Breast cancer is an important health care problem since it is the most frequently occurring cancer (1 out of about 10 women) and among the major causes of death in women in the Western world. Despite many studies, breast carcinogenesis is still not well understood. Although many breast cancer risk factors have been identified, they do not easily translate into molecular changes that help to understand why normal breast cells derail to form early lesions that then accumulate further genetic events that make them eventually progress to cancer. Discussing these risk factors is therefore beyond the scope of this chapter. We will also stay away from providing endless lists of possibly relevant individual genetic aberrations. Rather, we will try to integrate the fairly fragmentary knowledge of genetic aberrations and changes in gene expression into progression models based on long-standing morphologic progression models. This is quite challenging, since breast cancer is a very heterogeneous disease, far beyond the so-called ductal and lobular lesions that are best known. Nevertheless, these morphologic progression models have proven to provide a proper framework to depict and understand how different early lesions may progress to cancer, and help to place relevant genetic changes into the different progression routes to cancer. Further, they have been proven to be clinically relevant, in the sense that relative risk of these lesions to progress to cancer is known from long-term follow-up studies. This has become the base for clinical management of such lesions when found in a breast biopsy, for example, after a mammographic abnormality on breast screening.
The epithelial progression routes comprise those for ductal lesions, columnar cell lesions, lobular lesions, papillary lesions, apocrine lesions, mucinous lesions, medullary lesions, metaplastic lesions, secretory lesions, and adenomyoepithelial lesions. However, in this chapter we will focus on conceptual issues that are shared by the progression routes for these different lesions, and then shortly try to place different precursors within progression routes for epithelial breast cancer. In addition, carcinogenesis in hereditary cancer syndromes will be discussed separately.
The fibroepithelial route comprises progression from fibroadenoma and related lesions to malignant phyllodes tumors. Before going into some of these individual progression routes in detail, we will provide a general framework for breast carcinogenesis, integrating morphologic, immunohistochemical, and genetic findings.
The pathogenesis of invasive breast cancer has been under debate for decades, and many progression models have been proposed, incorporating the epidemiologic and genetic findings at the respective times. However, some of these models and concepts were not able to generate a global understanding for many aspects of this threatening disease.
In the general sense, (sporadic) breast carcinogenesis is not essentially different from carcinogenesis of other epithelial malignancies. Tumor suppressor gene inactivation through promoter methylation is likely among the earliest events in breast carcinogenesis.1,2 Besides, loss of heterozygosity may occur already early in breast carcinogenesis as a means of inactivating crucial genes.3,4
These events will, for example, affect DNA repair, which provides a background for accumulation of more genetic events. The resulting disruption of cell cycle control mechanisms and/or apoptosis-signaling pathways leads to disturbance of the balance between proliferation and apoptosis,5 in turn leading to growth of early lesions, which are usually polyclonal. Activating mutations in and amplifications of (proto)oncogenes, as well as chromosomal imbalances, and inactivating mutations in tumor suppressor genes (such as p53), usually kick in later, eventually leading to selective outgrowth of clones with the highest proliferation/apoptosis imbalance. Increased proliferation will lead to cells that are too far from the nearest blood vessels to sustain oxygen and nutrient supply levels, leading to hypoxia. This sets off a cellular survival program to cope with the hypoxic stress, inducing growth-factor—mediated angiogenesis and switch to anaerobic metabolism. In the stage of DCIS, angiogenesis is induced, often presenting as a network of vessels around the malignant ducts.6 However, lymphangiogenesis does not seem to play a role.6
In the latest pre-invasive stages, invasion-related genes become activated, allowing cells to break down the basement membrane and extracellular matrix to invade the surrounding stroma (see Chapter 2 for an extensive overview). This provides access to lymph and blood vessels (which occurs independently), allowing cells to enter the lymphatics to pass on to lymph nodes to form locoregional metastases, as well as the bloodstream to form distant metastases (see Chapter 3 for an extensive overview). For the latter, circulating tumor cells must home to the distant site, adhere to the endothelium, invade in the local tissue, and again create the optimal microenvironment to escape dormancy and grow out to clinically manifest metastases. This may need additional genetic hits.7 During this complex multistep process, aberrant cells must all the time escape from the immune response, in which down-regulation of Human Leukocyte Antigen (HLA) molecules plays an essential role. The concept of epithelial—mesenchymal transition (EMT), a currently very hot topic in molecular in vitro studies thought to play a main role in invasion and metastasis formation, is difficult to translate to human breast tumors, as loss of epithelial differentiation is very rare in human tumors including those from the breast.
Many genes play a role in the above process, and it would be impossible to mention them all while keeping an overview. Instead, let us just mention those perhaps most important. The estrogen and progesterone receptors are highly expressed in almost all preinvasive breast lesions, stimulating proliferation. Interestingly, expression of these steroid receptors is somewhat lower in invasive cancers. Many cell-cycle control proteins are involved. Cyclin D1 may be amplified or induced in an ER-dependent manner,8 and cyclin E is also often overexpressed.9 Cyclin-dependent kinases like CDK4 can be overexpressed,10 and CDK inhibitors like p27 and p21 may get deregulated.11 Often p53 is mutated,12 and c-myc may be amplified.13 Several DNA repair genes may be affected by sporadic inactivating mutations like BRCA1, BRCA2, ATM, and CHEK2. Many growth factor receptors like HER2 and epidermal growth factor receptor (EGFR) are often overexpressed,14 the former due to gene amplification, and many growth factors are overexpressed in stroma or epithelium to provide paracrine or autocrine loops.15,16 HIF-1a mediates the hypoxia response17 to increase cellular survival and strengthen malignant transformation.18 The E-cadherin gene is often inactivated (especially in lobular lesions, discussed below), leading to decreased cell—cell contact, facilitating invasion and metastatic spread. hTERT must be activated to ensure proper telomere length for sustained cell division.19 TWIST20 and Notch121 seem to play a role in metastases formation. As to Wnt signaling, it appears that DNA hypermethylation leads to aberrant regulation of the Wnt pathway in breast cancer rather than mutations in, for example, APC.22
MiRNAs are now recognized as a highly abundant class of regulatory RNA molecules. Iorio and associates identified 29 miRNAs to be deregulated in breast cancer by microarray analysis.23 MiRNA clusters that seem to be involved in breast carcinigenesis comprise miR-21, miR-29b-2, miR-125b, miR-145, miR-10b, miR-155, miR-17-5p, and miR-27b.23,24
Translocations in breast cancer occur probably rarely, but one leading to the ETV6-NTRK3 fusion gene, which encodes a chimeric tyrosine kinase, is very characteristic for the secretory type of breast cancer.25,26
Microarray studies have also shed light on breast carcinogenesis. Sørlie and associates analyzed 78 breast cancers by microarray analysis, and based on gene expression profiles, cancers could be classified into a basal epithelial-like group, a HER2-overexpressing group, and a group of luminal cancers that seemed to split up into at least 2 subgroups (Fig. 1-1).27 This classification appeared to have prognostic value. This paves the way for a molecular classification of breast cancer, which is, however, paralleled by expression of common immunohistochemical markers: basal cancers (CK5 and/or CK14 positive and ER/PR/HER2 negative), a group of cancers dictated by HER2 amplification/overexpression, and a group of cancers characterized by expression of ER while HER2 negative. This latter subgroup may be heterogeneous.
Figure 1-1
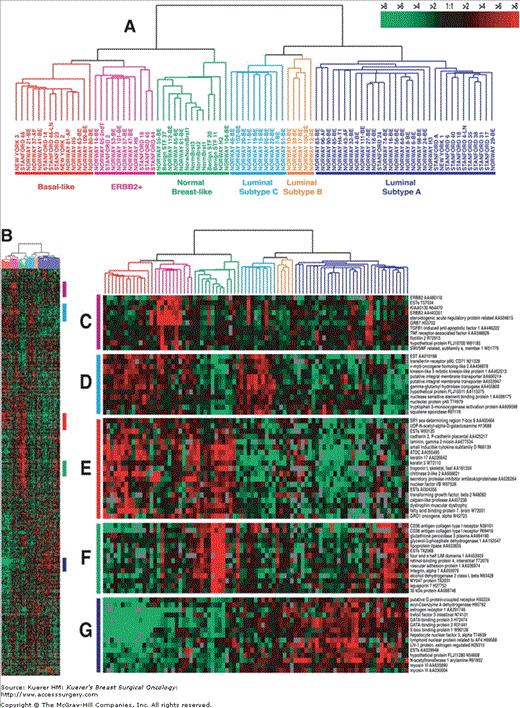
Gene expression patterns of 78 carcinomas, 3 benign tumors, and 4 normal tissues, analyzed by hierarchical clustering using the 476 cDNA intrinsic clone set.27 The cluster dendrogram showed 3 main subtypes of cancer: luminal, basal-like, and HER2+. Reproduced with permission from reference 27.
There is no established role for viruses in breast carcinogenesis. Some studies have suggested a role for oncogenic viruses such as human papilloma viruses (HPV) and Epstein—Barr virus (EBV), but results have been inconsistent. Some studies claim to have found EBV sequences in breast cancers by PCR, but this approach lacks specificity, since infected lymphocytes within the tumor easily lead to PCR products. Using an immunohistochemical approach, some studies have described presence of EBNA1, but the used antibody later appeared to cross-react with MAGE4.28 Several studies have reported presence of the oncogenic HPV types 16, 18, and 33 in invasive breast cancer, including a rare case of lymphoepithelioma-like breast cancer, a type previously thought to be associated with EBV infection.29 An association with cervical HPV induced lesions has been suggested.30 Although one study reported that HPV 16 E6/E7 proteins converted noninvasive human breast cancer cell lines to invasive cells,31 the etiologic role of oncogenic HPV infections remains to be proven.
Although some of these genetic events occur more often early, or late, in carcinogenesis, breast carcinogenesis cannot be viewed as a stepwise fixed genetic progression model, as has been suggested for colorectal carcinogenesis. Rather, breast cancer is to be viewed as the result of accumulation of various major and minor genetic events in a fairly random order, which we like to refer to as the “bingo principle” analogous to winning the “prize” (in this case cancer) in this popular game.
Nevertheless, these global mechanisms need adjustment to the very specific background of the normal human breast. This general concept has been challenged and needs modification according to latest genetic, morphologic, and immunohistochemical findings.
Most (pre)invasive lesions of the breast are thought to derive from the transition zone between the ducts and the functional unit of the breast, the lobule, which is composed of acini that are lined by an outer myoepithelial layer and an inner luminal or glandular layer. However, more recent studies give evidence that the inner, glandular layer contains a putative stem or progenitor cell compartment, which gives rise to the above-mentioned cells. These cells have been recently described and characterized more in detail. It is noteworthy that many characteristics of these cells are shared in mouse and human cells. A hallmark of these cells is the expression of high molecular weight cytokeratins (Ck 5/14), EGFR, and the lack of estrogen receptor expression. In contrast, more differentiated glandular cells are defined by the expression of low molecular weight cytokeratins (Ck8/18) and positivity for the estrogen receptor, whereas myoepithelial cells maintain the expression of Ck5/14 in addition to the expression of smooth-muscle actin (SMA) and p63. At present, the relationship between these cells and breast cancer—specific stem cells is unclear. Regardless of that, all these cells and presumed intermediate cells within the extremes of differentiation are targets for carcinogenic hits and potential precursor cells for the variety of morphologically different breast cancer subtypes. However, these cells can serve as a tool to explain the presence of monoclonal patches within a breast lobule or parts of the ductal tree. In addition, the description of nonrecurrent genetic changes within the morphologically normal breast tissue, requiring a large subset of affected cells, favors the idea of long-living cells as targets of the initial hit starting the genetic cascade towards an overt malignancy.
The finding of genetic changes within morphologically normal breast tissue is nowadays not only associated with an increased local recurrence risk but had also an tremendous influence on the validity of progression models of breast cancer and especially the relationship toward proposed precursor lesions. The finding of identical genetic alterations within two different precursors can therefore not automatically be interpreted as proof of a direct relationship, but might rather be regarded as a suggestion that both lesions have derived independently from a common precursor.
It has been a long-noted interesting phenomenon that pre-invasive lesions tend to progress while maintaining their morphologic differentiation status, usually referred to as “progression within grade.”32 This means that well-differentiated (grade I) pre-invasive lesions tend to progress to well-differentiated invasive lesions, and that poorly differentiated (grade III) pre-invasive breast lesions usually progress to poorly differentiated invasive lesions. Even distant metastases are usually of the same grade as the primary tumor. Naturally, intermediate lesions (grade II) occur, but to discuss them would complicate the depicted concepts considerably, so for the sake of clarity we will further focus on the extreme ends of differentiation.
With the establishment of new global, genetic screening techniques such as comparative genomic hybridization (CGH), a pattern of genetic alterations, characteristic of distinct grades and morphology, has emerged. The loss of chromosomal 16q-losses occurred as the hallmark of well-differentiated intraductal as well as invasive breast cancer cases. However, this finding, as will be discussed later, was not new. The oldest descriptions of 16q losses were based on classical cytogenetic approaches using G-banding of metaphase chromosomes. It became obvious that the loss of 16q was often due to an unbalanced chromosomal translocation t(1;16)33 and was associated with a decreased rate of lymph node metastasis, an increased expression of the estrogen and progesterone receptors34-37, a low tumor proliferation rate and an improved overall survival35,38-40. In a few cases, t(1;16) was the sole cytogenetic abnormality,41 underlining the importance of 16q losses. Since the breakpoint of the recurrent t(1;16) was located in the centromeric heterochromatin of chromosome 16, no specific genetic gene fusion transcript resulted from this chromosomal alteration, and speculation about gene dosage effects regulating the expression of respective candidate genes started. However, the consequences of this hypothesis have not been pursued, perhaps due to lack of suitable technical approaches to answer the question.
In parallel to the extensive characterization of breast cancer by conventional cytogenetics, the definition of the 16q-specific tumor suppressor gene was based on LOH analysis with a multitude of microsatellite markers covering the whole 16q arm. It was finally shown that probably 3 different shortest regions of overlap for the loss of 16q sequences in breast cancer exist. It is noteworthy that the different studies revealed partially different, and partially overlapping, results.42 Of even higher importance was the finding that 16q losses seemed to occur as rather early events in breast carcinogenesis, shown by the detection of 16q losses in ductal (DCIS) and lobular carcinoma in situ (LCIS)43,44 as well as in atypical hyperplasias.4,45
However, a part of these results might have been biased due to two circumstances. On the one hand, classical cytogenetic results could only be generated for a limited number of cases, due to technical difficulties in culturing breast cancer cells. On the other hand, the application of LOH analysis generally only allows a limited statement about the general chromosome 16q status, as discussed more extensively later in the chapter. With the introduction of technical approaches enabling a global overview of unbalanced chromosomal alterations in paraffin-embedded invasive carcinomas and their precursors, these restrictions could be overcome in a single step.
First studies on genetic changes in DCIS demonstrated that, besides gains of 1q, 16q-losses are the most recurrent changes in DCIS, already detectable at a precursor level of invasive breast cancer. However, the distribution of 16q losses in the wide morphologic spectrum of DICS showed that this distinct chromosomal alteration could predominantly be detected in well and intermediately differentiated DCIS, whereas in poorly differentiated DCIS the incidence of this change was rather low.46-48
In further studies it could be shown that this grade-dependent distribution of 16q losses was maintained in invasive carcinomas. Especially tubular, tubulo-lobular, and ductal invasive grade 1 breast cancers were characterized by 16q losses, whereas ductal invasive grade 3 carcinomas showed this alteration in a significant lower frequency.49-51 More detailed studies in ductal invasive grade 3 carcinomas could further show that this chromosomal alteration is also rather uncommon in HER-2 amplified and/or overexpressing carcinomas52 and in the recently rediscovered basal, triple-negative breast carcinomas.53,54 Data gathered in extensive studies involving more than 100 breast cancer cases further showed that 16q was associated with prognostically favorable features, such as a low proliferation rate in invasive and in situ breast cancer, and the expression of ER and PR. However, the explanation of 16q losses in grade 3 breast cancers allows two conclusions. On one hand, it might be that this subgroup has evolved through grades 1 and 2 ER-positive carcinomas; on the other hand, some studies can lead to the conclusion that 16q losses in these tumors are a reflection of cytogenetic instability in different subclones within a tumor.32 More recent studies of Korsching and associates support the first hypothesis.55
Interestingly, the underlying mechanisms of 16q losses in grades 1 and 3 ductal invasive breast cancer cases seem to differ significantly. Several studies with polymorphic markers could detect no difference in frequency of 16q LOH between invasive tumors of different histologic grades. However, by combining 16q LOH fluorescence in situ hybridization with chromosome 16—specific probes and CGH, it could be demonstrated that losses of chromosome 16q occur preferentially in well-differentiated (grade I) carcinomas, whereas in poorly differentiated grade III tumors, LOH was accompanied by mitotic recombination. These results clarified the discrepancies between CGH and LOH for 16q in breast cancer.56
The exceptional role of 16q in lobular differentiated breast cancer and its ultimate precursor, LCIS, was well known for years. With the routine use of global screening techniques such as different CGH methods, the genetic homology between LCIS and invasive breast cancer became even more prominent. About 60% to 70% of LCIS and lobular invasive breast cancer is characterized by a loss of 16q. Interestingly, as described also for early intraductal breast lesions such as well-differentiated DCIS and ADH, the loss of 16q has been described as the sole abnormality in synchronous, bilateral LCIS without associated invasive breast cancer.57
Integrating all these data into a unifying model of breast carcinogenesis, it becomes evident that the very simplified model derived from the understanding of colorectal carcinogenesis is of little help for breast cancer. Rather, the distribution of 16q loss in preinvasive or invasive breast lesions clearly points towards the existence of different pathways, associated with different malignancy grades. One could therefore propose low-grade and high-grade pathways in ductal breast carcinogenesis. The latter is characterized by a multitude of different genetic alterations and protein expression patterns (p53, HER2, CK5) in invasive breast cancer and its associated DCIS. In contrast, hallmarks of the low-grade pathway are the loss of 16q, the expression of ER, and a likewise lower degree of genetic instability.
These observations are therefore not only of tumor biological interest but also significantly influence our understanding of the classification of early breast lesions and invasive breast cancer. The most recent classification of breast cancer precursor lesions was proposed by the WHO in 2003. In analogy to a multitude of other “intraepithelial neoplasia” classification systems, such as in the cervix or squamous epithelium, these systems suggest a linear progression of grade 1 to grade 3 and finally to invasive carcinoma. However, as discussed above, this simple concept, transferred from other tumor entities, does not seem to hold true for breast cancer. Since the distribution of 16q losses changes significantly throughout grade, it is unlike that well-differentiated DCIS progresses towards poorly differentiated DCIS in a high frequency. The morphologic association of well-differentiated DCIS with tubular, tubulo-lobular, and ductal invasive grade 1 carcinomas, in contrast to the poorly differentiated DCIS/ductal invasive grade 3 carcinoma pathway, also points against this hypothesis. In consequence, our current understanding of breast cancer has to incorporate the presence of multiple genetic pathways in the progression of in situ and invasive breast cancer, as reviewed by reference.58
The role of stem cells in the breast carcinogenetic process is not entirely clear. Breast stem cells are pluripotent, and are characterized by expression of basal cytokeratins 5, 14, and 17, EGFR, and P-cadherin, while lacking expression of HER2, ER, and CK18. They have a variable location within ducts and acini. They may differentiate into luminal cells (expressing cytokeratin 8/18) or into myoepithelial cells (retaining CK5 and CK14 expression, and acquiring expression of contractile proteins such as smooth-muscle actin and calponin). These expression patterns of stem cells and glandular cells parallel those of different types of invasive breast cancers (Fig. 1-2). In this sense, (adeno)myoepithelial carcinomas, metaplastic carcinomas, BRCA1-associated carcinomas, medullary carcinomas, and basal carcinomas have expression patterns like stem cells, being further characterized by a high frequency of EGFR amplifications, p53 mutations, and BRCA1 dysfunction (by mutation or promoter methylation) and a low frequency of 16q loss. Tubular, lobular, and non—high-grade invasive ductal carcinomas share their immunophenotype with glandular cells, usually further showing 16q loss while lacking p53 mutations, EGFR amplifications, and BRCA1 dysfunction. Basoluminal and ductal invasive grade 3 cancers fall in between, typically with HER2 amplification.
Figure 1-2
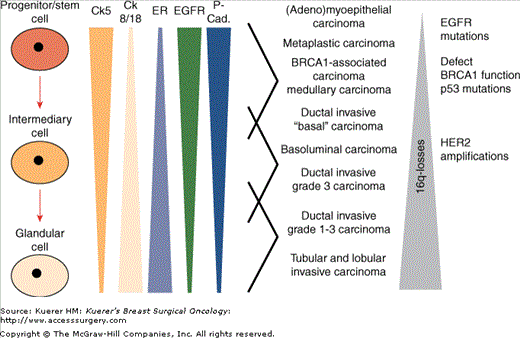
Associations of immunohistochemical expression patterns in physiological cellular subgroups within the normal breast and distinct subgroups of invasive breast cancer. (Reprinted with permission from Korsching E, Jeffrey SS, Meinerz W, et al. Basal carcinoma of the breast revisited: an old entity with new interpretations. J Clin Pathol. 2008;61:553-560.)
This gives rise to proposing carcinogenetic relationships between these normal breast cells and different histologic subgroups of invasive breast cancer. Two theories are possible (Fig. 1-3). In the “Linear cell of origin theory”, vastly overlapping expression profiles between physiological progenitor cells and invasive breast cancer supports the conclusion that the respective carcinoma subgroups originate in their respective physiological counterparts: stem cells, intermediate cells, or luminal cells. Whereas 16q-losses are rare events in breast cancer types with expression of CK5/14, the incidence increases with lower tumor grade. In contrast, EGFR amplifications, p53-mutations and defects/losses of BRCA1 are almost exclusively seen in high grade carcinomas that show CK5/14 expression. HER2-amplifications are rare in CK5/14 positive carcinomas. The “stem cell hypothesis” proposes that stem cells are the major target in the pathogenesis of different breast cancer subtypes. A multitude of genetic alterations can take place in these cells, starting distinct cellular expression programs, including the change of cytokeratin expression pattern characterizing distinct subgroups of invasive breast cancer. As often with different hypotheses, there may be truth in both of them.
Figure 1-3
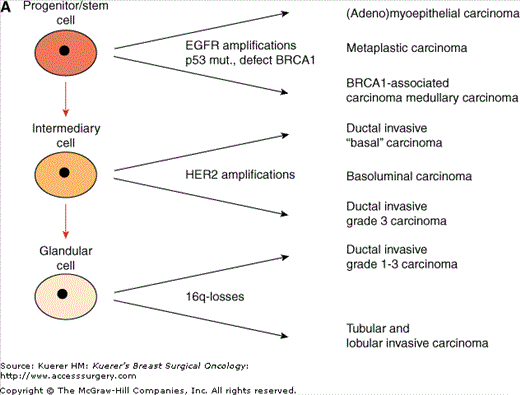
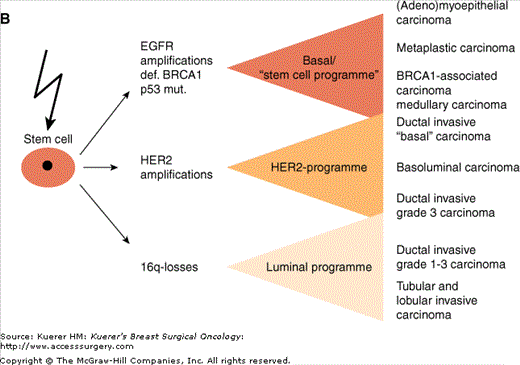
Different putative histogenetic models of the relationship between different subgroups of invasive breast cancer and breast stem cells. A. The “linear cell of origin theory” proposes that the respective carcinoma subgroups originate in their respective physiologic counterparts of the normal breast: stem cells, intermediate cells, or luminal cells. B. The “stem cell hypothesis” proposes that stem cells are the major target in carcinogenesis and progress after a multitude of genetic alterations that start distinct cellular expression programs to distinct subgroups of invasive breast cancer. (Reprinted with permission from Korsching E, Jeffrey SS, Meinerz W, et al. Basal carcinoma of the breast revisited: an old entity with new interpretations. J Clin Pathol. 2008;61:553-560.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree