The purpose of this chapter is to provide general guidelines on specimen evaluation and highlight some clinically relevant issues, which may help to increase the efficiency and overall quality of pathologic assessment.
As outlined in the protocol for the examination of breast specimens released by the College of American Pathologists,1 it is important that, together with the specimen, the pathologist receives specific information identifying the patient and the anatomic site from which the specimen was obtained, including its laterality and specific location in the breast (quadrant designation, subareolar location, etc), the date and type of procedure that was performed, and the name of the treating physician to whom the final report should be addressed.
In the course of gross examination, the prosector needs to state if the specimen was received in a fresh state or in fixative and specify the latter. Usually, a breast specimen consists of only 1 piece, but if more than 1 piece is present, the number should be stated, and the size of each piece measured in 3 dimensions. A specimen consisting of 3 or more pieces should not be inked. The gross description of the specimen needs to include any orientation markings identified on the specimen (see also “Margin Evaluation” later in the chapter). If a tumor is present, its size needs to be measured and reported. It is best to give the 3 dimensions of a tumor, but no definite consensus exists on this and the CAP recommends specifying the largest diameter.1 Description of the tumor also includes comments on its outline (eg, circumscribed, ill defined), characteristics of the cut surface (eg, bulging, retracted, papillary), consistency (eg, firm, soft, rubbery, friable, mucoid), and color (eg, white, tan, brown-red). The relation of the tumor to the surgical margin should also be clearly specified. Gross examination of a fresh specimen allows better appreciation for subtle differences in the characteristics of the tissue, and enhances both visual and tactile inspection. It also minimizes exposure to formalin fumes, although it does not protect from blood-borne pathogens.
Recently published ASCO/CAP guidelines have recommended optimal fixation times and choice of fixative for breast tissue samples, greatly contributing to protocol standardization.2 Poor or uneven tissue fixation negatively affects tissue preservation and immunohistochemical assessment. Proteolytic tissue degradation due to delayed fixation can result in weak or absent immunoreactivity and nonspecific binding of the antibody. Shorter than optimal formalin fixation (or attempts to fix large tissue pieces without proper sectioning) can result in a mixed fixation pattern where cross-linking occurs only at the periphery of the tissue and the center is either coagulatively fixed by alcohol or remains unfixed.3 Mixed fixation is a common cause of different staining intensities at the periphery and in the center of the same section, and can lead to difficulties in histologic diagnosis and misinterpretation of ER, PR, and HER2 results. Very long fixation should also be avoided as it may cause excessive cross-linkage and damage some epitopes.3
According to the current ASCO/CAP guidelines, breast core biopsies should be fixed for a minimum of 1 hour2; however, some published data indicate that 1-hour fixation may be insufficient for accurate receptor analysis. In a study by Goldstein and associates, very short fixation times led to unreliable results in assessment of ER expression by immunohistochemistry.4 Most discrepancies occurred when core biopsy tissues were fixed for less than 3 hours, while excellent results were achieved after fixation of 6 hours or longer. Given the importance of receptor status in breast carcinoma, it seems prudent to fix core biopsies for at least 3 hours (preferably 6) before processing. It is also recommended to repeat ER/PR and HER2 tests on the surgical specimen if the core biopsy yielded negative or equivocal results.5,6
According to the current ASCO/CAP recommendations,2 tissue sections should be fixed in 10% buffered formalin for a period of 6 to 48 hours. It is best to examine and sample the surgical specimens in the fresh state, and immediately place the tissue sections in cassettes so that they can fix more uniformly. Alternatively, the tissue can be cut into 3- to 4-mm slices and placed in a container filled with large amount of fresh formalin, with large pieces of gauze or few crumpled sheets of paper towel inserted between the slices to maximize exposure to formalin. The upper limit of fixation time is less well defined, and further studies should help to determine if it can be safely extended beyond 48 hours.
Percutaneous needle core biopsy is now the primary modality for diagnostic evaluation of breast lesions. Nonpalpable, radiographically suspicious areas in the breast can be targeted under stereotactic, ultrasonographic, or magnetic resonance imaging (MRI) guidance. The diagnostic accuracy of the procedure increases with increasing caliber of the needle and number of tissue cores.
Standard 14-gauge needles provide good sensitivity. The false-negative rate of ultrasound-guided 14-gauge needle biopsy was 1.6% in a recent study of 1352 cases.7 Larger tissue samples can be obtained with 11- or 8-gauge needles coupled with vacuum-assisted devices.
Most investigators agree that a minimum of 5 cores should be collected from suspicious breast lesions to ensure accurate sampling and diagnosis.7 In a study by Liberman and colleagues, five 14-gauge tissue cores were sufficient for diagnosis in 99% of mass lesions and 6 cores were informative in 92% of mammographic lesions with calcifications.8 The extent of sampling is dictated by the clinicoradiologic findings, and fewer than 5 cores may be sufficient in some cases.
Once collected, the core biopsy tissue is examined by a radiologist who documents the presence of the target calcifications. Pathologic evaluation is best accomplished if the cores are divided into those with and those without radiographic calcifications. Segregation of the cores allows for separate embedding and step sectioning of the appropriate block(s) if the initial levels fail to demonstrate calcifications. The pathologist should attempt to correlate histology with radiologic findings, bearing in mind that calcifications smaller than 100 μm may not be relevant as these cannot be detected radiographically,9 unless they are clustered together. Calcifications associated with malignant lesions tend to be relatively large, ranging in size from 100 to 300 μm.9 The pathology report needs to specify where the calcifications are located (in situ or invasive carcinoma, benign breast lesions, stroma, blood vessel wall). It is also important to review the accompanying specimen x-ray to determine if the amount of calcifications on x-ray is similar to what is seen on the slides, and this correlation is particularly important in cases with benign histologic findings. In order to avoid false-negative results, the pathologist needs to thoroughly search for calcifications whenever necessary. The tissue blocks are usually x-rayed en face to identify calcifications still embedded in the tissue, and multiple-level sections are obtained from the index block(s). There is no advantage in leaving tissue unsampled in the block if no calcifications are identified histologically, and levels through the block(s) should be ordered as necessary. Alternatively, blocks can be x-rayed on edge, allowing to identify the calcifications and to determine their depth in the tissue; in some cases this information is useful to assess whether it is opportune to melt the block and re-embed the tissue after it has been flipped, so that the calcifications are closer to the cutting surface and the tissue is not wasted. Calcifications are rarely affected by biopsy procedure and tissue processing, but occasionally they may be displaced, shattered, or lost (“knocked off”) when the paraffin block is trimmed in preparation for sectioning.10,11 This seems to occur more commonly in cases with larger calcium deposits and/or calcium oxalate crystals. Calcium oxalate crystals are often found in apocrine cysts or adjacent stroma, where they can be associated with a giant cell reaction. These crystals are translucent and can be missed on light microscopic examination, but they are birefringent under polarized light. Calcium crystals are water-soluble, and small calcifications have been reported to disappear within 3 days if the tissue is kept in a container with aqueous solution such as 10% formalin.12
Communication between the pathologist and the radiologist is essential and information about the radiographic appearance of the lesion and its differential diagnosis will help the pathologist to better assess adequacy of the specimen. Whenever the findings in the core biopsy are felt to be discordant or nondiagnostic, the pathologist should alert the radiologist and communicate in the final report the limitations of the specimen.
Measurement of the size of invasive carcinoma on core biopsy material may not be accurate. In many cases, the actual tumor is larger than the core fragments and its size is best measured in the excision specimen. In contrast, information about the tumor size should be provided if the invasive focus is small, such as microinvasive carcinoma, as subsequent excision may not contain additional evidence of stromal invasion.
Core biopsy should be viewed as a screening method aimed to identify lesions requiring surgical intervention. For that reason, a low threshold for excision is recommended for any abnormal or suspicious finding. The goal is not to remove the atypical foci, but to rule out the presence of frank malignancy in the adjacent tissue. Surgical excision should be performed for any type of epithelial or nonepithelial atypia, including atypical ductal hyperplasia (ADH), atypical columnar cell lesions (flat epithelial atypia, FEA), and histologically suspicious stromal and vascular proliferations. No consensus exists at present on the need for excision after diagnosis of lobular neoplasia (ALH/LCIS) in a core biopsy.13,14 Several studies have documented the association between columnar cell atypia (FEA) and lobular neoplasia on core biopsy and finding of carcinoma on subsequent surgical excision.15-19
Accurate diagnosis and careful wording of the report are equally important. The pathologist should be aware that even in the absence of cytologic atypia, the use of the word “papillary” in the final diagnosis of the breast core biopsy will be followed by excision of the lesion. It is reasonable to remove and evaluate in its entirety any sizable, radiologically detected papillary lesion to rule out atypia or overt malignancy; however, minute incidental papillomas or foci of florid papillary usual ductal hyperplasia should not be overinterpreted. Therefore, it is best to avoid confusing terminology, such as “papillary hyperplasia.” The diagnosis of “radial scar” or “complex sclerosing lesion” is also usually followed by surgical excision, and so is the diagnosis of “fibroepithelial lesion.” The latter term should be reserved for fibroepithelial proliferations characterized by increased cellularity, mitotic activity, and/or cytologic atypia, suspicious for phyllodes tumor.
Surgical margin status is an important predictor of local recurrence and affects survival of early-stage breast cancer, but no consensus exists on what constitutes an optimal margin. Its definition ranges widely, from “no tumor on ink” to “at least 1.0 cm clearance.” It is intuitive that a wider margin will contribute to better outcome, but even “wide negative margins” cannot guarantee complete tumor removal due to architectural complexity of the mammary ductal system, multifocality of some tumors, and limitations of margin assessment methods.
In a study of 60 mastectomy specimens, Faverly and associates explored the 3-dimensional structure of the mammary ducts and the patterns of distribution of ductal carcinoma in situ (DCIS). Each mastectomy specimen was sectioned at 5-mm intervals and x-rayed, and the slices containing radiographically or grossly suspicious areas were carefully mapped and examined by conventional microscopy and stereomicroscopy to determine the extent of intraductal carcinoma and connections between different foci. The study demonstrated that 70% of low-grade lesions were multifocal, whereas 90% of high-grade DCIS showed continuous duct involvement.20 These findings suggest that negative margins may be easier to achieve and more reliable in cases of high-grade DCIS as compared to low-grade carcinoma. In the same study, the gaps between the foci of carcinoma ranged from 0.1 to 4 cm, but most foci (82%) were less than 0.5 cm apart; hence, a 0.5-cm margin would have been adequate for 82% of patients.20 Studies aimed to improve the conservative management of breast carcinoma have consistently demonstrated the benefit of radiation therapy in achieving local control, and a margin smaller than 5 mm is considered adequate in this setting.21 A recent study evaluated over 1000 patients with DCIS treated with lumpectomy and radiation and showed that the recurrence rate at 10 years was 8% in patients with a 2-mm margin.22
Several methods of margin evaluation have been proposed. They are outlined next, with discussion of advantages and disadvantages of each method.
The most common method of margin assessment is the perpendicular (or radial) margin technique, which allows for precise measurement of the distance separating the tumor from the closest inked margin (Fig. 15-1). With this method, the specimen is received oriented with side specification (right vs left breast). Usually, the surgeon designates the superior margin with a short suture (or a single metal clip), and the lateral margin with a long suture (or 2 metal clips). The 6 margins are differentially inked by the prosector. Ink should be applied lightly, with gentle dabbing, so not to artificially disrupt the tissue. Excess ink should be gently removed by pressing a gauze sponge on the specimen, to reduce the problem of running ink. Brief application of acetone on the inked surface seems to enhance staining and reduce the problem of running ink.
Figure 15-1
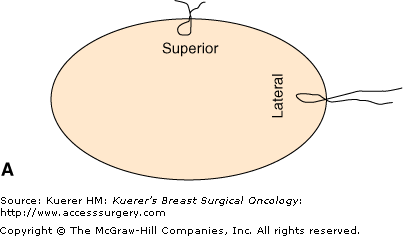
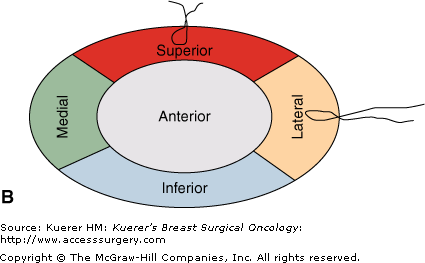
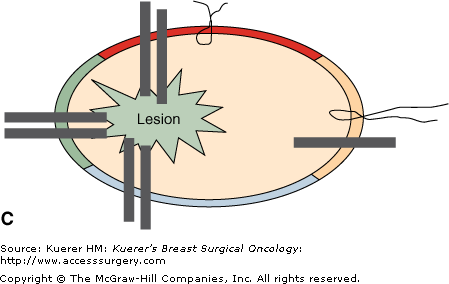
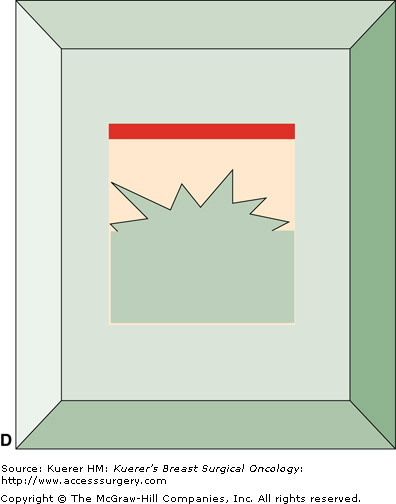
Perpendicular (radial) margin evaluation method. The specimen is received oriented (A) and the 6 margins are differentially inked (B). The tissue is then sequentially cut into 2- to 3-mm slices perpendicular to the long axis of the specimen, so that the perimeter of each slice contains 4 margins identified by different color inks (C). The margin sections are taken perpendicular to the inked surface (C). The extent of individual margin sampling is dictated by its proximity to the lesion. The tissue is embedded in such a manner that the distance from the lesion to the inked margin can be measured (D).
It is best to follow an established coloring scheme for margin designation, but color-coding should be always specified in the gross description for reference. Any color scheme is acceptable, as long as it specifically identifies each of the 6 margins. Medial and lateral margins can be inked with the same color and submitted in separate tissue blocks with margin designation. The inked specimen is then sequentially cut into 2- to 3-mm slices perpendicular to its long axis, so that the perimeter of each tissue slice contains 4 margins identified by ink of different colors. The extent of margin sampling is determined by its proximity to the index lesion. Margins within 0.5 cm of the index lesion are best evaluated in their entirety, and more distant margins can be representatively sampled. Sections should be taken preferably from fibrous, not fatty areas. With this method, the pathologist can report the exact microscopic distance from the tumor to each individual margin and distinguish between a truly positive margin (“tumor on ink”), a close margin (1-2 mm), and a margin 2 mm or greater, allowing the surgeon a greater level of judgment in determining the need of reexcision.23 The disadvantages of this method include imprecise margin orientation and seepage of ink. Breast tissue is soft and may be artifactually disrupted when the specimen is compressed to obtain a specimen radiograph, or in the course of gross examination. Seepage of ink inside the specimen and different color inks running into each other occur frequently, leading to possible overinterpretation and false-positive margins.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree