Breast magnetic resonance imaging (MRI) has attained a solid position in the evaluation of the breast, and many believe it is currently a necessary component of any breast imaging practice. In the past decade, many advances have contributed to the more routine use of this robust tool for cancer detection, including newer faster imaging sequences with improved image quality as well as new biopsy equipment that allows percutaneous needle biopsy of suspicious lesions. Additionally, societies have created guidelines for breast MRI, thus improving standardization in the performance, interpretation, and recommended use of this technology. Many of our current algorithms in the detection and treatment of breast cancer have been changed by the availability of breast MRI.
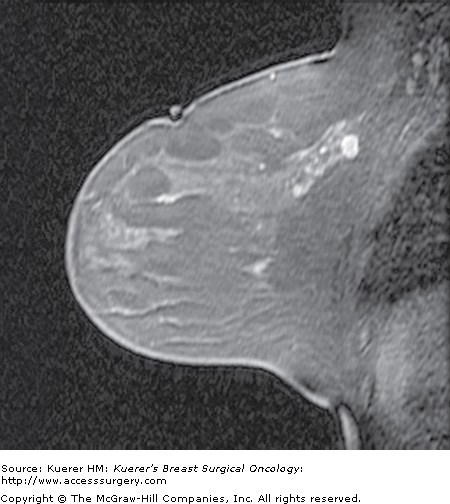
Clinical indications for breast MRI1 include screening for breast cancer in the high-risk patient (Fig. 35-1), assessing response to chemotherapy in the patient with known breast cancer undergoing new-adjuvant chemotherapy (Fig. 35-2), assessing residual disease in a conserved breast with positive margins (Fig. 35-3), assessing possible recurrence in the treated breast when there is clinical or imaging suspicion (Fig. 35-4), screening the contralateral breast in the patient with known breast cancer (Fig. 35-5), and assessing for underlying cancer in a patient with occult primary breast cancer (Fig. 35-6). Breast MRI can also be valuable in the evaluation of inconclusive findings on conventional imaging (Fig. 35-7). The final clinical indication where MRI may be helpful but where there is less clinical evidence is assessing extent of disease in the preoperative setting (Fig. 35-8).
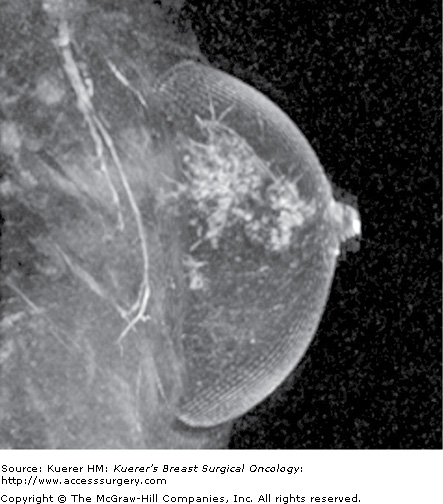
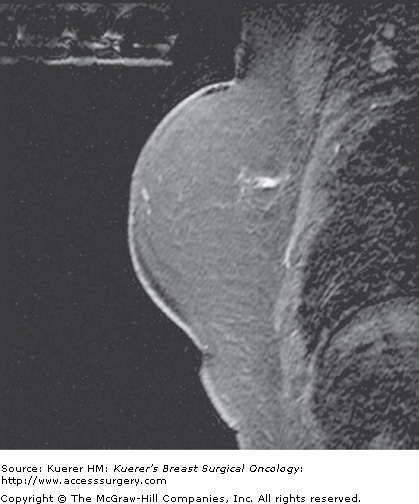
Figure 35-3
Residual disease assessment. Postoperative magnetic resonance imaging was performed for widely positive margins a few days following surgery. A seroma cavity is identified in the central breast with an air-fluid layer (the patient is scanned prone). Along the posterior aspect of the seroma cavity toward the chest wall, there is lobular enhancement that represents residual disease. The amount of residual disease proved too much for reexcision (proved first by percutaneous biopsy), and the patient underwent mastectomy.
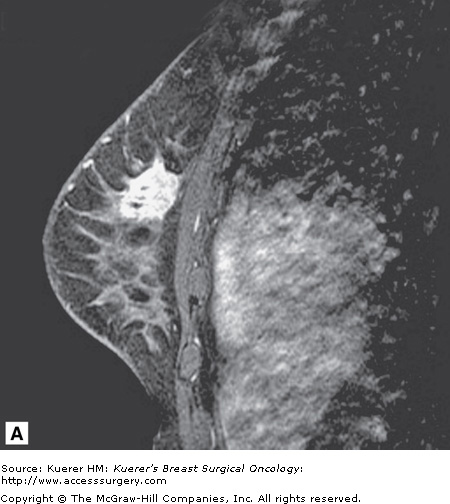
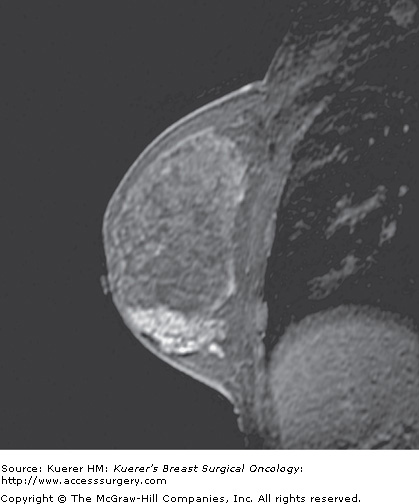
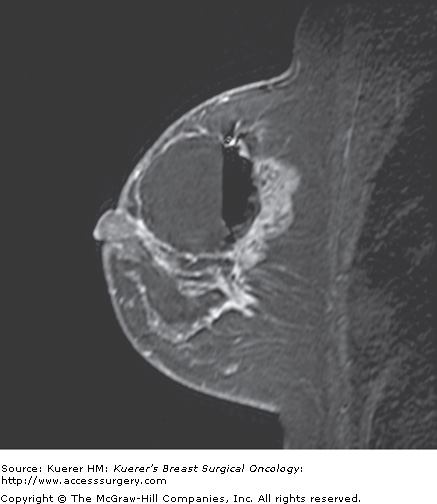
Figure 35-4
Recurrence following breast conservation. A 63-year-old woman with a history of ductal carcinoma in situ in the posterior breast (lumpectomy site clips are visible as black signal void) 2 years ago was treated with surgery and radiation. Screening magnetic resonance imaging demonstrates a new suspicious irregular mass in the anterior breast that proved to represent invasive mammary carcinoma with ductal and lobular features.
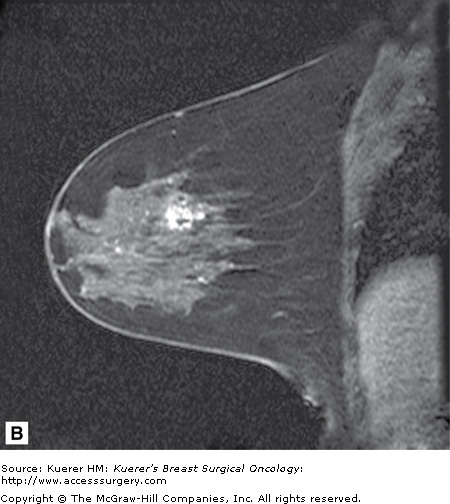
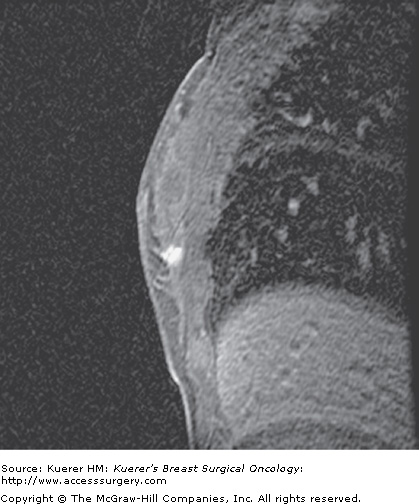
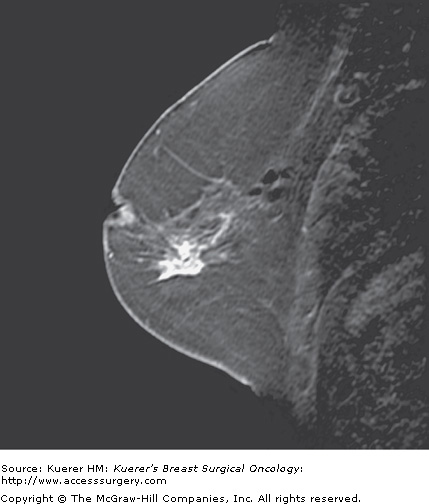
Figure 35-5
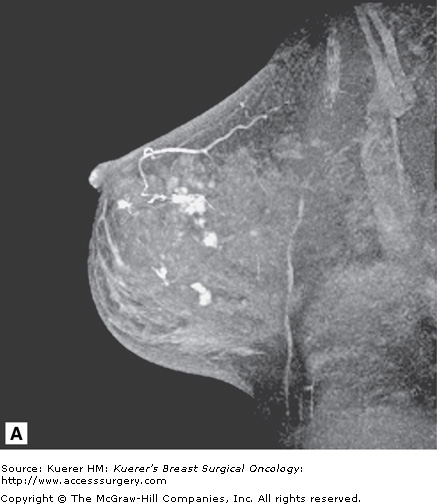
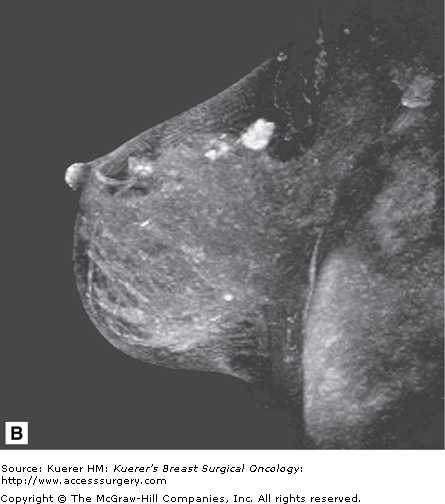
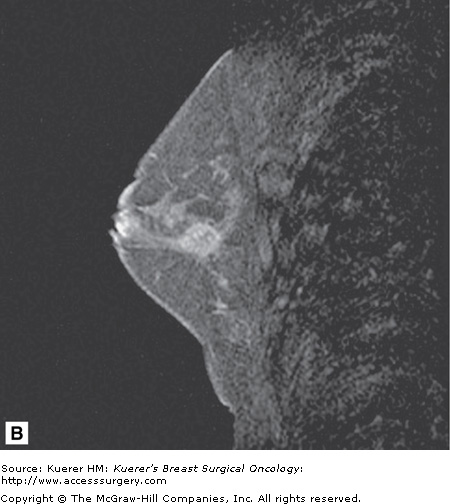
Screening of the contralateral breast. A. A 38-year-old woman with biopsy-proven moderately differentiated invasive ductal carcinoma of the right breast demonstrated on 3-dimensional magnetic resonance imaging (MRI) underwent bilateral MRI for preoperative staging. MRI demonstrated multiple additional findings in the index breast that proved to represent multicentric disease on biopsy. B. Contralateral MRI breast screening demonstrates an unsuspected mass with satellite lesions that proved to represent moderately differentiated ductal carcinoma of the opposite breast as well. The patient elected to undergo bilateral mastectomy.
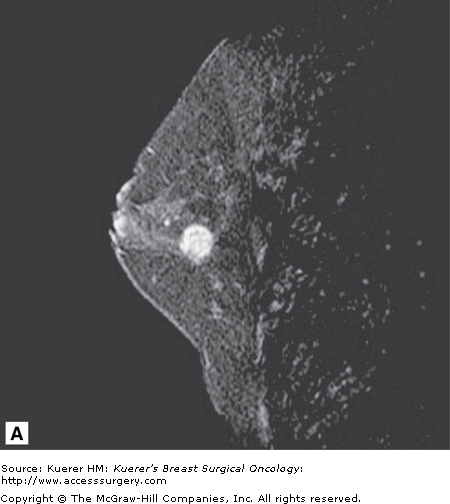
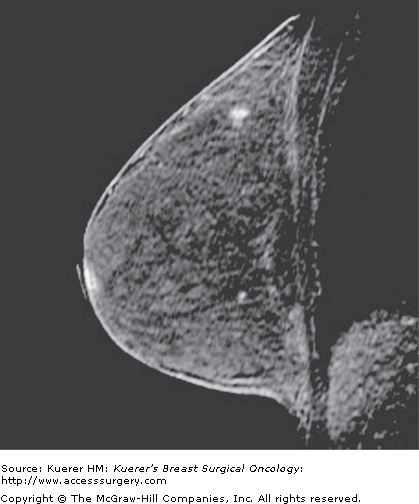
Figure 35-6
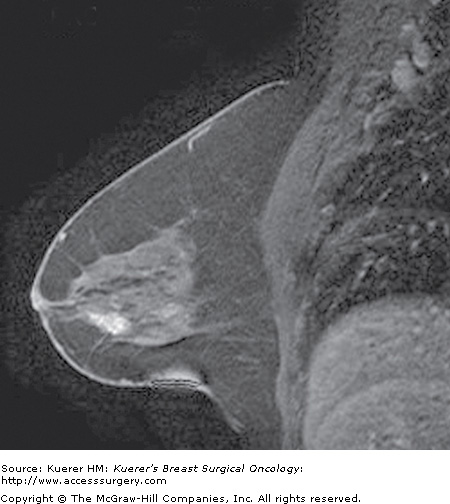
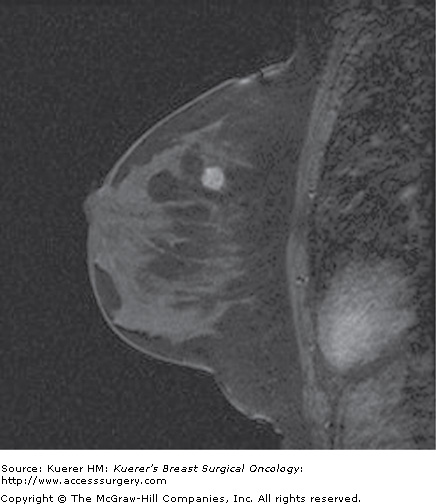
Occult primary breast cancer. A 60-year-old woman status postexcision of enlarged right axillary node showing metastatic adenocarcinoma from breast primary. Clinical examination, mammogram, and ultrasound of the breast were negative without suspicious findings. MRI demonstrated the underlying small primary cancer in the anterior breast.
Figure 35-7
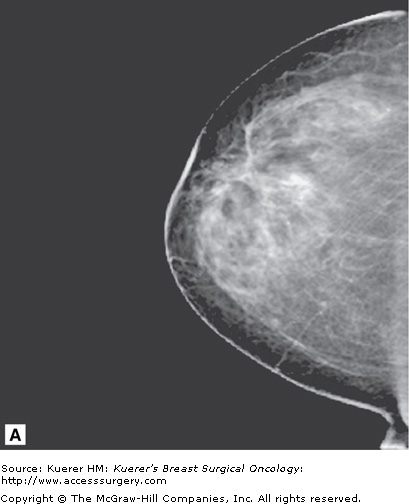
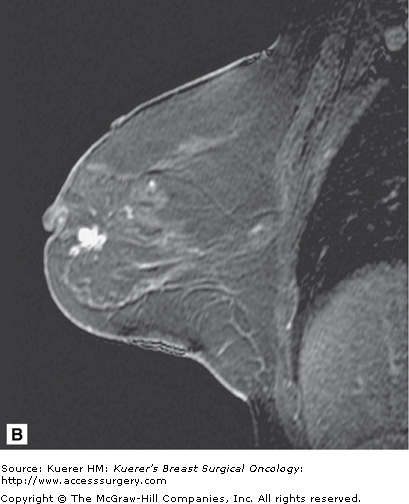
Inconclusive findings on mammography. A 66-year-old woman with increased distortion on mammography at a site of prior benign biopsy that yielded lobular carcinoma in situ (LCIS). Magnetic resonance imaging demonstrates suspicious mass at the prior benign biopsy site. Pathology yielded LCIS with several foci of invasive lobular carcinoma measuring 2 mm with negative sentinel nodes.
Figure 35-8
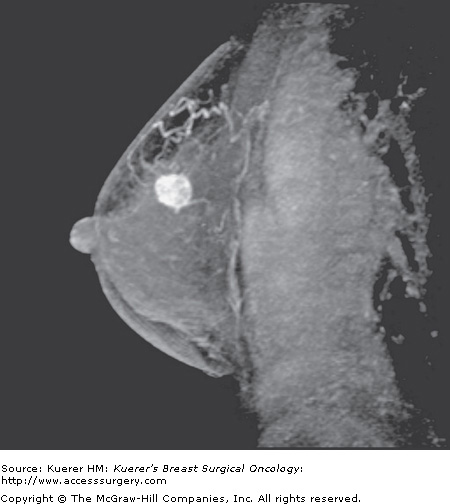
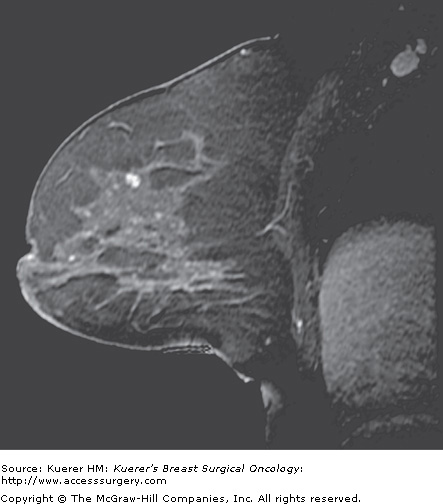
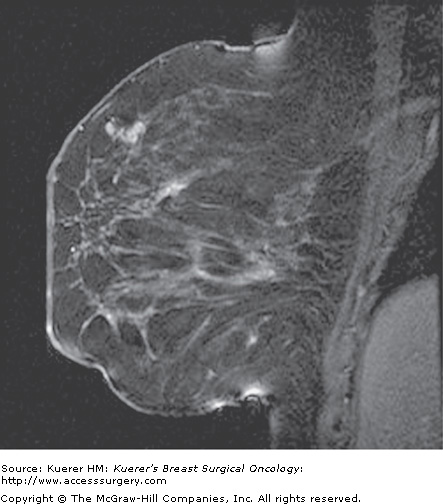
Preoperative evaluation of disease extent. A 44-year-old woman with palpable moderately to poorly differentiated invasive ductal cancer identified and biopsied under ultrasound guidance. Mammogram was dense without suspicious finding. Magnetic resonance imaging performed for assessment of disease extent demonstrates a unifocal cancer without additional suspicious finding. Breast conservation confirmed a 1.5-cm cancer with associated ductal carcinoma in situ, and margins were free of tumor.
Breast MRI uses an intravenous contrast agent for cancer detection and therefore relies almost exclusively on the associated neovascularity associated with carcinomas.2,3 The administration of an intravenous contrast agent such as gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) allows these lesions to be well visualized. Leaky capillaries and arteriovenous shunts allow contrast agents to rapidly accumulate and then rapidly leave the lesion over time, resulting in the characteristic washout time intensity curves that can be seen with some but not all malignancies (Fig. 35-9). Detection of invasive breast carcinoma is extremely reliable on MRI as the sensitivity approaches 100%.4,5 Because the sensitivity for cancer detection is high, the negative predictive value of breast MRI is high. If no enhancement is present in the breast, and any possible technical mishap such as intravenous contrast extravasation has been excluded, there is an extremely low likelihood that invasive carcinoma (but not ductal carcinoma in situ[DCIS], as discussed in the following paragraph) is present. Specificity is lower than sensitivity, and therefore false positives can pose a problem in interpretation.
False positives can be caused by high-risk lesions such as lobular carcinoma in situ (LCIS) (Fig. 35-10), atypical ductal hyperplasia (ADH) (Fig. 35-11), and atypical lobular hyperplasia (ALH), as well as benign masses such as fibroadenomas (Fig. 35-12), papillomas (Fig. 35-13), and lymph nodes (Fig. 35-14). Additionally, fibrocystic changes (Fig. 35-15), sclerosing adenosis (Fig. 35-16), duct hyperplasia (Fig. 35-17), and fibrosis (Fig. 35-18) can result in a benign biopsy. With experience, many of these lesions can be diagnosed as benign; however, false positives will always be an issue on MRI as they are on mammography and sonography. Despite the reputation of high detection of cancer, false-negative examinations with MRI do exist. It should be noted that false negatives have been reported with some well-differentiated invasive ductal carcinomas as well as invasive lobular carcinoma.6
Figure 35-11
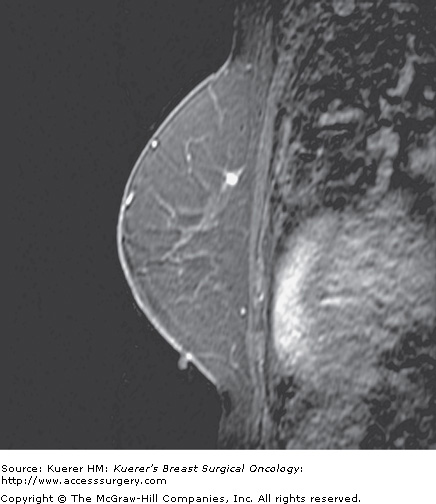
Atypical duct hyperplasia. A 49-year-old woman with a prior history of benign biopsy yielding lobular carcinoma in situ and atypical ductal hyperplasia (ADH) undergoes screening magnetic resonance imaging (MRI) examination. An enhancing mass in the superior breast was identified that underwent MR intervention yielding ADH.
Figure 35-14
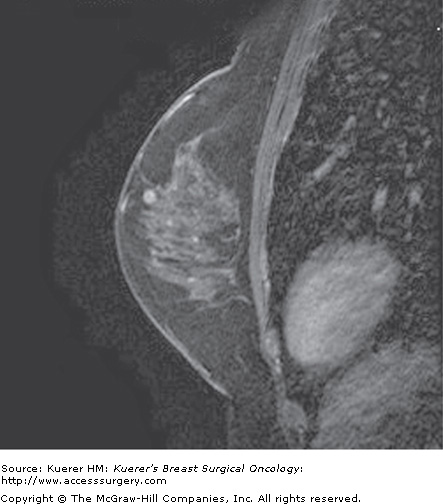
Lymph node. A 38-year-old woman with a prior history of benign biopsy yielding lobular carcinoma in situ, intraductal papillomas, and pseudoangiomatous stromal hyperplasia undergoes screening magnetic resonance imaging. In the supraareolar region there is an enhancing mass that represents a lymph node.
Not all DCIS is detected on MRI. Despite the sensitivity being very high for invasive carcinoma, the sensitivity of detection of DCIS has been reported in prior literature to be somewhat lower,7 possibly secondary to more variable angiogenesis associated with DCIS lesions. But more recent evidence8 suggests that the sensitivity for DCIS detection may actually be higher than previously reported now that high-resolution scanning techniques are more available and widely used and the patterns of DCIS on MRI are more recognized (Fig. 35-19). However, the sensitivity is still below that of invasive carcinomas.9 Morphology may be more important than kinetics in the evaluation of DCIS; thus a slightly modified interpretation approach is taken when evaluating for DCIS.10 Although more work needs to be performed in the MR assessment of in situ disease, MRI does not currently have as high a negative predictive value for DCIS as with invasive cancer. Therefore, MRI is not able to exclude DCIS with current technology and cannot be used to exclude the need for biopsy of suspicious calcifications. Nevertheless, MRI can detect mammographically occult DCIS and is able to play a valuable role possibly in the preoperative assessment of DCIS, where extent of disease may be underestimated by mammography (Fig. 35-20).
Figure 35-20
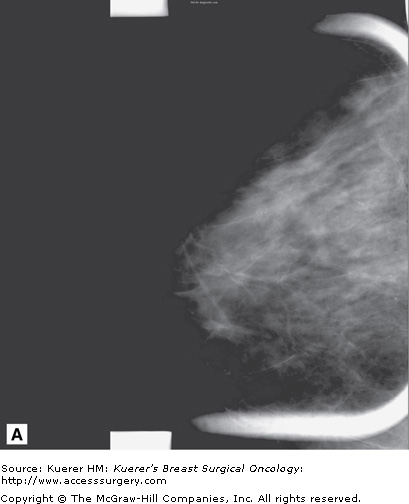
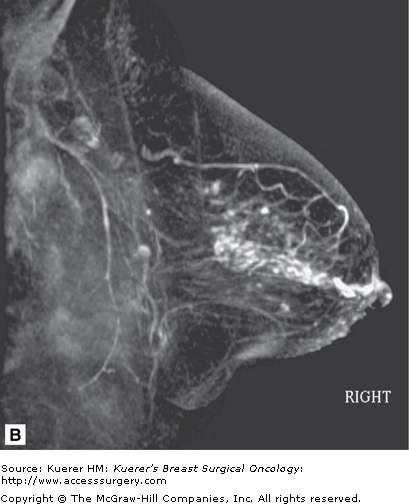
Ductal carcinoma in situ (DCIS) underestimated on mammography. A. Few calcifications are seen in the retroareolar region of the breast on mammography. B. Three-dimensional magnetic resonance imaging of the breast demonstrated more extensive ductal enhancement that corresponded to the full extent of DCIS.
Because of the potential issue of a false-negative examination, a negative MRI examination should not deter biopsy of a suspicious lesion (BI-RADS 4 or 5) on mammography or ultrasound. Mammographically suspicious findings, such as suspicious calcifications, spiculated masses, or areas of distortion, warrant appropriate biopsy, regardless of a negative MR examination. The MRI should ideally be interpreted in conjunction with all other pertinent imaging studies such as the mammogram and the ultrasound to arrive at the best treatment option for the patient. With these limitations, breast MRI is best used as an adjunct test to conventional imaging, complementing but never replacing basic mammography and sonography.
During the past few decades, as breast MRI has been incorporated into the clinical evaluation of the breast, it became apparent that standardization of image acquisition and terminology is extremely important. The American College of Radiology (ACR) Committee on Standards and Guidelines published a document for the indications and performance of breast MRI in 2004. Recently, the Breast Imaging Reporting and Data System (BI-RADS) lexicon11 has added a section regarding breast MRI that has already been revised, and further revisions are in progress. These efforts have been important in establishing the standards of reporting and the standards for patient selection. The existence of standardized guidelines in image acquisition and interpretation has helped disseminate this technology from academic centers into the community.12,13 Furthermore, the ACR is supporting efforts to establish a voluntary accreditation process for performing breast MRI. The accreditation process will further standardize the acquisition of the MRI examination and will ensure that high-quality imaging will be performed on referred patients to the accredited centers.
In 2006, the American Cancer Society (ACS) modified for the first time the screening recommendations to include screening of high-risk women with breast MRI.14
An important recent recommendation of breast MRI is in the screening of high-risk patients who have at least a ≥20% lifetime risk of developing breast cancer. Because mammography has an overall false-negative rate of up to 15% in the general population, it is evident that all cancers are not detected by conventional means. The rate of false-negative examinations may be even higher in premenopausal women with dense breasts (reaching 50%),15 and therefore exploration into alternative screening methods such as MRI has occurred. MRI has high-resolution capabilities, full documentation of the examination, and the potential to detect preinvasive DCIS and small invasive cancers that are usually node negative (Fig. 35-21). Studies that include patients with an overall cumulative lifetime risk of developing breast cancer of approximately 30% show that MRI is able to detect cancer in approximately 1% to 3% of patients.16-23 There are clear-cut indications for MRI screening in a small minority of patients at the highest risk of developing breast cancer. For the remaining majority, more data is needed to determine who benefits from this additional screening test.
Figure 35-21
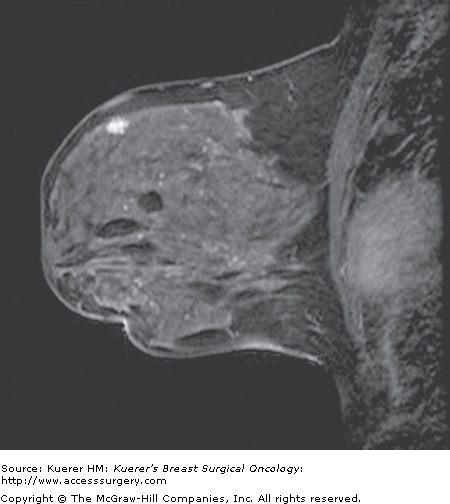
Screen detected cancer on magnetic resonance imaging (MRI). A 43-year-old woman with history of LCIS undergoes MRI screening. A new enhancing mass was identified in the superior breast that could not be seen on mammography or with targeted ultrasound. MR intervention demonstrated invasive ductal carcinoma. At surgery a 9-mm cancer was found with negative sentinel nodes.
The use of breast MRI in the high-risk population is limited to those women with the documented BRCA1 or BRCA2 gene or those women with a family member who is a documented carrier but they themselves are untested; any woman with a greater than 20% to 25% lifetime risk (as defined by the BRCAPRO or other models dependent on family history); women with a history of mantle radiation; women with a breast cancer syndrome such as Li-Fraumeni, Cowden, and Bannayan-Riley-Ruvalcaba. There is very little published clinical information that exists for screening patients who are at increased risk based on a prior benign biopsy yielding LCIS, ADH, or ALH. The ACS did not issue a recommendation for or against screening in these clinical scenarios because there is not yet enough compelling clinical data. The decision whether or not to screen patients with these high-risk lesions should be made on an individual basis by the referring clinician.