Fig. 21.1
Pre- and post-NACT MRI showing a complete clinical response
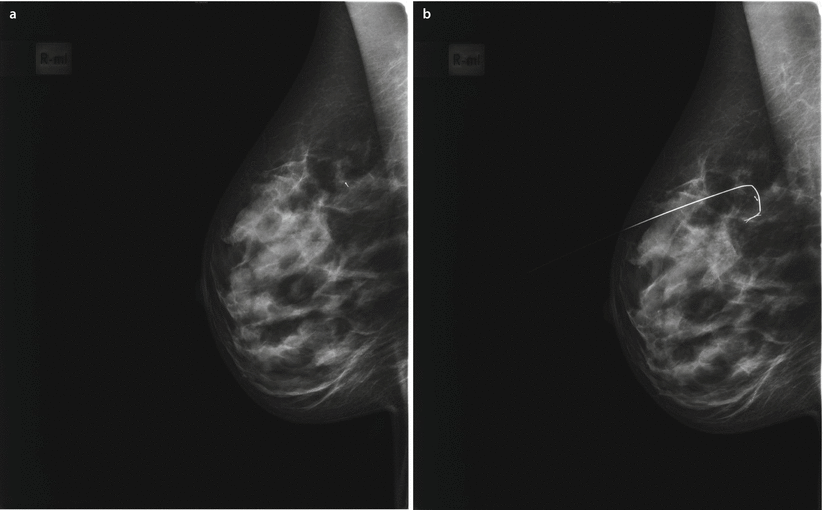
Fig. 21.2
a Clip placed in the centre of the original tumour bed and b needle localization of the clip
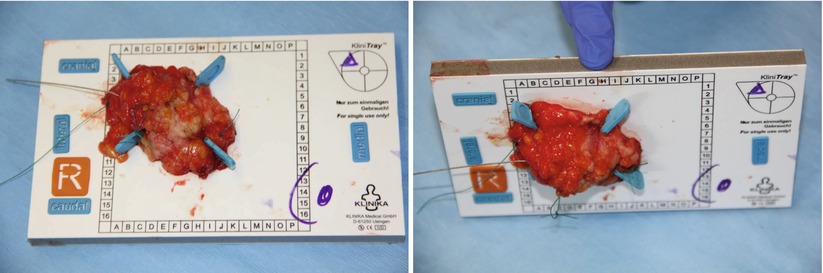
Fig. 21.3
Handling and orientation of the specimen – the specimen is fixed on a template and prepared for radiography in two planes to identify the clip
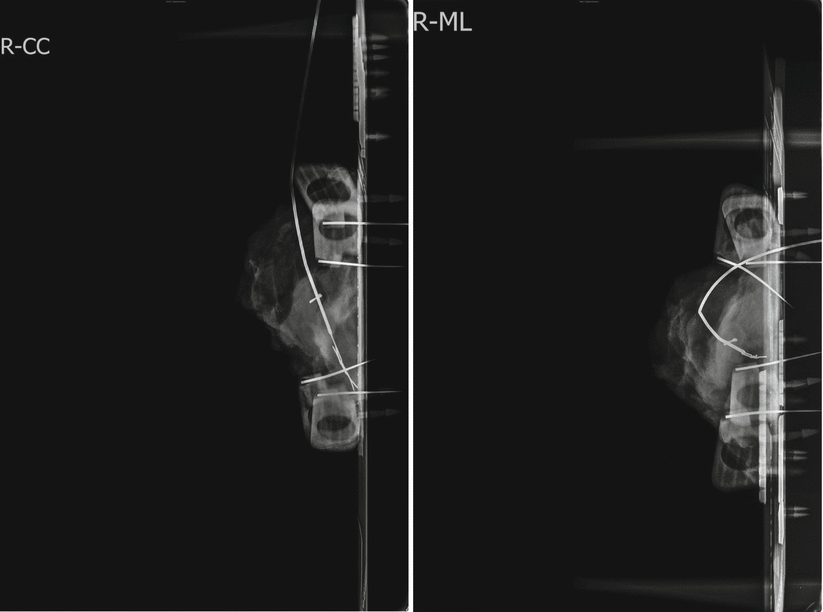
Fig. 21.4
Specimen radiography in two planes. The clip is located in the centre of the specimen with the wire in place
In cases of an unfavourable relation between the target resection volume and breast size, oncoplastic techniques can be applied to ensure an adequate resection volume and a good cosmetic outcome (see ► Chap. 19). Surgical solutions for eventual reresections and the necessity for unexpected, more extensive surgery should, however, be anticipated.
A close interdisciplinary cooperation between surgeon and pathologist is required to allow a high-quality histopathological evaluation. Ideally patients are discussed in a preoperative conference/multidisciplinary tumour board. The following information should be communicated to the pathologist:
- 1.
The specimen must be clearly marked as a post-PST specimen.
- 2.
Clear orientation of the specimen is mandatory.
- 3.
Results of previous core biopsies should be available.
- 4.
Clinical tumour size before and after chemotherapy (information given in cm or mm, rather than T-stage).
- 5.
Location of the tumour/tumour bed/after chemotherapy (ideally by a diagram or drawing).
- 6.
Information on close margins based on intraoperative findings (specimen radiography).
21.8 Pathologic Evaluation
The pathologic evaluation should include information on the adequacy of surgery (identification of the tumour bed) (e.g. clip) and the resection margins. Several reporting systems have been established to allow standardized histopathologic information on tumour response after chemotherapy [24–26]. The residual cancer burden (RCB) that assesses tumour extent, cellularity, size of lymph node metastases and presence of treatment effects in the breast and the lymph nodes after PST is widely used within clinical trials today [27, 28]. The RCB provides a standardized and reproducible tool to define tumour response after PST with a good association with clinical outcome in terms of DFS and overall survival.
21.9 Timing of Surgery and Radiotherapy
Surgery after PST should be planned after the nadir of the leucocyte count, in general 2–4 weeks after the last course of chemotherapy. Radiotherapy should be planned within a timeframe of 2–3 weeks after surgery [29].
21.10 Recent Development of Breast Surgery After PST
Refinements of neoadjuvant regimen and the introduction of targeted therapies such as trastuzumab or pertuzumab have improved pCR rates and outcome after PST considerably. Histopathologic complete response is observed in between 20 and 40% of the patients today, and a pCR rate as high as 74.6% has been achieved for HER-positive and ER-negative patients [12]. Astonishingly, these constantly improving response rates do not translate into a higher rate of BCT which ranges between 13% and 69% [30–31]. The reason for the persisting high mastectomy rates after PST is still unclear. Probably the consensus with regard to the extent of breast cancer surgery after PST is not yet widely accepted by the majority of breast surgeons. However, patients who are candidates for mastectomy prior to PST and whose clinical evaluation after chemotherapy reveals a good response to PST so that BCT appears feasible should be spared by the mutilating procedure of mastectomy wherever possible.
According to a study from the MD Anderson, four factors are associated with an increased recurrence rate in cases of BCT after PST: N2 or 3 disease, the presence of lymphovascular invasion (LVI), residual pathologic tumour size >2 cm and a multifocal residual pattern of disease [33]. These factors were summarized in a prognostic score ranging from 0 to 4 according to the number of risk factors involved. This prognostic index for locoregional recurrence after BCT was evaluated retrospectively in an independent cohort of 551 patients. The 5-year LLR rate was 92%, 92%, 84% and 69% when the index was 0 (no risk factor present), 1 (1 risk factor), 2 (2 risk factors) and 3–4 (3–4 risk factors present). When the prognostic index was 3–4, the 5-year LRR-free survival was significantly lower for patients treated with BCT compared with mastectomy [34].
21.11 Complications for Breast Surgery After NACT
The effect of PST on postoperative complications has not yet been investigated prospectively. In a retrospective analysis of 44,533 patients registered in the American College of Surgeons National Surgical Quality Improvement Programme database, the overall wound complication rate was low (3.4% vs 3.1%) and independent from the use of neoadjuvant chemotherapy. Smoking, functional dependence, obesity, diabetes, hypertension and mastectomy were the main risk factors for wound complications [35].
21.12 Future Perspectives
In view of the constantly improving response rates to systemic treatment regimens (including new targeted drugs) and the increasing sensitivity of imaging techniques to assess the post-PST tumourload, the issue of whether breast surgery is required at all in cases of clinical complete response is a current matter of debate. This relates to the effect of surgery on local control but also to the diagnostic purpose of breast surgery to assess pCR.
Clinical and imaging procedures are not associated with an acceptable sensitivity to assess pCR. Clinical complete response was associated with a 25% sensitivity to predict pCR for physical examination and mammography and 50% for ultrasound and MRI [36]. Shin and colleagues reported an accuracy of pCR prediction of 38% for mammography, 13% for ultrasound and 75% for MRI [37].
Heil and colleagues investigated the false-negative rates (FNR) and the negative predictive values (NPV) (to predict pCR after PST) for core needle biopsy (CNB) and vacuum-assisted biopsy (VAB) in a prospective study of 164 patients [38]. The FNR was, however, as high as 49.3% and the NPV 71.3%. In a small cohort of 16 patients within this study in whom VAB was performed, no FN case was observed. Future studies are being designed to examine the role of minimally invasive procedures to assess pCR.
21.13 Conclusion
Primary systemic treatment is becoming increasingly important in the treatment of breast cancer and has a high potential to tailor future systemic and locoregional treatment decisions. PST allows a more individualized and risk-adapted treatment of the patient. The treatment strategy of PST requires a high standard regarding the radiologic diagnostic workup, the planning of surgical strategies and the pathologic workup of the specimen. Close interdisciplinary cooperation is an important precondition to ensure that the great potential of a primary systemic treatment strategy can successfully be employed to improve the treatment of an individual patient.
References
1.
Killela BK, Yang VQ, Mougalian S, Horowitz NR, Pusztai L, Chapar AB, et al. Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: results from the National Cancer Database. J Am Coll Surg. 2015;220(6):1063–9. Epub 2015/04/15Crossref
2.
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–85.CrossrefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







