.52 Although high-risk patients may still benefit from prophylaxis, the selection criteria of such patients and the optimal anticoagulant regimen are not defined.
Prophylaxis in Medical Patients
Randomized trials have studied the efficacy and safety of anticoagulant prophylaxis in outpatients receiving chemotherapy (Table 134.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








