© Springer International Publishing AG 2018
Marissa Howard-McNatt (ed.)Changing Paradigms in the Management of Breast Cancer https://doi.org/10.1007/978-3-319-60336-0_1414. Breast Cancer Treatment in Young Women
(1)
Mount Sinai Beth Israel Hospital, 10 Union Square East, Suite 4E, New York, NY 10003, USA
Keywords
Premenopausal womenBreast cancerFertilityBRCAPanel testingSexualityIntroduction
Breast cancer affects one in eight women. Of the women who are diagnosed with breast cancer, 25% are premenopausal, and 15% are under the age of 45 [1]. Premenopausal women face different issues than postmenopausal women. All women under the age of 50 who are diagnosed with breast cancer may have a genetic mutation and should undergo genetic testing according to the NCCN guidelines. This is a complex psychosocial issue , in the era of panel testing. Patients may have a low penetrance cancer mutation, which does not mandate prophylactic contralateral surgery, but this may be difficult to explain to patients in the midst of a breast cancer diagnosis. Additionally, as women delay childbearing, many premenopausal breast cancer patients may not have had children. Patients should be offered fertility preservation, which in turn can be expensive and can delay treatment. Finally, there are many psychosocial issues that younger breast cancer patients face, which are unique to younger patients.
Younger women may have more aggressive subtypes of breast cancer. Some of these subtypes, including triple-negative breast cancer, are more prevalent in young women, especially BRCA 1 carriers, African-Americans, and Latino women [2]. African-American women have been shown to be diagnosed at earlier ages [2]. All of these factors contribute to adjuvant treatment, which usually involves chemotherapy in triple-negative breast cancers.
Fertility
Fertility preservation is a crucial factor for many patients to consider while undergoing treatment during their childbearing years. One of the many side effects of chemotherapy in young women is an increased risk of infertility. Chemotherapy leads to follicle loss in the ovaries due to apoptosis. Since women are born with all of their follicles, once follicles are damaged or lost, there is no way to regenerate new ones. In addition, certain hormonal treatment modalities, such as tamoxifen, have been shown to delay pregnancy for an average of 5 years due to risk of teratogenicity. As many as 73% of women aged 36–40 undergoing certain chemotherapy regimens face amenorrhea, and even if menstruation returns, fertility does not. There are many methods of fertility preservation for women who are undergoing breast cancer treatments.
The primary method of fertility preservation is embryo cryopreservation, which must take place prior to chemotherapy treatment [3]. Embryo cryopreservation is a three-step process. Initially, hormones are taken to mature the follicles, followed by in vitro fertilization (IVF), and finally the embryo is frozen for implantation at a later time. A second viable option for patients is oocyte cryopreservation, in which the oocyte, or egg, is frozen as an alternative to the embryo. This method of fertility preservation may be a more plausible option for women without a male partner to fertilize the oocyte. In 2012, oocyte cryopreservation became a standard recognized treatment by the American Society of Reproductive Medicine, in addition to embryo cryopreservation. However, there are some risks associated with undergoing these cryopreservation procedures. Primarily, chemotherapy must be delayed for an average of 2–6 weeks, until the follicles have matured. Delaying the initiation of chemotherapy may allow more aggressive cancers to progress. In addition, for patients with estrogen receptor-positive (ER+) cancers, the supplementation of additional hormones that increase estrogen levels can lead to progression of the cancer.
Recently, additional methods of fertility preservation have been investigated. One method that requires no hormonal stimulation, no male partner, and a shorter treatment delay is ovarian tissue cryopreservation for reimplantation. Women undergo an oophorectomy, and the removed ovarian tissue is preserved through cryopreservation, until it is later implanted back into the patient. While many of the previous risks are not a threat with this type of procedure, there is a chance that cancerous cells may be reintroduced into the body through ovarian tissue. This method is still under investigation and is not yet considered a standard treatment. Similarly, there is another investigational option, in which the cryopreserved ovarian tissue is matured in vitro, followed by IVF, and a subsequent uterine transfer (IUI). This option may be beneficial as it limits the risk of cancer cell reintroduction. This method is not yet considered standard treatment.
A less invasive method of fertility preservation, gonadotropin-releasing hormone (GnRH) agonist , has been shown to help some patients; however the supporting data is controversial. This hormone agonist acts as a competitive inhibitor and is thought to prevent follicles from undergoing apoptosis, while minimizing ovarian and uterine perfusion, and protecting germline stem cells, therefore reducing the infertility itself from occurring. Unfortunately, despite various research trials, there is no consensus on the benefits of this treatment [3].
Due to the time-sensitive nature of cancer treatment, it is important for women to be aware of their fertility preservation options in order to make a prompt decision on which method if any to proceed with. With such a large number of young women facing breast cancer and chemotherapy, fertility preservation is becoming a substantial aspect of cancer treatment for many.
Genetic Testing
Genetic counseling and testing is standard of care in premenopausal breast cancer patients. Diagnosis at a young age, specifically below 50 years old, should raise concern for a genetic mutation, in addition to a strong family history of the same or similar cancers and multiple affected family members in multiple generations. In fact, 6.2% of screening mammography populations are considered to be at high risk for a hereditary breast cancer gene, significantly elevating their lifetime cancer risk [4].
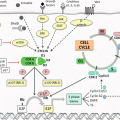
BRCA 1 (17q21) and BRCA 2 (13q12.3) are two genetic mutations most indicative of elevated breast cancer risk. These normally function as tumor suppressor genes likely involved in DNA repair and regulation of the cell cycle. When mutated, the genes fail to properly function and lose the ability to appropriately control cell division and tumor growth. To date, over 1000 mutations have been discovered. Since the BRCA genes are inherited in an autosomal dominant fashion, only one copy of the mutated gene is necessary to cause an increased cancer risk. Approximately 1/500–1/800 individuals in the general population have either the BRCA 1 or BRCA 2 mutation, which has an associated increased risk of not only breast cancer but also ovarian, pancreatic, prostate, and colon cancers and melanoma [5]. Furthermore, within the Ashkenazi Jewish population, the prevalence rate of BRCA mutations is as high as 1/40. Compared to the average population which has an 8% risk of developing breast cancer and less than a 1% risk of developing ovarian cancer by age 70, BRCA-positive patients have up to an 87% risk of developing breast cancer and a 44% risk of ovarian cancer [5].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree