Cancer and Coagulopathy
INTRODUCTION
The association between cancer and thrombosis was first proposed by Armand Trousseau (Figure 19-1) when he recognized the condition of thrombophlebitis migrans, as a forewarning of occult malignancy (1). In 1865, he remarked, “Should you, when in doubt as to the nature of an affection of the stomach, should you when hesitating between chronic gastritis, simple ulcer, and cancer, observe a vein become infected in the arm or leg, you may dispel your doubt, and pronounce in a positive manner that there is a cancer …” (1). Although the association of hemostatic disorders and cancer has been studied extensively over the past 100 years, venous thromboembolism (VTE), defined herein as pulmonary embolus (PE) or deep vein thrombosis (DVT), remains a major cause of morbidity and mortality in cancer patients.

FIGURE 19-1 Armand Trousseau.
This chapter will explore the pathogenesis of thrombosis in cancer as well as the epidemiology and risk factors. The chapter will also focus on novel risk assessment models and the emergence of new biomarkers to classify patients at high risk of developing VTE. Current diagnostic and management strategies for VTE in cancer patients and the challenges of antithrombotic therapy in this population will be examined. This update will evaluate the results of several randomized controlled trials aimed at assessing the clinical benefit of antithrombotic prophylaxis in cancer outpatients. Finally, new therapeutic developments in this area will be addressed.
PATHOGENESIS
The pathophysiological mechanisms of thrombosis in cancer patients are complex and involve multiple clinical and biological factors including tumor cells, the hemostatic system, inherited and acquired thrombophilia, and exogenous contributors such as chemotherapy and radiotherapy (2). Tumors contribute to thrombosis through the expression of procoagulant factors including tissue factor, cancer procoagulant, and adhesion molecules. Recent experimental models of human cancers have shown that an integral feature of neoplastic transformation from cancer cells is through activation of clotting proteins (3–6). The role of tissue factor-bearing microparticles (MP) contributing to thrombin generation has also been explored in vitro and in vivo studies. Zwicker et al. found that VTE developed in 34.8% of cancer patients with elevated levels of MP compared to 0% in those without detectable levels (7). Tumor cells can also induce platelet activation and aggregation through secretion of proteases. Tumor-related release of various cytokines, growth factors, and proteases including tumor necrosis factor α (TNFα), interleukin 1β, and vascular endothelial growth factor (VEGF) contribute not only to angiogenesis and inflammation but also to the activation of the hemostatic system. Furthermore, tumor cells interact directly with the host blood vessels, endothelial cells, leukocytes, and monocytes leading to host cell inflammatory responses (2). These many and varied interactions lead to both a direct and an indirect activation of the clotting system, an increase in thrombin generation, and ultimately a hypercoagulable state.
EPIDEMIOLOGY
Venous thrombosis is a common complication in patients with cancer. Although the exact incidence of VTE in cancer patients is unknown, it occurs in approximately 15%, with reports ranging from 4% to 30% (8, 9). These numbers likely underestimate the problem as VTE often causes no symptoms. In a recent study, clinically unsuspected PE was present in up to 4.4% of oncology patients undergoing CT scans for other indications (10). If symptoms are present, they are often nonspecific or attributed to a patient’s underlying malignancy.
Certain malignancies exhibit high rates of VTE, such as hematological malignancies and neoplasms, especially if high grade, of the pancreas, gastrointestinal tract, ovary, brain, colon, kidney, lung, and prostate (11–15). However, it is unclear if the high rates are due to the underlying properties of particular cancers or merely reflect the high prevalence of certain cancers. Nevertheless, it is well documented that cancers diagnosed at the same time as an episode of VTE are more likely to have distant metastases and lower survival rates (16, 17). One study showed that cancer patients with VTE had a 1-year survival of 12% as compared to 36% in cancer patients without VTE (17). Similarly, patients who develop VTE within a year after a cancer diagnosis are more likely to have advanced stage and poorer prognosis when compared to analogous cancer patients without VTE (17). A study of over 235,000 cancer patients showed that after adjusting for age, race, and stage of disease, VTE at the time of or within 1 year of cancer diagnosis was a significant predictor of death within that year (16). VTE is the second leading cause of death in cancer patients, with cancer progression being number one (18). It also appears that cancer patients with VTE are two to three times more likely to have recurrent VTE and two to six times more likely to experience hemorrhagic complications from anticoagulant therapy than noncancer patients with VTE (19, 20). These findings clearly indicate that VTE may be more aggressive and difficult to treat in cancer patients than in noncancer patients.
The association between cancer and thrombosis is further supported by many studies, suggesting that an idiopathic VTE is often associated with occult cancer. Approximately 10% of patients who present with an idiopathic or unprovoked VTE are diagnosed with cancer within the next 1–2 years (21). These provocative findings raise the unanswered question as to whether all patients with idiopathic VTE should undergo extensive cancer screening. The SOMIT study attempted to address this matter (22). Patients with an idiopathic VTE were randomized to either extensive or nonextensive cancer screening and followed for 24 months. Subjects in the extensive screening arm seemed to have a shorter delay in the diagnosis of cancer, their cancers were detected at earlier stages, and they had a lower cancer-related mortality (22). Unfortunately, this trial was stopped prematurely due to recruitment issues leaving these conclusions unsubstantiated. In a more recent prospective cohort study of 630 patients with a first episode of idiopathic VTE, extensive screening, which included abdominal and chest CT and mammogram, detected six additional cancers (2.0%; 95% CI, 0.74–4.3), compared to limited screening (23). At the 2.5 years of follow-up, cancer was diagnosed in 3.7% in extensive screening group and 5.0% in limited and there was no significant difference in death rates. Thus, this study concluded that the low yield of extensive screening and lack of survival benefit did not support routine screening for cancer in patients with an idiopathic VTE.
A recent systematic review of this question found that an extensive screening strategy employing an abdominal and pelvic CT statistically significantly increased the number of undiagnosed cancer from 49.4% to 67% in patients with an unprovoked VTE (24). However, this review could not address the complication rates, cost-effectiveness, or morbidity and mortality difference associated with an extensive screening approach. The use of PET-CT was recently investigated to screen for occult malignancy in 40 patients who presented with an unprovoked VTE (25). Twenty-five patients (62.5%) had abnormal findings requiring additional evaluations and of these, only one occult malignancy was discovered. This malignancy, however, was detected in a patient with unexplained abdominal pain and unintentional weight loss of 40 pounds, which are symptoms concerning for a malignancy. Hence, larger studies are needed to evaluate the cost-effectiveness of PET-CT in this population.
Current recommendations are to provide age appropriate cancer screening for patients who present with idiopathic VTE, and any additional testing should be driven by what is discovered in a thorough history and physical examination. Given the SOMIT observations, albeit underpowered, future studies evaluating extensive cancer screening for patients with idiopathic VTE are warranted.
RISK FACTORS
Many inherited and acquired risk factors are associated with the development of VTE and are listed in Table 19-1. Cancer patients may have additional risk factors related to their malignancy, including surgery, immobilization, chemotherapy, some forms of hormone therapy, and the presence of indwelling central venous catheters (CVCs). Without appropriate prophylaxis, cancer patients have twice the risk of developing postoperative DVT and three times the risk of developing a fatal PE than patients without cancer (26). Long-term immobilization, often due to lengthy hospital stays, also increases the risk of developing VTE. Furthermore, comorbid conditions, distant metastases, advanced age, obesity, prior history of VTE and elevated platelet count are associated with increased VTE risk (12, 13, 27, 28).
In addition to patient-related risks, there are treatment-related risks. Tamoxifen, estrogen, thalidomide, L-asparaginase, cisplatin, and VEGF inhibitors are a few of the cancer therapies associated with high rates of thromboembolic complications, especially when used in combination with other chemotherapeutic agents. In a trial involving over 2600 women with early stage breast cancer, the incidence of developing VTE was 0.2% with placebo and 0.9% with tamoxifen (29). Another trial involving women with advanced stage breast cancer showed that the incidence of VTE was 2.6% with tamoxifen alone versus 13.6% with tamoxifen plus chemotherapy (30). Similarly, in studies involving multiple myeloma, treatment with thalidomide alone had a risk of 2%. The risk increased to 33% with the addition of chemotherapy (31). Cancer patients who receive either cytotoxic or immunosuppressive therapy have a 6.5-fold increased risk of developing a VTE when compared to noncancer patients, and a twofold increased risk compared to cancer patients not receiving chemotherapy (32). In a recent systematic review of 8216 cancer patients, those receiving cisplatin-based chemotherapy had a significantly increased rate of VTE compared to patients who did not (RR, 1.67l 95% CI, 1.25 – 2.23; p = 0.01) (33). Furthermore, venous thrombosis, and in particular, cortical sinus thrombosis, is a frequent complication of L-asparaginase treatment, and it is related to inhibition of the synthesis of anticoagulant factors, protein C and protein S.
Erythropoiesis-stimulating agents (ESAs) are often given to patients with chemotherapy induced anemia. However, recent studies show that ESAs administered to patients with cancer increase not only risk of VTE but also risk of mortality (13, 34, 35). As such, the FDA label now limits the use of ESA to patients receiving chemotherapy for palliative intent. ESAs are no longer indicated for patients receiving chemotherapy for curative intent.
Many new antiangiogenic agents are under investigation and used in practice to treat a variety of cancers. In a systematic review of 15 RCT, patients receiving bevacizumab, the recombinant humanized monoclonal antibody to VEGF, had an increased risk of VTE compared to controls (RR 1.3; 95% CI, 1.13–1.56; p<0.001) (36). Similarly in a meta-analysis of anti-epidermal growth factor receptor (EGFR) agents, the associated RR of VTEs was 1.32 (95% CI 1.07–1.63; p = 0.01) in patients who received anti-EGFRs versus controls (37). The risk was highest with the use of cetuximab and panitumumab (13).
CVCs are another common risk factor for VTE. These devices are commonplace among cancer patients who require long-term chemotherapy. The reported incidence of catheter-related thrombosis ranges from 5% to 75%, and this wide range likely reflects the distinct types of malignancy, the kind of catheter used, and the duration of its implantation (38). In addition, the complications associated with CVC-related thrombosis can result in loss of catheter function, postphlebitic syndrome of the upper extremity, PE, and even mortality. There have been major efforts to identify disease management approaches to decrease the risk of VTE with CVC, and these mechanisms are discussed in the Prevention section of this chapter.
RISK PREDICTIVE MODELS
Trying to predict the risk of VTE in cancer patients is a major clinical challenge. Patients at high risk of developing VTE may benefit from prophylactic anticoagulation, whereas patients at low risk may have unnecessary and unfavorable consequences from this practice, such as bleeding. Therefore, the development of risk assessment tools and predictive biomarkers to identify high-risk patients is clinically relevant and important.
One novel and promising tool is the Khorana score, which uses baseline clinical and laboratory variables to predict the risk of chemotherapy-associated VTE (39). The score assigns points to cancer site (2 points for very high-risk sites such as pancreatic or gastric and 1 point for high-risk sites such as lung, ovarian, or bladder), platelet count ≥350 × 109/l (1 point), hemoglobin ≤ 10 g/dl or the use of erythropoietin-stimulating agents (1 point), leukocyte count ≥11 × 109/l (1 point), and body mass index ≥35 kg/m2 (1 point). A score of ≥3 is considered high risk and correlates with a rate of symptomatic VTE in 6.7% of patients undergoing chemotherapy. This model was recently modified in another study to include platinum or gemcitabine-based chemotherapies (40).
The Ottawa score is another clinical prediction rule aimed at identifying recurrent VTE risk in patients with cancer-associated VTE (41). The independent variables include sex, primary tumor site, tumor-node-metastasis (TNM) stage, and prior VTE. High-risk predictors include female, lung cancer, and history of VTE and are given 1 point each. Low-risk predictors include breast cancer and stage 1 cancer of any origins and are given –1 and –2 points, respectively. A score of ≤0 correlates with a low clinical probability of recurrent VTE (<4.5%), whereas a score of ≥1 correlates with a high clinical probability of recurrent VTE (≥19%). Future prospective trials are warranted to demonstrate the reproducibility, generalizability, and safety of the Khorana and Ottawa scores as well as to determine their effectiveness as a tool for treating cancer patients at risk of VTE.
BIOMARKERS
Identifying biomarkers to help predict the risk of VTE in cancer patients is one of the largest growing areas of research. Data from the prospective Vienna Cancer and Thrombosis Study (CATS) demonstrated that elevated levels of P-selectin were predictive of VTE in cancer patients (42). The probability of developing VTE at 6 months was 11.9% in patients with high levels of P-selectin compared to 3.7% in patients with low levels. This group also found that patients with elevated D-dimer and high prothrombin fragment 1+2 (F 1+2) compared to patients with nonelevated levels were associated with an increased risk of VTE (15.5% vs 5%) (43). Thrombin generation is another potential biomarker studied by the Vienna CATS group (44). Elevated peak thrombin levels conferred an 11% risk of developing VTE compared to 4% in patients with lower levels. An expansion of the original Khorana score to include D-dimer and P-selectin appears to improve the VTE risk prediction tool (45). There was a 26-fold higher probability of developing VTE in patients with a high score compared to patients with a low score. However, this expanded risk score requires further validation studies but may be limited by the lack of widely available P-selectin assays.
In the last few years, the role of tissue factor-bearing MP in connection with cancer progression and thrombosis has been investigated. In an immunohistochemical study, high levels versus low levels of MP in pancreatic cancer patients correlated with the development of VTE (26.3% vs 4.5%) (46). Similarly, Zwicker et al. found that VTE developed in 34.8% cancer patients with detectable levels of MP compared to 0% in patients with undetectable levels (7).
Biomarkers may improve the stratification of cancer patients with regard to their risk of VTE. The efficacy and safety of prophylactic anticoagulation in patients with these elevated biomarkers needs to be addressed in well-designed RCTs.
CLINICAL MANIFESTATIONS
Cancer patients can present with a wide range of thromboembolic events. The two most commonly recognized are DVT and pulmonary embolism (PE). However, symptoms and signs may result from migratory thrombophlebitis, nonbacterial thrombotic endocarditis, disseminated intravascular coagulopathy (DIC), thrombotic microangiography, and arterial thrombosis. Cancer patients may also present with multiple clinical sequelae as was originally reported in 1977 by Sack et al. in a review of 182 cases of neoplasia associated with alterations in blood coagulation (47). Figure 19-2 is an expansion of the original Venn diagram created by Sack et al. which represents the interrelations between the various clinical phenomena. Discussion of all these clinical presentations is beyond the scope of this chapter and, therefore, only DVT and PE will be presented in detail.
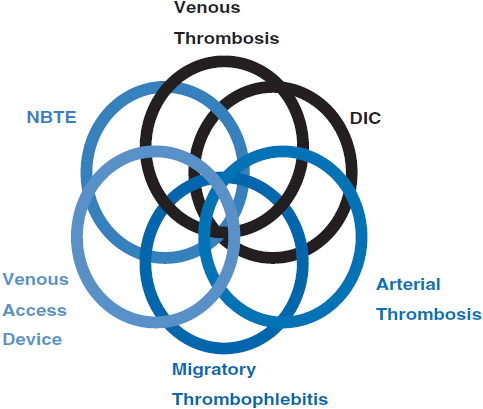
FIGURE 19-2 Venn diagram of relationships between clinical signs.
Patients with DVT may experience complaints of leg pain, swelling, tenderness, discoloration, venous distension, or a palpable cord. Nonspecific symptoms of PE include dyspnea, tachypnea, tachycardia, pleuritic chest pain, cough, and wheeze. Signs may include hemoptysis, hypotension, syncope, coma, pleural effusion, or pulmonary infiltrates. Each of these clinical features can be a manifestation of other cardiac or pulmonary processes, such as pneumonia or heart failure, making the diagnosis of PE difficult.
Data from the MASTER registry in Italy demonstrated that the clinical presentation of acute VTE in cancer patients is different and more extensive than in patients without cancer (48). The incidence of bilateral DVT and rates of iliocaval thrombosis were higher in the cancer patients. The management of VTE in the cancer patients was also more problematic with a higher incidence of hemorrhage and need for inferior vena cava (IVC) filters.
Other consequences of DVT and PE include acute morbidity or even death. Some of the short- and long-term complications due to thrombotic events consist of extension of the clot, embolization, postthrombotic syndrome, pulmonary hypertension, and recurrent VTE. Furthermore, there may be significant morbidity associated with long-term anticoagulation or with placement of an IVC filter. The psychological stress and fear that patients face when suffering from a thromboembolic event must also be appreciated. Moreover, the presence of VTE or its complications may cause delays in chemotherapy or other treatments, which may have considerable consequences for the patient. In one of the largest outcome studies of DVT in cancer patients, the most common complication was bleeding, which occurred in 13% of patients (49). PE, death from DVT, and death from anticoagulation were also observed. The mean length of stay was 11 days with a mean cost of hospitalization of $20,065. VTE as the cause of death was also shown in an autopsy-based study where one out of every seven cancer deaths in the hospital was due to PE (50). Over 60% of those who died of a PE had either limited metastatic or local disease indicating a reasonable chance of prolonged survival if not for the fatal PE (50).
DIAGNOSIS OF VTE
Diagnosing VTE in cancer patients, as in other patients, may be difficult. The signs and symptoms of VTE are often variable and nonspecific, and available diagnostic tests have varying sensitivities and specificities. Moreover, commonly employed models for predicting the probability of VTE have limited value in cancer patients because of the significant additional risk factors at play. Ultimately, a diagnosis requires a combination of modalities.
If VTE is clinically suspected, a common first test is the D-dimer, which reflects the degradation product of cross-linked fibrin. This test is highly sensitive but not specific (51). Because it is elevated in a variety of situations including malignancy, acute VTE, underlying lung abnormalities, recent surgery, hospitalization, and aging, the primary value of the D-dimer test is a negative result, which constitutes strong evidence against significant thrombosis (51). Two recent studies demonstrated that the combination of a normal D-dimer and a low clinical pretest probability was useful in excluding the diagnosis of DVT in cancer patients (52, 53). If the D-dimer test is positive, however, additional diagnostic tests should be performed.
Duplex venous ultrasound (US) imaging, the most widely available modality for diagnosing DVT, is highly sensitive for detecting proximal vein thrombosis but less so for detecting calf vein clots. Therefore, if a patient presents with symptoms suggestive of a calf vein thrombosis but has a negative US test, a repeat test at 3–5 days may be warranted, especially if symptoms persist and no alternative diagnosis has been established. If the US is inconclusive, magnetic resonance imaging (MRI) of the lower extremities is usually definitive.
If PE is suspected, a variety of imaging tests may aid in making the diagnosis, including lung radionuclide scans (VQ), spiral computed tomography (CT), pulmonary angiography, MRI, and magnetic resonance angiography (MRA). Although pulmonary angiography has been the gold standard, it is invasive and often unavailable or impractical and, therefore, has been replaced by CT angiography.
VQ scans, formerly a frequently used diagnostic tool, are now employed only when there is a contraindication to CT scans with contrast, such as renal failure or iodine allergy. VQ scans are helpful when they are either positive or negative, but their results are often inconclusive or confounded by underlying lung abnormalities.
The spiral CT or computerized tomographic angiography (CTA) is highly sensitive for large emboli. Multidetector scanners are able to visualize the subsegmental arteries effectively. Simultaneous imaging of the lower extremities, which is helpful in identifying an associated DVT, can further increase the diagnostic sensitivity of CTA. Moreover, the spiral CT may help identify alternative etiologies for a patient’s symptoms if a PE is not identified.
One of the newest diagnostic tools to diagnose PE, MRI/MRA of the chest, has been incompletely evaluated and standardized. Currently studies are underway to assess its accuracy and safety.
TREATMENT
The management of VTE in cancer patients is a challenge due to the frequent presence of a hypercoagulable state, physical obstructions to blood flow, patient immobility, and the general impression that these patients are relatively resistant to anticoagulant therapy. The goals of treatment are to prevent fatal PE, recurrent VTE, and long-term VTE complications. This section will outline current treatment recommendations for both the acute and long-term treatment of VTE in cancer patients based on the seventh American College of Chest Physicians (ACCP) Conference guidelines and Journal of National Comprehensive Cancer Network (JNCCN) (54, 55).
 ACUTE TREATMENT
ACUTE TREATMENT
The initial treatment for DVT and PE are similar. There are three options for anticoagulation: unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), and fondaparinux sodium. The use of newer anticoagulants to treat VTE in cancer patients is discussed under the section of New Developments.
LMWH is a fragment of UFH and exerts its anticoagulant effect through antifactor Xa and antithrombin activities. It is cleared from plasma by metabolism in the liver, and a small portion is excreted in the urine. LMWH has largely replaced the use of UFH as the initial treatment for VTE because of its similar efficacy, superior safety profile, and pharmacokinetic advantages that allow for once or twice daily subcutaneous administration without laboratory monitoring, lower risk of complications such as heparin-induced thrombocytopenia (HIT) or heparin-induced osteoporosis, and potential for outpatient treatment. LMWH, a weight-based therapy, may need to be episodically monitored in two particular situations: in patients who are at the extremes of body weight or in those suffering from renal failure, the latter situation because of LMWH clearance by renal excretion. This monitoring involves measuring the antifactor Xa activity 3–4 h after subcutaneous injection with a therapeutic goal of 0.5–1.3 U/ml.
If a patient requires a short-acting initial anticoagulant or one that needs to be carefully monitored, is anticipating an invasive procedure, or has a contraindication to LMWH such as severe renal failure, UFH is the favored treatment. UFH, a glycosaminoglycan, exerts its anticoagulant effect at several steps in the formation of fibrin clots. Specifically, when combined with antithrombin III, it inactivates activated factor X and inhibits the conversion of prothrombin to thrombin. UFH is metabolized in the liver and can be reversed with the antidote, protamine sulfate, if necessary. However, unlike LMWH, UFH is usually given intravenously and needs to be monitored frequently, and the dose must be adjusted with the use of nomograms to maintain an activated partial thromboplastin time (aPPT) of 1.5–2.5 times the normal.
A third anticoagulant option is the synthetic pentasaccharide, fondaparinux sodium, which works by indirectly inhibiting factor Xa. It has similar efficacy and safety for the initial treatment of PE and DVT as UFH or LMWH. It is an attractive medication because of its once daily subcutaneous administration and linear pharmacokinetic profile. In addition, it does not cause a syndrome akin to HIT to date. However, it cannot be reversed and its 17-h half-life makes it an unreasonable option in patients who require a short-acting therapy. In addition, because fondaparinux is excreted unchanged in the urine, monitoring is essential in patients with mild or moderate renal insufficiency, and the medication is contraindicated in patients with severe renal failure. The efficacy of fondaparinux for the initial VTE treatment in cancer patients is limited. Results from a post-hoc analysis of the MATISSE trials suggest that fondaparinux may be more efficacious than UFH but less efficacious than LMWH (56).
Other options for the initial treatment of DVT and PE that are not anticoagulants include thrombolytic therapy, thromboendarterectomy, and IVC filters. The use of thrombolytics is controversial in DVT and currently should be considered only for patients with massive iliofemoral thrombosis and at risk for limb gangrene (54, 57). A meta-analysis has helped clarify the use of thrombolytics for the initial treatment of PE (58). While there was no overall benefit of thrombolytics in terms of recurrent PE or death, patients who were hemodynamically unstable (systolic blood pressure <90–100 mmHg) had a significantly lower rate of recurrent PE and death, but a significantly higher rate of major bleeding (58). The use of thrombolytics has also been considered in patients who are hemodynamically stable but exhibit evidence of severe right ventricular dysfunction. Results thus far are not definitive.
Thrombolytic drugs in current use include tissue-type plasminogen activator (t-PA), urokinase, and streptokinase. T-PA, the most commonly used of the group, is given as a 100-mg infusion over 2 h, followed by heparin. In patients refractory to t-PA, one should consider the presence of a saddle embolus, which might require thromboendarterectomy.
IVC filters are another option for the initial treatment of VTE. They are primarily used in patients with recurrent DVT or PE on anticoagulation, or in patients at high risk of bleeding on anticoagulation. There is little published evidence to document an improvement in outcome after their use. Moreover, if necessary, removable filters are preferred.
 LONG-TERM TREATMENT
LONG-TERM TREATMENT
Similar to the initial treatment for VTE, the long-term treatment for VTE in cancer patients can be complicated by the concomitant need for chemotherapy, hormone therapy, invasive procedures, or CVCs. In addition, cancer patients have higher rates of recurrent VTE and bleeding with traditional anticoagulant therapy than noncancer patients, which adds a further challenge to their management. For many years, the long-term treatment recommendation for VTE in cancer patients was similar to that of the general population, the vitamin K antagonist (VKA), warfarin (Coumadin). After the initiation of UFH, LMWH or fondaparinux, warfarin is started on day 1 and adjusted to maintain an INR of 2–3. Given the slow onset of action, there needs to be a 5- to 7-day overlap between the two medications.
Although warfarin has the advantage of being an oral medication, it has significant disadvantages. It requires regular laboratory monitoring, has significant drug interactions because of its cyp 3A4–dependent metabolism, and is influenced by nutritional status. Fortunately, several trials have shown that LMWH is an attractive alternative.
A randomized controlled trial (RCT) demonstrated a clear benefit to LMWH compared with warfarin in cancer patients for long-term treatment or secondary prophylaxis after VTE (59). After 6 months of therapy, cancer patients who received LMWH had a significantly lower rate of recurrent VTE (9%) than those who received warfarin (17%), with no difference in the rates of bleeding (Figure 19-3). In addition, a post-hoc analysis of the patients with nonmetastatic solid tumors revealed a survival advantage in the LMWH group. Twelve-month cumulative mortality in this population was 20% in the LMWH group versus 36% in the warfarin group (60).
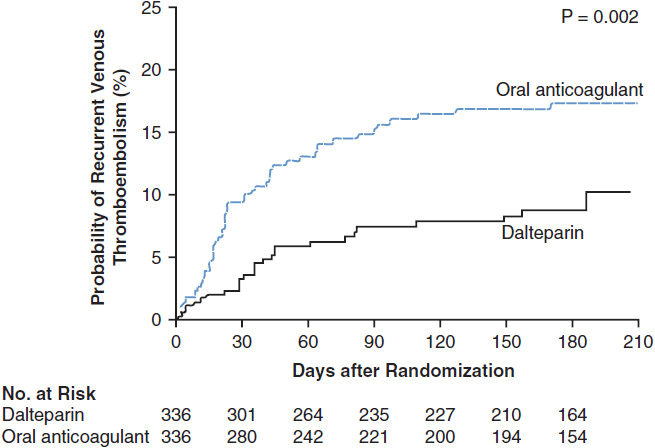
FIGURE 19-3 Recurrent VTE. Kaplan-Meier estimates of the probability of symptomatic recurrent VTE among cancer patients who were randomized to secondary prophylaxis with dalteparin vs warfarin treatment for acute VTE (22). (From Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003; 349: 146–53. Copyright © 2003. Massachusetts Medical Society. All rights reserved.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






