Breast cancer is the most common malignancy affecting American women excluding skin cancers. It is the second leading cause of cancer-related deaths, having been surpassed by mortality from lung cancer. The American Cancer Society estimated that there would be 182,460 new cases of breast cancer and 40,480 breast cancer-related deaths in 2008.1 Breast cancer mortality in the United States has declined substantially over the past 30 years, from 31.4 deaths per 100,000 women per year in 1975 to 25.9 deaths per 100,000 women per year in 2001.2 The reduction in breast cancer mortality has been attributed to advances in treatment options and the combination of increasing utilization of screening mammography and improved mammographic quality.3,4 Mammography remains the only study proven to detect early breast cancer and decrease breast cancer–related deaths.
An additional important goal of screening mammography is to identify cancers when they are small, and subsequently at an earlier stage. In a study conducted by Barth and associates,5 the tumor size of patients who had breast cancer detected by mammography was 1.5 cm, which was statistically significantly smaller than the cohort group whose breast cancer was detected by physical exam and in which tumor size was on average 2.9 cm. The screening-detected cancer patients were also more likely to be node negative and less likely to receive chemotherapy. Similar results have been reported in populations with high mammographic screening rates, such as in Rhode Island.6 Ductal carcinoma in situ (DSIS), which is believed to progress to invasive carcinoma in certain patients, is clinically occult and only identified by mammography.
The benefits from screening mammography for women age 40 to 70 years have been proven in 8 randomized controlled trials (RCTs) during the past 40 years.7-14 These trials were conducted in Europe (Edinburgh, Malmö, Gothenburg, Stockholm, Swedish Two County), Canada (includes 2 trials: age 40-49 and age 50-59), and the United States (Health Insurance Plan Project). Table 5-1 summarizes the results of these trials. All but one of the RCTs report statistically significant results as measured by mortality reduction as end-point. Reported reductions in breast cancer mortality range from -2% to 32%. In addition, long-term follow-up of 3 trials (HIP, Gothenburg, and Malmö) each found statistically significant reductions in breast cancer mortality.15-17 The only RCT to actually show mortality in the screening group is the Canadian study for women aged 40 to 49 years. This study has been highly criticized for the poor image quality and for including patients with clinically evident advanced breast cancer.18 The benefits of mammographic screening are in general accepted as valid. Controversy remains for screening women younger than the age of 40 years; in this group there are few data from RCTs. Therefore, decisions concerning screening practice in this age group must be based on less rigorous evidence and on a more individual basis.
| Trial | Age (yr) | Screening Interval (mo) | Mortality Reduction (%) |
|---|---|---|---|
| Swedish Two County13 | 40–74 | 24–33 | 32 |
| Edinburgh7 | 45–64 | 24 | 29 |
| Malmö8 | 45–69 | 18–24 | 23 |
| Stockholm9 | 40–64 | 24–28 | 20 |
| Gothenburg10 | 40–59 | 18 | 23 |
| HIP14 | 40–64 | 12 | 23 |
| Canadian11 | 40–49 | 12 | -3 |
| Canadian12 | 50–59 | 12 | -2 |
Mammographic screening has suffered repeated attacks, some of which have made headlines in the lay media; for example the 2001 front-page coverage by The New York Times of the Cochrane Collaboration report.19 The most flagrant assault on screening mammography in a reputable journal was reported in The Lancet by 2 members of the Cochrane Collaboration, Gotzsche and Olsen.20,21 In their reanalysis and meta-analysis of the largest RCT, they claimed that mammography caused increased mortality. It did not take long for a number of different experts to identify the biased and faulty analysis that the Cochrane Collaboration employed.18,22,23 Gotzsche and Olson excluded all the trials that showed benefit because per their analysis those studies were of poor quality. Their conclusions were based on the Canadian study results, which most experts have found to otherwise be flawed. In a more recent analysis of the Swedish Two-County Trial 20-year follow-up data, Tabar and associates reported a 44% reduction in breast cancer mortality in women aged 40 to 69 years.24 Although skepticism will reemerge regarding the benefit of screening mammography, it is doubtful that additional RCTs could be performed; it would be unethical to randomize patients to no mammographic screening when multiple large trials and clinical experience have already shown the benefits of mammographic screening.
For average-risk women, the majority of professional organizations in the United States recommend screening mammography beginning at the age of 40 and to continue annually. The American Cancer Society25 in addition recommends that women in their 20s and 30s undergo clinical breast examination once every 3 years. Although there is no evidence to recommend routine breast self-examination (SBE), the American Cancer Society recommends that women be informed about the benefits and limitations of breast self-examination to begin at age 20. If a woman chooses to do SBE, then any changes noted are to be reported to her health care provider. Table 5-2 summarizes the current American Cancer Society guidelines.
| Annual mammogram starting at age 40 |
| Breast clinical exam age 20-39 every 3 years, annual clinical exam beginning at age 40 |
| Optional breast self-exam beginning at age 20 |
| Women to be informed of the benefits and harms of mammographic screening |
| High-risk women: additional annual breast MRI as adjunc-tive to mammography |
The time between screens is an important factor in decreasing breast cancer mortality. If too long a time were placed between screening intervals, early detection would be compromised. As noted in Table 5-1, screening intervals in all major RCTs differ, and ranged from 12 to 33 months. Most of the major American professional organizations (including the American Medical Association, American College of Radiology, and American Cancer Society) recommend screening intervals of 12 months.
The principal mammographic imaging findings of breast cancer include clustered microcalcifications, masses, architectural distortions, and asymmetries (Fig. 5-1). Comparison to prior mammograms is essential, as some cancers may only be detected by perceiving them as a subtle change from prior studies. In addition, the availability of prior mammograms for comparison can reduce the number of unnecessary call-backs for stable benign findings.26 After a finding is detected in a screening mammogram, the patient is called back for diagnostic evaluation. Additional imaging evaluation is performed to determine if the lesion is real, and if real, to plan for biopsy. Imaging modalities available for diagnostic evaluation include special view mammography, ultrasound, and in selected cases, contrast-enhanced breast MRI.
Figure 5-1
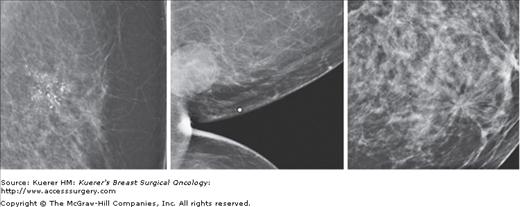
From left to right: spot compression magnification view of clustered pleomorphic calcifications, cleavage view of a round mass, spot compression view of architectural distortion. All of these abnormalities are biopsy-proven invasive ductal carcinomas that were identified at the time of screening mammogram.
The benefits of screening of younger women, defined as age 40 to 49, has been controversial because of the relatively small numbers of women in this age range who have enrolled in the individual trials. In 1997, a meta-analysis of women aged 40 to 49 years in all 5 Swedish trials found a statistically significant 29% reduction in breast cancer deaths.27 When taking into account all 8 randomized controlled clinical trials, and with an average follow-up of 12.7 years, this same meta-analysis documented an 18% reduction in breast cancer deaths. Given that younger women have a longer life expectancy and the fact that cancers tend to grow more rapidly in younger women, earlier detection of these cancers may theoretically be advantageous. However, this must be balanced by the limitations of screening mammography in younger women, which include a lower frequency of breast cancer, reduced sensitivity of mammography, slightly increased radiation risk, and higher recall rates. Screening of younger women will be of most benefit in women at high risk, especially those known to carry the BRCA1 or BRCA2 gene mutation. Observational studies of high-risk women aged 30 to 39 years show a cancer detection rate similar to that for women aged 40 to 49 years.28,29
When to stop screening evaluation is a controversial topic. There is no specific recommendation regarding at what age to stop screening mammography. In general it is agreed that when comorbidities outweigh the benefits of screening, then cessation of screening should take place. It is also agreed that age is a strong risk factor for breast cancer; the older the women, the greater the risk of breast cancer. Only 2 of the 8 RCTs (Malmö and Swedish Two-County Trials) included patients over the age of 65. There is, therefore, insufficient evidence to recommend for or against screening older women. However, since the cumulative risk of radiation is less, breast parenchymal density diminishes, and the risk of developing breast cancer increases, women should continue routine annual screening as long as there are no compromising comorbid health conditions.
In April 2007, the American Cancer Society published new guidelines for screening high-risk women.30 Risk is greatest for women with genetics mutations including BRCA1 and BRCA2, p53, Cowden disease, and those women who underwent radiation therapy for lymphoma at an early age. Methods for estimating risk based on medical and family history include the Gail, Claus, and BRCAPRO mathematical models. Women who have a 20% to 25% lifetime risk for breast cancer based on family history calculated by any of these models are considered high risk. Women with a personal history of breast cancer or a prior diagnosis of atypical ductal or lobular hyperplasia also have increased risk of malignancy. Table 5-3 summarizes the factors placing women at increased risk for breast cancer. For the women with increased risk of breast cancer, in addition to annual mammography, contrast-enhanced breast MRI is recommended. One strategy that can be considered is to stagger the mammogram and breast MRI so that the woman can have surveillance at a shorter interval time.
| BRCA1 or BRCA2 mutation, or untested first-degree relative of known carrier |
| Chest radiation from age 10-30 for Hodgkin lymphoma |
| Lifetime risk of 20-25% as determined by statistical risk assessment models such as BRCAPRO or Gail |
| Other genetic mutations including p53 and Cowden disease |
In general, screening of women under 40 years of age is restricted to high-risk subgroups (women with a 20% lifetime breast cancer risk at or before the age of 30 years, or breast cancer risk at a given age equivalent to that of the average woman at the age of 40 years).
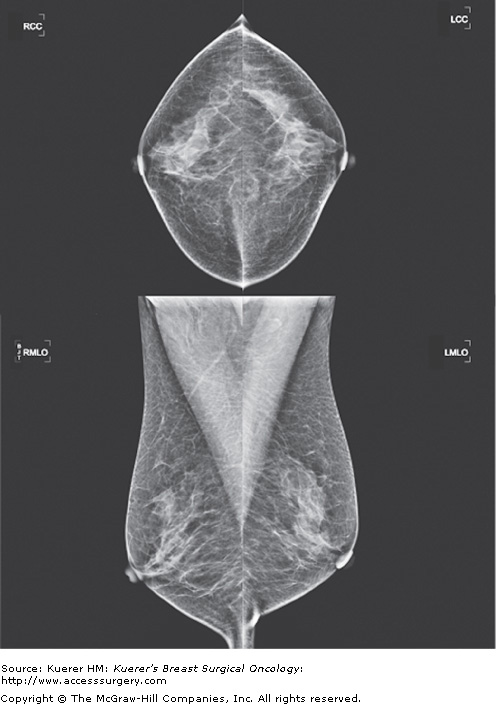
The standard screening mammogram consists of 2 views of each breast. The mediolateral oblique (MLO) projection is usually obtained at a 45-degree angle along the border of the pectoralis major muscle (Fig. 5-2). The cranio-caudal (CC) projection is obtained with the nipple on profile and breast tissue compressed with the image receptor parallel to the floor (Fig. 5-2). The breast tissue is positioned by highly skilled technologists to maximize the breast tissue visualized and with proper compression to decrease radiation exposure.
The Mammographic Quality Standards Act of 1992 (MQSA) requires that facilities be accredited by the Food and Drug Administration (FDA) to provide high-quality screening and diagnostic mammographic services. In addition to image quality, MQSA also defines qualification standards for interpreting physicians, technologists, physicists and facility inspectors. As part of MQSA, a Breast Imaging Reporting and Data System (BIRADS) final assessment category is assigned to each interpreted study (Table 5-4). BIRADS was developed by the American College of Radiology to standardize mammographic reporting, reduce confusion in interpretation, and facilitate monitoring of outcomes.31 It is composed of a lexicon of descriptive terms and definitions for each of the breast imaging modalities. It also provides a standardized reporting language and provides a structure for managing the medical audit and outcomes.
| Assessment Category | Assessment | Definition |
|---|---|---|
| 0 | Incomplete | Need additional imaging evaluation or prior mammograms for comparison |
| 1 | Negative | No masses, architectural distortion, or suspicious calcifications |
| 2 | Benign | Interpreter chooses to describe a benign finding |
| 3 | Probably benign | Finding has less than 2% risk of malignancy; sequential short-term follow-up (every 6 months for 2 or more years) is recommended |
| 4 | Suspicious | Risk of malignancy is greater than category 3; biopsy is recommended |
| 5 | Highly suggestive of Malignancy | Greater than 95% probability of malignancy |
| 6 | Known cancer | Biopsy proven malignancy |
The presence of breast implants presents a challenge to the radiologist interpreting the mammographic screening evaluation. In addition to the standard mammographic views, women with breast implants undergo additional views in each breast in order to obtain better compression and to visualize a greater amount of breast tissue. The implant-displaced views are obtained by displacing the implant posteriorly toward the chest wall and “pinching” the breast tissue anteriorly within the image receptor area. This technique was first described by Eklund and has become standard for the mammographic evaluation of the augmented breast.32
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree