Fig. 29.1
Dual chamber expandable implant

Fig. 29.2
Two textured surface implants, one with a round shape and the other an anatomic cohesive gel implant
29.3 Safety Issues and Complications
29.3.1 Safety: Systemic Disease
The health-related safety of silicone implants was questioned based on animal studies in the 1990s that suggested a possible link between silicones and connective tissue disease [24, 25]. After case reports describing cancer and connective tissue disease in women with breast implants, the US Food and Drug Administration (FDA) requested a moratorium on silicone gel breast implants in 1992. The FDA deemed that the manufacturers had not adequately addressed public concerns about complications as implant rupture and silicone leakage and required them to submit premarket approval applications that contained data on safety and effectiveness. Silicone implants were subsequently permitted only for breast reconstruction after mastectomy and for replacement of existing implants. This resulted in an increase in the use of saline-filled implants in the United States, while silicone implants continued to be used in Europe and many other countries. In 1999, the Institute of Medicine (IOM) released a report on published literature and ongoing studies regarding breast implants, entitled «Safety of Silicone Breast Implants» [26]. The report made a distinction between local complications and systemic health concerns. It concluded that local complications were the primary safety issue and that there was no evidence that silicone breast implants caused systemic health effects. A meta-analysis published in 2000 [3] found no evidence of an association between breast implants and connective tissue disorders. In 2006, silicone gel implants were reintroduced in the United States. The FDA approved two different silicone gel breast implants (fourth generation) based on the manufacturers’ clinical studies, which had followed hundreds of women for 3–4 years. As a condition of approval, the manufacturers of silicone gel-filled breast were required to evaluate long-term performance and safety after 10 years [20–23].
The first so-called core study results at 10 years were published in 2014 for Allergan’s® round silicone-filled breast implants (Allergan®, Irvine, California, US) [23]. Of 715 included subjects, 98 were postmastectomy reconstruction patients, and 15 had revisions of reconstructions. Across all cohorts, 56.2% of implants were smooth, and 43.8% were textured. Follow-up over the course of the 10 years was complete in 73.0% for the reconstruction group. The risk of having an implant replaced with a different size/style implant was 31.5% for the reconstruction group. By the end of the study, 63% in this group still had an implant, and 41% retained the same implant that was first implanted.
The Mentor® (Mentor®, Santa Barbara, California, US) core studies [27] included 2003 women with 9 years of follow-up, divided into approximately equal proportions of women with round versus shaped implants. As in the Allergan® studies, the majority of patients had implants for breast augmentation, 441 were reconstruction patients and 129 had undergone revision related to breast reconstruction. In this study, the cumulative rate for any reoperation after primary reconstruction was 45% for round and 52% for shaped implants. The risk for capsular contracture was 20% and 13%, respectively, and for implant loss 30% and 35%, respectively.
Numerous studies have been published reporting data on silicone gel implants and systemic disease without finding strong evidence regarding an association between implants and cancer, immunological or systemic disease. A systematic review from 2016 [28] aiming at evaluating specific long-term health outcomes including cancer, connective tissue, rheumatologic and autoimmune diseases, neurologic disorders, reproductive and mental health issues found 32 studies meeting their eligibility criteria. There were possible associations with a decreased risk for primary breast and endometrial cancer and an increased risk for lung cancer, rheumatoid arthritis, Sjögren’s syndrome and Raynaud’s syndrome. The evidence, however, was most often not specifically regarding silicone gel implants as other implants were also included. In addition, many studies were not adequately adjusted for potential confounders. Thus, the authors concluded that «further investigation is required to determine whether any true associations exist between silicone gel implants and long-term health outcomes».
29.3.2 Safety: Incidence of Breast-Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL)
Anaplastic large cell lymphoma is a rare condition that can affect various tissues. It may present as a nodal lymphoma or in various other locations. The first case of breast-implant-associated ALCL was reported in 1997 [29], and today almost 200 cases are known [30]. According to the FDA, breast lymphomas are very rare with three cases in 100 million women per year diagnosed in the United States (reviewed in [31]).
One well-described finding associated with BIA-ALCL is a late-onset seroma surrounding the implant. Other symptoms are nonspecific swelling, pain, tenderness or capsular contracture [31, 32]. A late seroma should raise the suspicion of BIA-ALCL, and cytology should be obtained by fine-needle aspiration. Biopsy or resection specimens with BIA-ALCL show large pleomorphic tumour cells of T-cell lineage that strongly and uniformly express CD30, as shown by immunohistochemistry or flow cytometry [33]. Treatment is by surgical removal of the implant and complete capsulectomy. The capsule surrounding the implant may be thickened and fibrous or deceptively normal in appearance. Patients who present with a palpable mass or extension of the tumour outside the capsule might benefit from adjuvant treatment after surgical removal of the mass [31, 33, 34]. After clinical follow-up [33] of 87 patients with BIA-ALCL, the 5-year overall survival was 89%. Patients with lymphoma confined to the capsule and undergoing complete surgical excision had better overall and event-free survival than those who were treated with partial capsulectomy, chemotherapy or radiotherapy. Those patients who died had local or regional extension of the disease, while no patient developed disseminated disease. Miranda and colleagues [35] reviewed the literature for all published cases of BIA-ALCL from 1997 to 2012. They found that most patients with BIA-ALCL confined to the fibrous capsule achieved complete remission. Patients receiving breast implants should be advised of the risk of developing BIA-ALCL, as well as the common presenting symptoms.
29.3.3 Safety: PIP Implants
In 2010, the French authorities suspended the sale of implants from the French manufacturer Poly Implant Prothèse (PIP) because of a high rate of ruptures and silicone leakage. PIP implants were introduced in France in 1991 and obtained the Conformité Européenne (CE) Marking in 1997. In 2010, several laboratory studies on PIP breast implants conducted by the French health authorities (AFSSAPS) showed that these had not been manufactured according to the documented procedures provided to obtain the CE Marking: The barrier layer had been removed from the shell in 2007, and the medical grade silicone gel was replaced by inferior industrial grade gels. The contents of the PIP silicone breast implants tested negative for cytotoxicity and genotoxicity, but an in vivo test for irritancy was positive [36]. In an update from the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) in 2014, it was stated that several cyclic siloxanes (D4, D5 and D6) had been identified at higher concentrations in PIP devices than in other silicone breast implants. Investigating the possible toxicological consequences of cyclic siloxanes release from damaged PIP implants was not possible as these chemicals are frequently found also in women without breast implants, as siloxanes are present in many domestic products. The cyclic siloxanes D4, D5 and D6 are nontoxic and not irritant in standard tests. In some women, however, implant gel bleed or rupture has been associated with an inflammatory reaction either locally or in regional lymph nodes. Neither implant rupture nor local inflammation has been found to be associated with breast cancer or anaplastic large cell lymphoma [37]. The committee concluded that removal of all intact PIP implants was not recommended but left the final decision to affected women and their surgeon or physician.
The reported PIP implant rupture rate in the literature varies between 3.9% and 31.6% [38–40]. Comparison is difficult as the authors use different definitions, and patient groups are not homogeneous. Most included patients had undergone breast augmentation. An Italian report including 578 women who had PIP implants for postmastectomy reconstruction found 18.5% ruptured implants after a mean implant lifespan of 57.5 months [41]. In the Netherlands, 224 PIP implants were evaluated with a mean implant age of 122 months. One third of the women who had undergone breast augmentation with PIP implants were shown to have at least one ruptured implant after 10 years; 45.9% had bilateral rupture, and 13.5% had extracapsular leakage. Most implant ruptures were asymptomatic [40].
29.3.4 Complications
Complications related to breast implant surgery are common. Reported incidences vary widely in the literature; diverging study designs, mixtures of patient groups and definitions of follow-up variables make comparisons difficult. Naturally, complication rates after breast augmentation are lower than after breast reconstruction. Obesity, smoking, large breast size, hypertension and diabetes [42–45] are associated with an increased risk of wound complications and reconstructive failure, which should be thoroughly discussed before planning surgery. Tobacco users should be recommended to stop smoking 4 weeks before and 4 weeks after surgery, as this substantially reduces the risk of complications [46].
The Danish Registry for Plastic Surgery of the Breast prospectively collects pre- and postoperative data for women undergoing breast surgery. In one report, 189 immediate breast reconstructions (therapeutic in 167 women) were analysed, 40 one-stage and 149 two-stage procedures. None of the women had radiotherapy, and median follow-up was 3.9 years. A postoperative complication was reported in 144 procedures (76%), 74 (39%) of whom also had a reoperation during the follow-up period. Immediate complications, i.e. infection, haematoma and seroma, occurred primarily within the first postoperative year, with risk estimates of 19.0%, 11.1% and 12.2%, respectively. Overall, the estimated risk for reoperation within the first postoperative year was 23.3%, increasing to 40.6% within 8 years [47]. In a recent review on breast reconstruction, common complication rates such as mastectomy skin flap necrosis (2–8.7%), haematoma (0.4–1.7%) and infection (2.5–4.95%) are discussed [45].
Today, the importance of avoiding complications is further stressed by the hypothesis that local and systemic inflammatory mediators may interact with tumour cells and stimulate tumour growth. A retrospective study in 229 patients with breast cancer and immediate breast reconstruction with different techniques showed a significantly lower 5-year recurrence-free survival rate in patients who had suffered a postoperative wound complication compared with those who had not [48].
Bleeding in the early postoperative period should be evacuated as a haematoma increases the risk for capsular contracture, infection and an inferior cosmetic result. Infection is a serious complication reported after approximately 6% (0–29%) of reconstructions, leading to additional surgery and implant removal in 3% (1.5–8%) [49–51].
Mastectomy skin flap necrosis is most commonly seen after skin-sparing or nipple-sparing mastectomy and immediate reconstruction. Reported frequencies are varying, reported in 2–30% of patients. Careful dissection preserving the subcutaneous fat layer and if possible the internal mammary perforators reduces the risk of necrosis. Fluorescent angiography has been suggested as a means of evaluating tissue perfusion, and care must be taken when choosing the size of implant or expander and not putting too much strain on the skin envelope [52].
The frequency of rupture is not easily assessed, as clinical evaluation and patient history are unreliable. Magnetic resonance imaging (MRI) has the highest accuracy of detecting silicone implant rupture with sensitivities ranging from 77% to 95% and specificities from 85% to 100%, while the corresponding figures are lower for ultrasound (47% to 74% and 55% to 96%, respectively). MRI is recommended for implant follow-up by the FDA, but the evidence for this recommendation is not generally accepted, and the cost is high [53]. In a 10-year follow-up of silicone implants for augmentation and reconstructive purposes, the overall Kaplan-Meier rupture rate was 13.0% for individual patients and 7.7% for implants by serial magnetic resonance imaging [23]. ◘ Figure 29.3 shows an MRI of a ruptured implant.
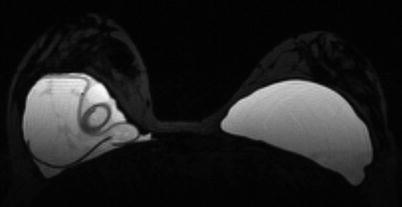

Fig. 29.3
MRI of a ruptured implant
Silicone lymphadenopathy is a foreign body reaction due to silicone leakage into the tissues surrounding the breast implant. Patients may present with a palpable mass in their axilla or in the chest wall which should be investigated as any other suspicious finding using ultrasound, fine needle aspiration or biopsy. The incidence and prevalence in women with breast implants are largely unknown. In a review [54] of 178 cases with silicone lymphadenopathy, the mean age of all implants at removal was 11 years. This figure was lower (2–6 years) in Poly Implant Prothèse (PIP) implants. Silicone lymphadenopathy does not warrant treatment unless it is symptomatic or interferes with breast cancer detection.
29.4 Capsule Formation: A Foreign Body Response
First of all, it is important to understand the difference between the formation of a capsule surrounding any foreign object implanted into the body and the contracture of that same capsule. The formation is initiated by the recognition of a foreign body, i.e. the implant, by the host immune system, staging an inflammatory response in order to eliminate the intruder. The term «biofouling» describes the formation of a matrix around an implant that is fuelled by vascular injury and the deposition of blood proteins and thrombotic substances. Inflammatory cells, including phagocytes, macrophages and foreign body giant cells, are recruited to the site of injury but enter a stage of chronic inflammation due to the inability to remove the foreign body. The ensuing granulation tissue around the implant subsequently develops into a fibrous capsule (reviewed in [55, 56]). Attempts to mitigate the foreign body response include the mimicking of human tissue by newer biomaterials, the designing of modified surfaces as well as molecular and cell-based strategies.
One should not forget that the formation of a capsule contributes to the maintenance of the implant position, while capsular contracture may result in cosmetically inferior outcomes, pain, lack of softness and deformity. It is the most common reason for revision surgery following breast augmentation; even after revision surgery, most commonly including the division or removal of the capsule, recurrence of capsular contracture is frequent. The risk for capsular contracture is increased by bacterial contamination, subclinical infection with the development of a biofilm, smooth versus textured surface structure, subglandular versus submuscular placement of the implant, smoking, postoperative haematoma or seroma, long implantation time and radiotherapy (reviewed in [13, 44]). The rate of capsular contracture is 20–40.4% after immediate and 17% to 26.4% after delayed breast reconstruction [44, 57, 58] but depends significantly on follow-up time and method of evaluation. The bacteria most commonly found in cases of capsular contracture are Staphylococcus epidermidis, deriving from contaminated ducts and breast parenchyma and readily adhering to silicone surfaces [59]. Therefore, many strategies to avoid capsular contracture, such as funnel-aided implant insertion, nipple coverage and antiseptic or antibiotic irrigation, are aiming at the avoidance of bacterial contamination [60]. Histologically, the contracted capsule is characterized by increased thickness, collagen fibre alignment and the presence of contractile myofibroblasts [61]. Clinically, degrees of contracture are most commonly measured using the subjective Baker scale (◘ Table 29.1a) which was, however, not designed for implant-based breast reconstruction. An attempt at a modified scale was therefore undertaken by Spear and Baker in 1995 [62], adding one subgroup to the four-grade Baker scale (◘ Table 29.1b). Applanation tonometry, using a transparent standard-weight Plexiglas disk with concentric imprinted circles may present a somewhat less subjective method for the evaluation of breast compressibility [63]; breast MRI offers an even less subjective yet expensive measure. ◘ Figure 29.4 shows a mammogram showing capsular contracture and rupture, ◘ Fig. 29.5 shows a patient with asymmetry as a result of capsule formation on the right and ◘ Fig. 29.6 shows an explanted implant and capsule after explantation and capsulectomy.
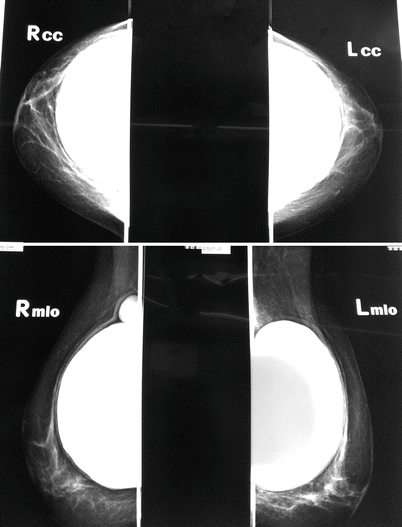
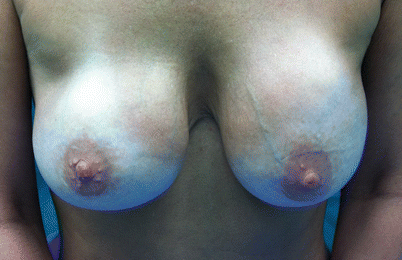
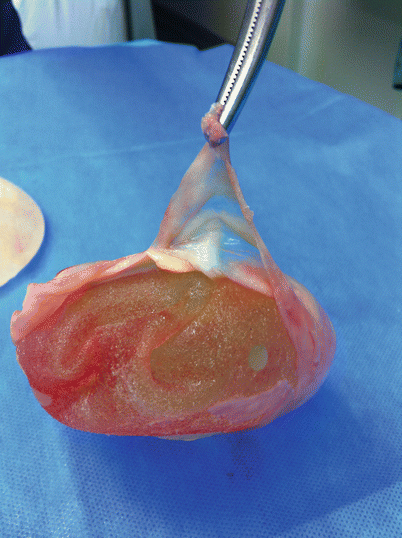
Table 29.1a
Original Baker classification of capsular contracture after breast augmentation
Class I | Breast absolutely natural; no one could tell breast was augmented |
Class II | Minimal contracture; I can tell surgery was performed, but patient has no complaint |
Class III | Moderate contracture; patient feels some firmness |
Class IV | Severe contracture; obvious just from observation |
Table 29.1b
Spear modification of Baker classification of capsular contracture after breast reconstruction
Class IA | Absolutely natural; cannot tell breast was reconstructed |
Class IB | Soft, but the implant is detectable by physical examination or inspection because of mastectomy |
Class II | Mildly firm reconstructed breast with an implant that may be visible and detectable by physical examination |
Class III | Moderate firm reconstructed breast. The implant is readily detectable, but the result may still be acceptable |
Class IV | Severe capsular contracture with an unacceptable aesthetic outcome and/or significant patient symptoms requiring surgical intervention |

Fig. 29.4
Mammogram showing capsular contracture and rupture

Fig. 29.5
Patient with asymmetry as a result of capsule formation on the right

Fig. 29.6
Explanted implant and capsule after explantation and capsulectomy
Radiotherapy is a strong risk factor for capsular contracture and has become a significant problem in breast reconstructive surgery with the increasing use of postmastectomy radiotherapy following an EBCTCG overview in 2005 [64] showing a benefit of postoperative irradiation even in patients with 1–3 positive axillary lymph nodes. Rates of Baker grade III/IV capsular contracture are significantly increased [65–67] as are overall revision rates, postoperative complications, inferior cosmetic results and reduced patient satisfaction [68–73]. The delivery of postoperative radiotherapy is not hampered [74–76] by the presence of a breast implant, at least not as long as no integrated magnetic dome is used that might disturb computed tomography (CT)-based radiotherapy planning. It has also been argued that in the event of a radiation field covering the internal mammary nodes, which may again become more common after recent oncological results [77], a breast implant increases the lung volume unintentionally irradiated. The effect of irradiation on the implant itself has been little described, but, recently, changes in a nanoscale surface analysis have been reported [78]. This being said, one should not forget that autologous flaps are also exposed to radiation damage in case of immediate autologous reconstruction and postoperative radiotherapy; this choice is therefore not very commonly used.
In the few reports comparing prior to post-reconstruction radiotherapy, the former has a significantly higher rate of complications and implant failure and thus predisposes more to delayed autologous reconstruction [73, 79, 80]. While prior radiotherapy therefore should be regarded as a strong caveat for implant-based options, postoperative radiotherapy is no longer viewed as a contraindication against immediate breast reconstruction in the well-informed patient [81]. A majority of patients still keep their implant-based reconstruction, and relatively few are converted to autologous tissue reconstruction [82]. It must be stressed, however, that the discussion of implant-based reconstructive surgery in the face of radiotherapy must weigh together all risk factors, patient wishes and planned oncological treatment in order to achieve safe reconstructive surgery.
29.5 Breast Implants: Indications for Use
Even though the most common use of breast implants is for breast augmentation in cosmetic surgery, implant-based breast reconstruction has become more and more common. Implants may be used for breast reconstruction in most women, both for immediate and delayed reconstructions. The advantages of implant reconstruction include a relatively short and economically advantageous procedure with no additional scars or other potential complications at a donor site [70]. If performed immediately, the three-dimensional breast envelope and in selected cases also the nipple-areola complex may be preserved, and a number of women may prefer the relatively limited extent of surgery and the fact that breast reconstruction already has begun once waking up from the primary cancer operation. There is no strong evidence favouring immediate versus delayed reconstruction [83], the discussion of which would exceed the scope of this chapter. Known disadvantages of implant-based reconstruction are the relative firmness and fixation of the breast to the thoracic wall, its inability to age with the patient and adapt to weight changes and recognised difficulties to achieving natural ptosis. Whether this dilemma, which is accentuated in the face of radiotherapy, may be mitigated by the use of acellular dermal matrix [84] and other materials to preserve natural breast ptosis and the inframammary fold will need to be shown in future trials. Another strategy managing possible irradiation consequences and maintaining the option of implant-based immediate reconstruction is the concept of immediate-delayed reconstruction, using a tissue expander in the immediate setting which may then be exchanged for autologous tissue with or without an implant once postoperative radiotherapy has been delivered [85–87].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







