Breast Cancer: Locally Advanced and Recurrent Disease, Postmastectomy Radiation, Systemic Therapies
Patients who present with locally advanced breast cancer require care from a multidisciplinary team that incorporates diagnostic imaging, chemotherapy, surgery, and careful pathology assessment, including molecular-based studies, radiation, and, if indicated, biologic and hormonal therapies. The treatment outcome for an individual patient may depend on the degree to which this multidisciplinary approach is integrated and the expertise of the treatment team. The input and coordination from each multidisciplinary discipline is especially important in the management of patients with locally advanced disease because such patients have the highest risk of disease recurrence without optimal treatment and require the most complex decision making.
Fortunately, the outcome for patients with locally advanced breast cancer has improved dramatically. Before the routine use of chemotherapy, patients treated with mastectomy, radiation, or a combination of the two had high rates of distant metastases and death.1–2,3 The introduction of adjuvant and neoadjuvant chemotherapy and hormone therapy regimens has significantly improved the prognosis. Furthermore, new systemic regimens and introduction of biologic therapies have offered additional improvements and further increased the importance of local-regional disease eradication.
This chapter focuses on the management of locally advanced breast cancer, with a focus on local-regional control and radiation therapy. There is no consensus on the definition of “locally advanced breast cancer,” but most commonly this term refers to stage III disease, meaning advanced primary or nodal disease without clinically evident systemic metastases. In addition to reviewing management strategies for stage III breast cancer, this chapter also reviews the role of postmastectomy radiation and systemic treatments for patients with all stages of invasive breast cancer. Management and outcome of locally recurrent breast cancer and selected unusual presentations of breast cancers are also discussed.
TABLE 57.1 ESTIMATES OF BREAST CANCER EXTENT OF DISEASE AT DIAGNOSIS FOR 2005–2006
 EPIDEMIOLOGY OF LOCALLY ADVANCED BREAST CANCER
EPIDEMIOLOGY OF LOCALLY ADVANCED BREAST CANCER
Between 1980 and 1987, the incidence of breast cancer increased by approximately 4% each year, in part because of the increase in use of screening mammography. Between 1987 and 1994 the incidence was constant and then increased again at a 1.6% rate up until 1999, after which time breast cancer incidence has decreased by 2% per year.4 Between 1988 and 2000 there was a steady increase in tumors diagnosed at a size of 2.0 cm or less, but since 2000 this incidence rate has decreased by 3.3% per year, and the rate of tumors of >5.0 cm has increased by 2% per year since 1992.4 A few reasons undoubtedly contributed to the decline in the percentage of cases of locally advanced disease at diagnosis during the late 1980s. First, mammographic screening resulted in a larger proportion of patients being diagnosed with earlier disease stages. A second important contribution was women’s health initiatives and public education efforts that prompted women to seek medical care at the first sign of a breast mass. Finally, the medical community has become better educated about appropriate standards for evaluating a breast mass.
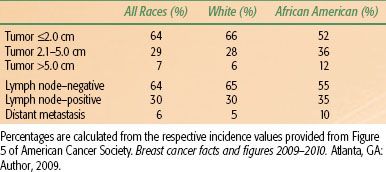
Table 57.1 gives estimates of the percentage of breast cancers diagnosed as T3 disease or with lymph node involvement according to data from the Surveillance, Epidemiology and End Results (SEER) Program, as reported by the American Cancer Society. Estimates indicate that 230,480 new cases of invasive breast cancer will be diagnosed in 2011.5 Using the percentages in Table 57.1 would yield an estimated 16,134 new cases with primary tumors of >5.0 cm and 69,144 new cases with lymph node–positive disease at diagnosis.
Table 57.1 also includes data concerning the distribution of disease in Whites and in African Americans. African-American women with breast cancer more commonly present with advanced primary disease and lymph node–positive disease than do White women with breast cancer.4 This has been explained on the basis of both socioeconomic factors and biology. Specifically, African-American women with breast cancer reportedly have less access to medical care and undergo screening mammography less often than do White women. African-American women also more often have breast cancers that are of higher nuclear grade and more frequently have estrogen receptor (ER)-negative disease compared with white women.6,7
Inflammatory breast cancer is an important subcategory of locally advanced breast cancer that has a unique epidemiology, presentation, and biology. Inflammatory breast cancers are rare, accounting for only 2% of all breast cancers in the United States.8 Estimates indicate that approximately 4,000 cases of inflammatory breast cancer would be diagnosed in the United States in 2010. During the 1990 s, the incidence of inflammatory breast cancer increased slightly. No known risk factors have been identified that are unique for the development of this form of breast cancer. However, the disease tends to occur in a younger population than does noninflammatory breast cancer. The proportion of African-American women with breast cancer diagnosed with inflammatory breast cancer is higher than the proportion of White women with breast cancer. Lymph node involvement at the time of diagnosis is much more common in patients with inflammatory breast cancer than in those with noninflammatory breast cancer, and it is more common for patients with inflammatory breast cancer to have distant metastases at diagnosis.8
 NATURAL HISTORY
NATURAL HISTORY
Natural History of Locally Advanced Breast Cancer
The outcomes of patients who present with locally advanced breast cancer were once poor, but improvements in treatments have changed the prognosis considerably. Currently, many patients with locally advanced disease can be cured. As the disease grows within the breast, the tumor may infiltrate or invade the dermis or the chest wall. Skin retraction may occur because of tumor invasion of Cooper’s ligaments, although this process can also be present in early-stage disease. Tumor growth can also lead to infiltration or obstruction of the lymphatic drainage of the breast and breast skin, causing edema of the breast, known as peau d’orange. In addition, primary tumor growth increases the risk of spread through the lymphatics to involve regional lymph nodes and/or spread hematogenously to involve distant sites such as the liver, lung, bone, and brain.
Most advanced primary tumors are associated with axillary lymph node involvement at the time of diagnosis. The axillary lymph node region is divided into three levels, defined according to their relationship to the pectoralis minor muscle: level I lymph nodes are inferolateral to the muscle, level II are beneath the muscle, and level III are superomedial to the pectoralis muscle (these lymph nodes are also called infraclavicular lymph nodes). Additional lymph nodes that may be involved in locally advanced breast cancer include Rotter’s nodes, which are located between the pectoralis minor and pectoralis major muscles, and the supraclavicular and internal mammary lymph nodes. Figure 57.1 shows computed tomography (CT) scans from patients with locally advanced breast cancer presenting with involvement of the level II axilla (Fig. 57.1A), an infraclavicular lymph node (Fig. 57.1B), a Rotter’s lymph node (Fig. 57.1C), and an internal mammary lymph node (Fig. 57.1D).
The clinical course of locally advanced breast cancer depends on several factors, including the specific disease characteristics at presentation, the biologic features of the disease, and the treatment given. Without treatment, almost all locally advanced breast cancers eventually metastasize to visceral organs and become life-threatening.1,2 Local disease progression can lead to ulceration of the breast skin, pain, bleeding, and infection. Progression of untreated regional lymphatic disease can cause pain, brachial plexopathy, arm edema, obstruction and thrombosis of the brachial vasculature, and skin ulceration.
Treatment advances have improved survival rates for women with locally advanced breast cancer. Before the use of systemic treatment became routine, patients with advanced disease treated with mastectomy, radiation therapy, or both had 5-year survival rates of only 25% to 45%.1–2,3 After the introduction of combined-modality treatments including surgery, radiation therapy, and chemotherapy, the 5-year survival rates approach 80% for patients with stage IIIA disease and 45% for patients with stage IIIB disease.9 More recent data suggest continued improvement with the addition of more-effective systemic regimens. For example, a recent SEER study examining patients with IIIB/C disease reported a 2-year breast cancer death rate of only 10%.10 The subset of patients with inflammatory breast cancer had a worse outcome than those with noninflammatory IIIB/C disease. In addition to stage, survival of locally advanced disease is dependent on other factors. For example, an elderly woman with a neglected ER-positive, hormonally responsive, stage III breast cancer that did not metastasize over a 1- to 2-year period of growth will have a much more favorable prognosis compared to a young woman with an ER-negative T4 tumor that presented with a history of rapidly progressive disease.
When considering improvements in the outcome of patients with stage III disease over time, it is important to recognize the effect of stage migration. Improvements in diagnostic imaging over time increase the likelihood of detecting metastatic disease, which results in some cases of stage III disease being reclassified as stage IV, which can have the effect of improving the outcome statistics for both stage III and stage IV disease. A similar effect was introduced by the 2003 change in the American Joint Commission of Cancer (AJCC) breast cancer staging system, which incorporated the number of positive lymph nodes in the pathologic disease stage. Specifically, many patients with four or more positive lymph nodes in the past would have had stage II disease but are classified as having stage III disease in the 2003 staging system. Indeed, a study that compared the stage-specific survival of 1,350 patients staged according to the 1988 AJCC staging system to the stage-specific survival of the same patients restaged according to the 2003 AJCC system found that the 10-year overall survival rates were significantly higher when the 2003 system was used, both for patients with stage II disease (76% [2003] vs. 65% [1988], p < .0001) and for those with stage IIIA disease (59% [2003] vs. 45% [1988], p < .0001).11 The reason for the better stage-specific outcome was that restaging into the 2003 system led to most of the patients with four or more positive lymph nodes being moved from the stage II to the stage III category.
FIGURE 57.1. Computed tomography images of patients with lymph node involvement at the time of diagnosis. A: Image from a patient with involvement of axillary lymph nodes in the level II axilla. The white arrows show the involved lymph nodes, which are just beneath the pectoralis minor muscle. B: An involved level III axillary lymph node (white arrow) that extents superomedially to the pectoralis minor muscle. C: An involved Rotter’s lymph node (white arrow), which is anterior to the pectoralis minor and beneath the pectoralis major muscle. D: Image from a patient with an involved internal mammary lymph node (white arrow).
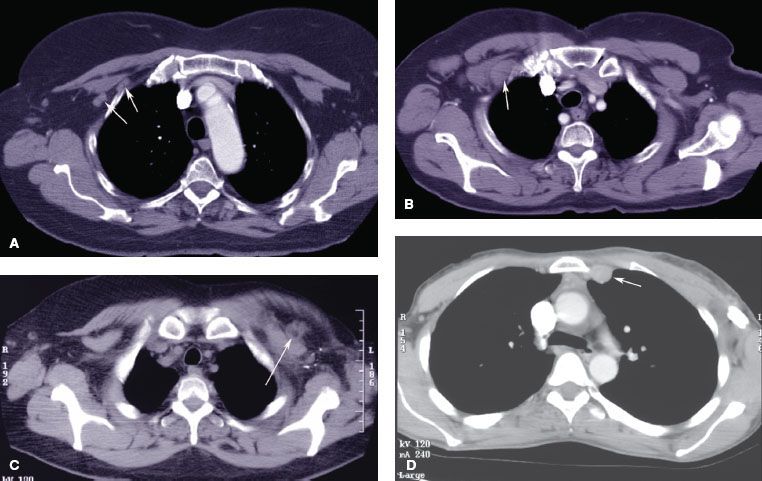
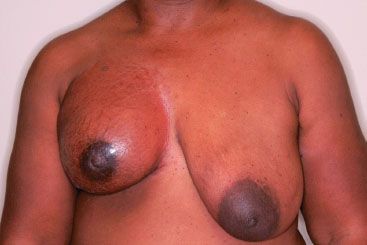
FIGURE 57.2. Photograph of a patient with a locally advanced noninflammatory right breast cancer at the time of diagnosis.
Natural History of Inflammatory Breast Cancer
Inflammatory breast cancer is a clinically defined subcategory of locally advanced breast cancer. The hallmarks of inflammatory breast cancer are rapid disease onset and the clinical findings of skin erythema, edema (peau d’orange), brawny breast induration, warmth, and asymmetric enlargement. Typically, extensive lymphovascular invasion by tumor emboli is present that involves the superficial dermal plexus of vessels in the papillary and high reticular dermis.12 It is critical to distinguish inflammatory breast cancers from locally advanced breast cancer with secondary lymphatic congestion. Neglected primary tumors can also lead to breast erythema, edema, warmth, and asymmetric enlargement, particularly when bulky axillary adenopathy impedes the normal lymphatic flow from the breast. However, the former has a history of rapid onset, whereas the latter tends to have a long interval between the first symptom and the presentation for medical treatment.
Despite the natural history of inflammatory breast cancer being one of rapid disease progression and early distant dissemination,12,13 in the United States, approximately 70% of patients with inflammatory breast cancer have only evidence of local-regional disease at the time of diagnosis.14 Patients with inflammatory breast cancer typically have a worse clinical outcome than do other patients with T4 disease, suggesting that inflammatory breast cancer is a distinct biologic entity.10 However, the prognosis for patients with inflammatory breast cancer has improved. Before the availability of combination chemotherapy, inflammatory breast cancer was almost uniformly fatal. Fewer than 5% of patients treated with surgery, radiation therapy, or both survived past 5 years, and the expected median survival time for such patients was <15 months.13 Local recurrence rates after surgery or radiation therapy were also high at approximately 50%.15,16 The introduction of doxorubicin-based chemotherapy improved outcomes.17,18 An evaluation of the outcome of patients with inflammatory breast cancer registered in the SEER Program found that breast cancer–specific survival rates for patients with inflammatory breast cancer improved continuously throughout the 1990s.19 Currently, local control rates for patients treated with chemotherapy, mastectomy, and postmastectomy radiation approach 70% to 80%, and 5-year survival rates are approximately 40%.17,18
 CLINICAL PRESENTATION OF LOCALLY ADVANCED BREAST CANCER
CLINICAL PRESENTATION OF LOCALLY ADVANCED BREAST CANCER
Locally advanced breast cancer most commonly is diagnosed after a palpable mass is detected within the breast. Advanced disease can cause symptoms such as local or regional pain, bleeding, paresthesia, and paresis. As previously indicated, it is critically important to determine the onset of symptoms and the rate of disease progression to reach an accurate diagnosis as to whether an advanced breast cancer represents an inflammatory carcinoma.
Diagnostic Workup
For patients with locally advanced breast cancer, the workup should start with a careful history and physical examination. The breast examination should include note of the breast symmetry, as well as careful inspection for involvement or edema of the skin. Peau d’orange can sometimes be subtle and at times can be best detected through gentle compression of the dermis between two fingers, which can elicit an increased prominence of the hair follicles and skin thickening compared with the skin overlying the contralateral breast. This finding may be missed on a quick visual inspection. Other times, physical examination findings are more obvious. Figure 57.2 shows a photograph of a patient with a neglected locally advanced breast cancer presenting with peau d’orange, inflammatory changes, breast retraction and involution, and effacement of the nipple–areola complex. Medical photographs are helpful to document the extent of visible abnormalities before treatment is begun and can be used to assess disease response to treatments.
The extent of palpable disease should also be measured and documented. Fixation of a breast mass to the pectoralis muscle or chest wall should be determined by assessing the mobility of the mass with the pectoralis muscle relaxed and contracted. Regional lymph nodes should be thoroughly evaluated by careful clinical examination with the patient in both supine and sitting positions. Clinical nodal evaluation may be supplemented with ultrasonographic imaging.
All cases of locally advanced disease require complete staging before initiation of therapy. Laboratory studies should include a complete blood cell count and serum chemistry profile with liver function tests. Radiographic studies should include a chest radiograph, a CT scan of the abdomen, a bone scan, and plain radiographs of symptomatic regions or areas of increased uptake on bone scans. Bone scans are recommended for all patients with locally advanced disease; up to 35% of patients with clinical stage III cancer can show abnormal bone scan results.20 If any neurologic symptoms suggestive of cerebral metastases are present, a contrast-enhanced CT scan or gadolinium-enhanced magnetic resonance imaging (MRI) scan of the brain should be obtained. Gadolinium-enhanced MRI is the preferred imaging technique if leptomeningeal carcinomatosis is suspected.
There is increasing interest in the use of [18F]fluorodeoxyglucose positron emission tomography (PET)/CT for disease staging of patients with locally advanced breast cancer, particularly those with inflammatory breast cancer. In a study of 41 women presenting with inflammatory breast cancer, PET/CT was able to detect distant metastases that were not found with other staging studies in 17% of the patients.21
Staging of Locally Advanced Breast Cancer
A comprehensive discussion of disease staging systems for breast cancer is provided in Chapter 56. Some staging considerations are particularly relevant to patients with stage III disease. Stage III breast cancer can represent either T3 disease (tumors >5.0 cm) with involved lymph nodes, N2 or N3 disease, or T4 disease.22
Specific aspects of both primary tumor and nodal staging in locally advanced breast cancer warrant additional consideration. Specifically, T4 disease may represent invasion into the chest wall (T4a), tumors associated with breast edema or skin ulceration or satellite nodules (T4b), both invasion and T4b characteristics (T4c), or inflammatory breast cancer (T4d). Invasion of disease into the pectoralis major muscle without chest wall invasion and dimpling or fixation of the overlying skin does not qualify as T4 disease. Both clinical and pathologic staging systems have been established for N2 and N3 disease. Clinical N2 disease signifies either involved axillary lymph nodes that are fixed to one another or to surrounding structures (N2a) or involved internal mammary lymph nodes without concurrent disease in the axilla (N2b), as determined by physical examination or imaging studies. Clinical N3 disease is disease that involves the infraclavicular region (N3a), both the axilla and internal mammary lymph nodes (N3b), or the supraclavicular region (N3c). Pathologic N2 disease represents involvement of 4 to 9 axillary lymph nodes with at least one focus measuring >2.0 mm (N2a) or clinical involvement of internal mammary lymph nodes with pathologically negative axillary lymph nodes (N2b). Pathologic N3 disease represents involvement of an infraclavicular lymph node or 10 or more involved lymph nodes with at least one focus measuring >2.0 mm (N3a), clinical involvement of internal mammary lymph nodes with 1 to 9 axillary lymph nodes involved, pathologic involvement of a sentinel internal mammary lymph node with four or more axillary lymph nodes involved (N3b), or a metastasis in the supraclavicular region (N3c).22
Neoadjuvant chemotherapy is recommended for most patients with locally advanced breast cancer. The initial extent of disease is an important factor for later local-regional treatment decisions and will be known only from the initial physical examination and radiographic findings. It is therefore imperative that disease in all patients be carefully assigned a clinical stage before any treatment is begun.
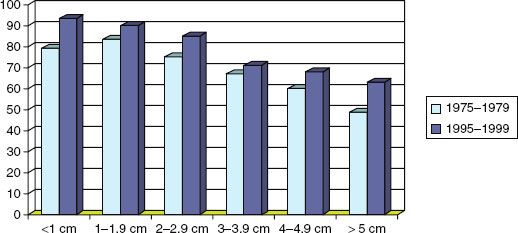
FIGURE 57.3. Five-year survival rates for patients with positive axillary lymph nodes according to tumor size and year of diagnosis. (Data from Elkin et al.30; reprinted from Buchholz TA. Locally advanced breast cancer. In: Haffty BG, Wilson L, eds. Handbook of radiation oncology. Sudbury, MA: Jones and Bartlett, 2008.)
 PATHOLOGY AND BIOLOGY OF LOCALLY ADVANCED BREAST CANCER
PATHOLOGY AND BIOLOGY OF LOCALLY ADVANCED BREAST CANCER
The histopathology of locally advanced disease is relatively similar to that of early-stage disease. Both infiltrating ductal carcinoma and lobular carcinoma can present as locally advanced disease. However, it is unusual for histologically “favorable” tumor types (e.g., tubular carcinoma, mucinous carcinoma, and medullary carcinoma) to present at advanced clinical stages unless the breast mass has been present for a long time.
The term “locally advanced breast cancer” encompasses a biologic spectrum of diseases. Locally advanced disease that has developed between interval (annual) screening mammograms is most often ER-negative, with high nuclear grade and high proliferative index. In contrast, patients who present with extensive local-regional disease after years of medical neglect more often are found to have ER-positive disease with low nuclear grade and low proliferative index.
Inflammatory breast cancer also has biologic characteristics that differ from those of noninflammatory breast cancer. Specifically, inflammatory cancer more often is of high histologic grade, shows high percentages of cells in S phase and aneuploidy, does not express the ER, and expresses high levels of p53 and epidermal growth factor.23,24 Of interest, most investigators have found that HER2/neu overexpression is no more common in inflammatory breast cancer than in noninflammatory advanced disease.23,25 Other, more recently discovered markers include the propensity of inflammatory tumors to overexpress RhoC GTPase and to not express the tumor suppressor gene WIPS3.26,27 Finally, others have described that inflammatory breast cancers with loss of MUC-1 may be associated with poorer survival than tumors that express MUC-1. If these findings are confirmed and validated, markers such as these may prove to be useful for diagnosis and possibly as future therapeutic targets.26,27 More recently, investigators have noted that overexpression of E-cadherin may play an important role in the tumor emboli formation that is typically noted in the dermal lymphatics, and preclinical work suggests that targeting E-cadherin may decrease invasiveness.28,29
 GENERAL MANAGEMENT AND TREATMENT RESULTS FOR LOCALLY ADVANCED BREAST CANCER
GENERAL MANAGEMENT AND TREATMENT RESULTS FOR LOCALLY ADVANCED BREAST CANCER
Locally advanced disease requires multimodality therapy aimed at eradicating all disease in the local-regional area and preventing distant disease recurrence. These goals are best achieved through the use of combined-modality treatments that include chemotherapy, surgery, and radiation. In addition, ER-positive disease should be treated with hormonal therapy, and HER2/neu–positive disease should be treated with trastuzumab. Combined-modality therapy has significantly improved the prognosis for patients with advanced breast cancer. As previously noted, the prognosis of patients with locally advanced disease treated in the era before chemotherapy was available was very poor, with 5-year survival rates of only 25%.1–2,3 In contrast, more recent single-institution studies have reported 5-year survival rates approaching 80% for patients with stage IIIA disease and 45% for patients with stage IIIB disease.9 National database studies also reflect improvements in survival over time. For example, a study evaluating the outcome of patients with lymph node–positive breast cancer demonstrated that 5-year survival rates were significantly better in the group treated in 1995–1999 than in the group treated in 1975–1979 (Fig. 57.3).30 The most recent data from the American Cancer Society estimated an 84% 5-year survival for patients diagnosed between 1999–2005 who had lymph node–positive disease without distant metastases.4 These survival statistics are likely to show continued improvement over time because since 1999 several positive phase III clinical trials have shown that a new systemic treatment strategy may improve outcome among patients with lymph node–positive breast cancer. What is particularly exciting is that some of these advances represent incremental improvements in outcome over previous advances, so that when the benefits are added together the improvements over time become quite significant. As evidence of this improvement, an interesting study recently reported that between 1950 and 1980, the U.S. Food and Drug Administration approved fewer than 5 new systemic treatments for breast cancer during each decade; in contrast, 6 new agents were approved during the 1980s, and 12 new agents were approved during the 1990s.31 The pace of change in available therapeutics has been even greater in the last decade. These new treatments are likely to continue to improve the prognosis for patients with advanced breast cancer during the decades to come, and the rate at which new agents for breast cancer treatment are introduced is expected to accelerate with identification of new therapeutic targets.
Overview of Treatment
Locally advanced breast cancer can present as either operable disease or inoperable disease. The current standard of treatment for all patients with inoperable breast cancer is to proceed with neoadjuvant chemotherapy as the initial therapy. Approximately 80% to 90% of patients with advanced breast cancer will show partial or complete clinical response to neoadjuvant chemotherapy,32,33 and most patients presenting with inoperable breast cancer become candidates for surgery after neoadjuvant treatment.
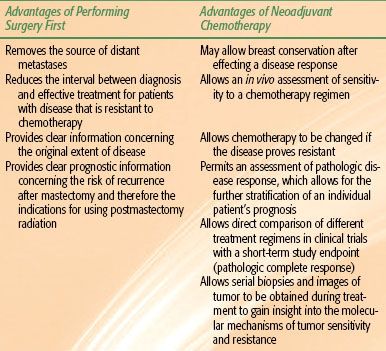
No criteria have been agreed upon to distinguish inoperable from operable disease. In general, neoadjuvant chemotherapy is preferred if an initial surgical procedure is not likely to completely resect all gross disease with achievement of negative surgical margins. This includes most patients with T4 disease and all patients with inflammatory breast cancer, and for such patients neoadjuvant chemotherapy can allow primary closure of the skin flaps of the mastectomy. For patients with operable stage IIIA disease, initial surgery followed by adjuvant chemotherapy or neoadjuvant chemotherapy are equally good options. The advantages of each are highlighted in the following.
TABLE 57.2 CONSIDERATIONS REGARDING THE SEQUENCING OF SURGERY AND CHEMOTHERAPY FOR PATIENTS WITH OPERABLE LOCALLY ADVANCED BREAST CANCER
Neoadjuvant Chemotherapy—Advantages and Disadvantages
Neoadjuvant chemotherapy has become an increasingly popular treatment strategy for patients with stage II or III breast cancer. The use of neoadjuvant chemotherapy has several potential advantages over the traditional sequence of surgery followed by adjuvant chemotherapy, but it also carries some disadvantages (Table 57.2). Several trials have clearly shown that neoadjuvant chemotherapy substantially reduces the size of the primary tumor and nodal metastases in >80% of cases. Accordingly, for patients with large primary tumors, the approach of using chemotherapy as the initial treatment has been shown to increase the probability that breast-conserving surgery can be performed.32,34–36 A second advantage of using chemotherapy first is that this sequence allows the response of the disease to a particular chemotherapy regimen to be assessed, which in turn could provide an opportunity to “cross over” to a different treatment regimen if disease in an individual patient shows little or no response to the first regimen. By doing so, a potentially effective second-line agent can be given rapidly, and the toxicity of an ineffective first regimen can be avoided.
Neoadjuvant chemotherapy has also been proven to be extremely valuable for clinical research. After several studies showed a strong correlation between the achievement of a pathologic complete response (pCR; defined as no residual cancer being found in the postchemotherapy surgical specimen) and survival,32–34 investigators began using pCR rates as a short-term surrogate of the success of a chemotherapy regimen. Phase III randomized trials in which pCR rates were used as the primary endpoint have allowed the activity of two chemotherapy regimens to be compared with relatively small study populations and very short follow-up times relative to studies comparing two chemotherapy regimens used in an adjuvant setting. Neoadjuvant chemotherapy can also facilitate translational research to investigate the mechanisms of chemotherapy-induced cell death and chemotherapy resistance. For example, it has proven feasible to study changes in tumor genomes in response to treatment and how such changes correlate with chemotherapy response through the use of cDNA microarrays from serial biopsy specimens.37 Such studies are likely to provide significant insights into the heterogeneity of tumor response and to identify new targets for therapies.
Some have asserted that treatment with neoadjuvant chemotherapy also provides additional prognostic information. Clearly the prognosis for patients with a pCR (defined as complete eradication of invasive disease and negative axillary lymph nodes at the time of surgical treatment) is better than the prognosis would have been before treatment. However, an equal percentage of patients will be found to have residual disease after chemotherapy, which confers a worse prognosis than originally anticipated. Therefore, the true value of the additional prognostic information from the use of neoadjuvant chemotherapy will come only when additional treatments become available that can positively influence prognosis for those with a high residual disease burden. Currently, there are a number of clinical protocols to evaluate new therapeutics specifically in patients who exhibit a poor response to neoadjuvant treatment.
One theoretical advantage of neoadjuvant chemotherapy that has not been borne out in practice was the hope that earlier delivery of chemotherapy might improve survival for patients with locally advanced breast cancer. Clearly most of the patients who present with advanced disease and subsequently die of that disease do so as a consequence of the progression of metastatic disease that was present at a microscopic level at the time of diagnosis. Therefore, the suggestion that initiating chemotherapy at diagnosis (when the micrometastatic tumor burden would be lowest) would improve outcome relative to delaying chemotherapy until after surgical resection was a rational one. This was further supported by preclinical animal studies showing that removal of the primary tumor could increase the growth rate of existing micrometastases and that treating animals with either chemotherapy or tamoxifen before resection of the primary tumor abrogated this adverse effect.38
Two large randomized trials have been conducted to test the hypothesis that neoadjuvant chemotherapy could improve survival in patients with operable breast cancer. The first of these trials was the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 study, in which 1,523 patients with operable breast cancer were randomly assigned to receive four cycles of doxorubicin and cyclophosphamide (AC) either before or after surgical treatment.32,34 After 16 years, the overall survival rates and disease-free survival rates were nearly identical between the two groups (p = .99 and p = .93, respectively).39 However, in an unplanned subgroup analysis the authors found an interaction between age and sequencing of chemotherapy. Patients younger than 50 years of age had a 16-year overall survival of 61% with neoadjuvant chemotherapy versus 55% with adjuvant chemotherapy. An opposite trend noting an improvement with adjuvant chemotherapy was noted in the patients 50 years old and older.39 A second randomized prospective trial, conducted by the European Organization for Research and Treatment of Cancer (EORTC), confirmed these results and again found equivalent rates of 10-year survival and distant metastases between the neoadjuvant chemotherapy and adjuvant chemotherapy treatment groups.40 A meta-analysis of data from 3,946 patients treated in nine randomized trials comparing neoadjuvant with adjuvant chemotherapy in breast cancer found no statistical difference in the risk of death (risk ratio, 1.00), disease progression (risk ratio, 0.99), or distant disease recurrence (risk ratio, 0.94).41
The increasing use of neoadjuvant chemotherapy in patients with clinically negative lymph nodes has also created a controversy with respect to the sequencing of chemotherapy and sentinel lymph node surgery. It is clear from the B-18 trial and other institutional studies that neoadjuvant chemotherapy leads to complete eradication of disease within lymph nodes in roughly 20% to 40% of patients.32,42 If this eradication occurs selectively within the sentinel lymph node but not in other involved axillary lymph nodes, there is the potential that the false-negative rate of sentinel lymph node surgery after chemotherapy may be higher than sentinel lymph node surgery performed prior to chemotherapy. In addition, the original extent of axillary disease is unknown when sentinel lymph node surgery is performed after chemotherapy. This can have implications with respect to radiation treatment field design or recommendations concerning whether to use postmastectomy radiation. In some instance, this can also have implications with respect to adjuvant chemotherapy treatment decisions.
There are some advantages to performing the sentinel lymph node surgery after rather than before neoadjuvant chemotherapy. Of importance, with this strategy, most commonly patients only have to go one surgery rather than two. Second, if a component of disease is removed prior to surgery, then the prognostic value of achieving a pCR is less certain. Finally, performing surgery prior to chemotherapy delays the administration of systemic treatments, particularly if an axillary metastasis is found and the patient then undergoes an axillary dissection. Finally, because patients will more commonly have pathologically lymph node–negative disease after neoadjuvant chemotherapy, they more commonly will not require a completion axillary dissection.43
A number of groups have studied the identification rates and false-negative rates associated with sentinel lymph node surgery. The largest experience has been from the NSABP B-27 trial, which randomized 2,411 patients to one of three neoadjuvant chemotherapy regimens. A total of 428 of these patients had lymphatic mapping attempted. Successful identification of a sentinel lymph node was made in 85%, and the false-negative rate was 11% (defined as the number of patients with positive nonsentinel lymph nodes with a negative sentinel lymph node divided by the total number of patients with positive axillary lymph nodes).44
A meta-analysis investigated 1,273 patients (21 published studies) treated with a sentinel lymph node biopsy with subsequent axillary dissection following neoadjuvant chemotherapy.45 These authors reported a pooled identification rate of 90% and false-negative rate of 12%. Because these outcomes are similar to those reported in multicenter studies in which sentinel lymph node surgery was performed prior to systemic therapy, delaying sentinel lymph node surgery until after chemotherapy appears acceptable.
Neoadjuvant Hormonal Therapy
There are fewer data concerning the long-term outcome of patients treated with hormone therapy prior to surgery. In part this is because most patients treated with neoadjuvant systemic treatments for an ER-positive breast cancer have disease extent that necessitates both chemotherapy and hormonal treatments. However, interest in neoadjuvant hormonal therapy increased after reports from studies that found that patients with ER-positive disease have a lower probability of achieving a pCR compared to those with ER-negative disease.46 For example, patients with lobular breast cancer, in whom >90% of tumors are ER-positive, have particularly low rates of pCR.47 In addition, some patients with ER-positive breast cancer that is locally advanced and/or lymph node–positive at presentation are not candidates for neoadjuvant chemotherapy due to comorbid medical conditions. For such patients, treatment with neoadjuvant hormonal therapy is a reasonable option.48
Responses to neoadjuvant hormonal therapy occur over a slower period of time than those to neoadjuvant chemotherapy, and the rates of pCR with hormonal therapy are lower than those achievable with neoadjuvant chemotherapy. After aromatase inhibitors became available for postmenopausal patients with ER-positive disease, neoadjuvant hormone therapy trials were developed to directly compare the activities of various agents. A 330-patient randomized trial run in the United Kingdom compared 3 months of anastrozole, tamoxifen, or combined anastrozole/tamoxifen and found response rates of 36% to 39%, with only 1% to 3% achieving a clinical complete response.49 In the subgroup of 124 patients who were not candidates for breast conservation at diagnosis, the rates of breast conservation after 3 months of neoadjuvant hormone treatment were highest in the anastrozole-alone arm. An Italian trial randomized patients to 3 months of anastrozole versus tamoxifen and also noted a higher rate of breast conservation after treatment with anastrozole alone versus tamoxifen alone.50 The overall response rates were similar to those in the United Kingdom study.
Breast Conservation Therapy After Neoadjuvant Chemotherapy
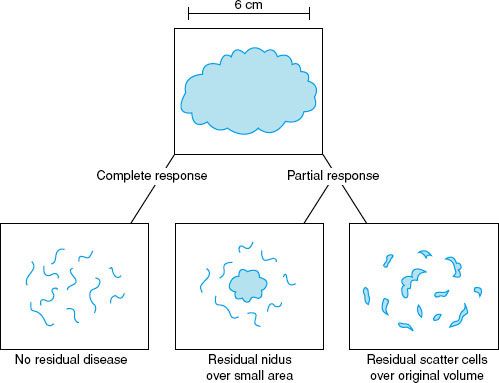
As noted, one of the potential benefits of neoadjuvant chemotherapy is that a large primary tumor will respond favorably to neoadjuvant chemotherapy, thereby rendering the disease amenable to a breast conservation surgical approach. One important consideration for performing breast-conserving surgery after neoadjuvant chemotherapy concerns the volume of surgical resection. This is less of a problem for patients with small initial primary tumors that shrink still further after neoadjuvant chemotherapy. However, for patients with T3 disease for whom initial breast-conserving surgery would be deforming, the volume of resection after neoadjuvant chemotherapy must be directed at the residual nidus rather than the original extent of disease. In some instances, neoadjuvant chemotherapy successfully shrinks large primary tumors to smaller residual niduses that can easily be resected in small volumes of tissue with good or excellent aesthetic outcomes. However, breast cancers can respond to neoadjuvant chemotherapy in a variety of ways, as shown in Figure 57.4. For tumors that shrink to a residual nidus, limited surgery would successfully resect the volume of residual disease, and the outcome, particularly for those with a pCR, would be expected to be excellent. However, in other cases, tumors respond favorably to neoadjuvant chemotherapy, but the residual disease is diffuse, multifocal, and scattered throughout the original tumor volume. In such cases, a small surgical resection carries the risk of leaving a residual disease burden within the breast. Careful selection of cases for breast conservation is therefore critical.
One of the first studies to provide findings regarding appropriate selection criteria for breast conservation after neoadjuvant chemotherapy examined 143 mastectomy specimens from patients given neoadjuvant chemotherapy to determine patterns of residual disease and their relationships to clinical factors.51 Only 23% of tumors had clinical and pathologic features that would have predicted success with breast conservation. Important selection criteria included resolution of skin edema, favorable clinical response to neoadjuvant treatment, lack of multicentricity, and lack of extensive lymphovascular space invasion.
FIGURE 57.4. Three potential pathologic outcomes of a primary tumor that responds to neoadjuvant chemotherapy. (From Buchholz TA, Hunt KK, Whitman GJ, et al. Neoadjuvant chemotherapy for breast carcinoma: multidisciplinary considerations of benefits and risks. Cancer 2003;98:1150–1160.)
Since those findings were published, several groups have studied the clinical outcome of patients treated with breast conservation after neoadjuvant chemotherapy. In general, the outcome results have varied considerably across series, for several possible reasons. First, the selection criteria vary considerably across studies, and not surprisingly studies that include patients with positive surgical margins or inflammatory breast cancer tended to report higher rates of local recurrence.52,53 Similarly, some studies in which patients who achieved complete clinical resolution of disease could elect to forgo surgery showed higher recurrence rates.54 Other studies, however—predominantly from institutions with well-coordinated multidisciplinary teams and careful pathologic analysis of the surgical specimen—reported excellent outcomes.55,56
Two of the most influential publications that addressed breast conservation after neoadjuvant chemotherapy were from the two largest randomized trials (NSABP B-18 and EORTC) comparing neoadjuvant chemotherapy with adjuvant chemotherapy for patients with stage II or stage III breast cancer. The conclusion in both studies was that neoadjuvant chemotherapy offered an advantage because breast conservation rates were higher in the neoadjuvant chemotherapy groups.32,34,36 However, it is important to recognize that approximately 60% of the patients enrolled in these studies were considered candidates for breast conservation at the time of diagnosis. Therefore in the B-18 study, the improvement in breast conservation rates from 60% to 68% for patients treated with neoadjuvant chemotherapy essentially showed that 20% of initial mastectomy candidates (8 of 40 patients) could undergo breast conservation surgery instead after neoadjuvant chemotherapy. Not surprisingly, this increase was directly due to a higher percentage of patients with T3 disease being offered breast conservation after first responding to chemotherapy. Both studies reported that the overall breast recurrence risk in patients treated with neoadjuvant chemotherapy was not statistically different from that in patients treated with surgery first.32,34,36 However, in the B-18 study, the breast recurrence rate in a subset of patients who initially would have required a mastectomy but were treated with breast conservation after a favorable response to neoadjuvant chemotherapy was twice that of the patients with smaller tumors who were treated with surgery first (15.7% vs. 7.6%, respectively).34
A meta-analysis of the nine randomized studies comparing neoadjuvant and adjuvant chemotherapy reported that the use of neoadjuvant chemotherapy was associated with an increase in the relative risk of local-regional recurrence relative to adjuvant chemotherapy (relative risk, 1.22; 95% confidence interval [CI], 1.04 to 1.43).41 This difference was largely influenced by the trials in which surgery was not performed and breast conservation after neoadjuvant chemotherapy was achieved with the use of radiation therapy alone (in those trials the relative risk was 1.53 and the 95% CI was 1.11 to 2.10).41 These findings indicate that patients who achieve a complete clinical response would still benefit from a surgical procedure in addition to radiation.
Two of the largest studies showing acceptable outcomes for breast conservation after neoadjuvant chemotherapy have been from the Istituto Nazionale Tumori in Milan, Italy, and the University of Texas MD Anderson Cancer Center.55,56 The Milan experience consisted of 536 patients treated with neoadjuvant chemotherapy for a primary tumor 2.5 cm in diameter or larger. Eighty-five percent of these patients subsequently had breast-conserving surgery. However, it is important to note that the initial tumor size in more than half of these women was <4 cm, and thus these women may have been candidates for breast conservation at diagnosis. The 8-year rate of breast recurrence as a first site of failure in those treated with breast conservation was 6.8%.55 In the MD Anderson series, 340 carefully selected patients were treated with breast conservation therapy after showing a favorable response to chemotherapy.56 Patient selection criteria for the breast-conserving approach included having no residual T4 breast skin abnormalities, negative surgical margins, no multicentric disease, no residual malignant calcifications on postoperative mammogram, and the willingness and ability to undergo both surgery and radiation therapy. With these criteria, the outcome was favorable, with 5- and 10-year local recurrence rates of 5% and 10%, respectively, despite the fact that 72% of patients in the study had clinical stage IIB or III disease. Four factors were found to be independently associated with breast cancer recurrence and local-regional recurrence: clinical N2 or N3 disease, lymphovascular space invasion, a multifocal pattern of residual disease, and residual disease >2 cm in diameter.56 Eighty-four percent of patients had none or just one of these factors, and the recurrence rate at 10 years in this group was only 4%.57 In contrast, the 4% of patients with three of these factors had a recurrence rate of 45%. Women with primary clinical T3 or T4 disease were at very low risk of recurrence if the tumor shrank to a solitary nidus or showed a pCR, but among patients with T3/T4 tumors that broke up and left a multifocal pattern of residual disease the breast cancer recurrence rate was 20%.56 More recently this same group validated this prognostic index in a more recent cohort of patients. The patients had respective 5-year locoregional recurrence–free survival rates of 92% (score, 0; n = 91), 92% (score, 1; n = 82), 84% (score, 2; n = 38), and 69% (score, 3 to 4; n = 13) (p = .01).58
The investigators from MD Anderson recently updated their experience and compared how treatment with neoadjuvant versus adjuvant chemotherapy affected rates of local-regional failures in 2,984 patients treated with breast conservation therapy between 1987 and 2005. After adjusting for differences in clinical stage, their multivariate analysis demonstrated no differences in local-regional recurrence between surgery-first and chemotherapy-first patients.59
When considering the outcome of breast conservation therapy for patients with locally advanced breast cancer, it is important to consider that patients with stage III breast cancer are at risk for local-regional recurrence even when mastectomy is performed. In addition, patients with advanced disease are at significant risk for distant metastases, which is an additional incentive to avoid removing the entire breast when breast-conserving surgery can be done with acceptably low recurrence rates. The investigators from MD Anderson applied the four prognostic criteria associated with breast recurrence in patients treated with neoadjuvant chemotherapy and breast conservation to a cohort of patients treated with neoadjuvant chemotherapy, mastectomy, and postmastectomy radiation.60 These investigators found that for patients who had none or one of these factors, the results with either local-regional treatment approach were excellent and equivalent. Among patients with two factors, a nonsignificant trend was evident toward fewer local-regional recurrences with mastectomy, and for the small cohort of patients with three or four factors, mastectomy provided a statistically significant benefit. This trend was also noted in their updated validation paper.58
Mastectomy
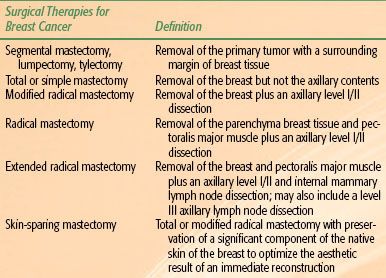
In the United States, mastectomy continues to be the most common local-regional treatment for breast cancer, particularly for patients with locally advanced disease. Several alternative mastectomy approaches are available for women with breast cancer (Table 57.3). A simple or total mastectomy resects the breast but not the axillary contents. For patients with clinical stage T1/T2 N0 disease who are not interested in breast conservation, a total mastectomy with a sentinel lymph node dissection may be the treatment of choice. A modified radical mastectomy (removal of the breast plus a level I/II axillary dissection) remains the standard of care for patients with clinically positive lymph nodes or locally advanced disease.
TABLE 57.3 TYPES OF MASTECTOMIES USED AS TREATMENTS FOR BREAST CANCER
Postmastectomy Radiation Therapy
Meta-Analyses of Postmastectomy Radiation Therapy Trials
Adjuvant radiation has been used after mastectomy for many decades. Indeed, some of the first randomized, prospective trials in medicine were done to investigate the efficacy of postmastectomy radiation. Despite this, significant controversy remains over the indications for its use. It is clear that mastectomy without radiation offers excellent local-regional control rates for most patients with noninvasive or early-stage, lymph node–negative disease. In contrast, patients with stage III breast cancer (four or more positive lymph nodes or T3/T4 primary tumors) have a clinically relevant risk of local-regional recurrence after mastectomy and thus would benefit from adjuvant radiation. What is less clear is whether radiation provides a survival advantage for patients with stage II breast cancer and one to three positive lymph nodes.
In 1987, Cuzick et al.61 published the first meta-analysis of data from postmastectomy radiation therapy trials and reported that radiation use was associated with a poorer overall survival rate. In a subsequent analysis, the same group reported that postmastectomy radiation therapy decreased the breast cancer death rate but increased the non–breast cancer death rate,62 which resulted in equivalent overall survival rates in the radiation and no-radiation groups. These analyses are mostly of historical interest because of the considerable heterogeneity in the surgical and radiation therapy treatments used in these trials as compared with modern treatment approaches. Moreover, these early trials often enrolled patients with early-stage disease, who would not be predicted to derive any benefit from radiation therapy. Finally, the early studies of postmastectomy radiation predated the use of systemic therapy.
After the Cuzick meta-analyses, the Early Breast Cancer Trialists’ Collaborative Group obtained the raw data from every randomized trial that investigated the role of radiation in breast cancer. Through the years, this group has published a series of meta-analyses that have provided important insights into the risks and benefits of postmastectomy radiation therapy.63 In the most recently published analysis, based on data from 9,933 patients, postmastectomy radiation therapy reduced the 15-year isolated local-regional recurrence rates for patients with lymph node–positive disease from 29% to 8%.64 Of importance, this reduction led to a 5% decrease in the 15-year breast cancer mortality rate (60% vs. 55%). In 2005, the group reanalyzed updated data, with the resulting publication expected in 2012. This updated further subdivided patients according to pathologic lymph node status, and the provisional findings have been presented.65 In this update postmastectomy radiation reduced 15-year rates of isolated local recurrences in pN0 patients from 5.8% to 2.4% (n = 1,277), in patients with one to three positive lymph nodes by 24.7% versus 5.3% (n = 3,316), and in patients with four or more positive lymph nodes by 40.6% versus 12.9% (n = 2,813). The improvement in local-regional recurrence with postmastectomy radiation was associated with a statistically significant improvement in death from breast cancer and overall survival for the patients with one to three positive lymph nodes and those with four or more positive lymph nodes but not for the patients with lymph node–negative disease.65
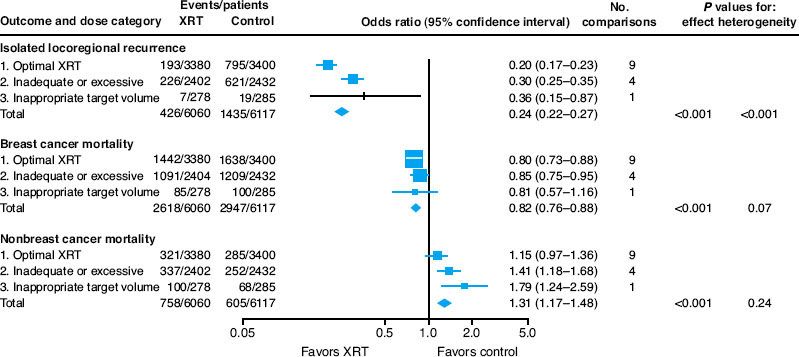
It is important to recognize the limitations of meta-analyses when considering the relevance of these reports to modern treatments for breast cancer. One problem with including trials dating back to the 1950s is that the radiation doses, fractionation patterns, treatment units, and field designs differ significantly from current standards. To minimize these confounding effects, Van de Steene et al.66 conducted a similar meta-analysis but excluded trials that began before 1970, trials with small sample sizes, trials with relatively poor survival rates, and trials that used radiation fractionation schedules that are no longer in standard practice. When these studies were excluded, use of postmastectomy radiation therapy was associated with an even greater overall survival advantage. Moreover, adjuvant radiation probably can improve survival even further if the risk of dying from micrometastatic disease is minimized through the use of systemic therapy. To investigate this question, Whelan et al.67 performed a meta-analysis of postmastectomy radiation trials that included systemic therapy for both treatment groups. In this analysis, the addition of radiation after mastectomy led to an even greater reduction in the risk of any recurrence (odds ratio, 0.69) and the risk of death (odds ratio, 0.83). Finally, another meta-analysis attempted to account for the quality of radiation delivery in these trials.68 In this study, the authors defined optimal dose as a between 40 and 60 Gy delivered in 2-Gy fractions and optimal treatment field arrangements as ones that included both the chest wall and the regional lymphatics. They then reanalyzed the data from the Early Breast Cancer Trialists’ Collaborative Group according to the quality of radiation treatments and demonstrated that proportional reduction in local-regional recurrence was 80% for trials with optimal dose and treatment fields, compared to 70% and 64% for trials that were suboptimal with respect to dose or field arrangements, respectively. In addition, there was a statistically significant improvement in breast cancer mortality in the trials that used optimal radiation dose and treatment fields but not in the other trials (Fig. 57.5).
In summary, these meta-analyses conclusively demonstrated that radiation has an important role in the management of locally advanced breast cancer. By reducing the risk of recurrence after mastectomy, radiation offers an incremental improvement in overall survival. Radiation seems to offer the greatest benefit when given using modern treatment techniques that minimize the risk of normal-tissue injury and maximize the probability of tumor control and when given to patients who also receive systemic treatments.
FIGURE 57.5. Data from a meta-analysis showing the association between postmastectomy radiation therapy and locoregional recurrence, breast cancer mortality, and mortality from other causes. The trials were analyzed and divided according the appropriateness of the radiation dose (labeled in the figure as “inadequate or excessive”) and design of the radiation treatment fields. This figure shows that the trials with the highest quality of radiation therapy achieved the greatest proportional reduction in locoregional recurrences, were the only trials associated with an improvement in breast cancer mortality, and were the only trials that did not find an association between radiation use and increased mortality from other causes. (From Gebski V, Lagleva M, Keech A, et al. Survival effects of postmastectomy adjuvant radiation therapy using biologically equivalent doses: a clinical perspective. J Natl Cancer Inst 2006;98(1):26–38.)
Phase III Randomized Trials Investigating Postmastectomy Radiation Therapy
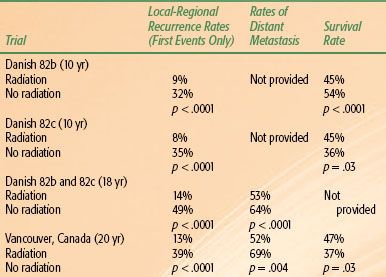
The three most recently completed randomized trials investigating the efficacy of postmastectomy radiation for patients with stage II or III breast cancer were conducted in the 1980s and have 15- to 20-year outcome data. The largest of these studies was the Danish Breast Cancer Cooperative Group 82b trial, which randomly assigned 1,708 premenopausal women with stage II or III breast cancer to receive mastectomy followed by nine cycles of cyclophosphamide, methotrexate, and fluorouracil (CMF) chemotherapy or mastectomy, radiation therapy, and eight cycles of CMF chemotherapy.69 At the same time, this group also conducted the 82c trial, in which >1,300 postmenopausal women were randomly assigned to undergo mastectomy and 1 year of tamoxifen or mastectomy, tamoxifen, and radiation therapy.70 Finally, a smaller trial, conducted in Vancouver, Canada, randomly assigned 318 premenopausal women with lymph node–positive disease to undergo mastectomy and CMF chemotherapy with or without postmastectomy radiation therapy.71 In all three of these studies, patients treated with radiation had a lower long-term risk of isolated local-regional recurrence than did patients randomized to no radiation therapy. Of importance, these improvements led to fewer patients developing metastatic disease and an improvement in overall survival.71,72 The results from these three trials are shown in Table 57.4.
Several important concepts can be ascertained from these studies. First, these studies, together with the Oxford meta-analyses, clearly demonstrate that by reducing local-regional recurrence, postmastectomy radiation therapy could improve overall survival. Second, these trials demonstrated that these patients had a clinically relevant risk of local-regional recurrence despite the use of either CMF chemotherapy or tamoxifen. These findings imply that the benefits of systemic treatments are predominantly to lower the competing risk of distant metastases, which makes the achievement of local-regional control more important.
One controversy that arose after the publication of these studies concerns the most appropriate indications for postmastectomy radiation. Specifically, these trials led to a debate as to whether postmastectomy radiation therapy is indicated for patients with stage II breast cancer with one to three positive lymph nodes. Most of the patients enrolled in the Danish and British Columbia trials had stage II disease with one to three positive lymph nodes.69–71 Accordingly, many have argued that these findings suggest that all patients with lymph node–positive disease should receive radiation after mastectomy. This argument is further supported by the recent analysis from the Early Breast Cancer Trialists’ Collaborative Group, which also noted a survival advantage in the patients with one to three positive lymph nodes.65 The difficulty in interpreting these data, however, is that many patients in these trials did not undergo a formal level I/II axillary dissection. In the Danish studies, the median number of axillary lymph nodes resected was only seven,69 which is approximately 50% of the number reported from studies conducted in the United States. In addition, 76% of the patients had <10 lymph nodes removed, and 15% had three or fewer lymph nodes removed.69 In the Vancouver trial, the median number of resected lymph nodes was 11.73 Given the less extensive axillary surgery done in these studies, it is highly probable that some of the patients in these studies reported as having had one to three positive lymph nodes would have had four or more positive lymph nodes if a standard axillary dissection had been performed. Correspondingly, their risk of a chest wall or supraclavicular recurrence would be higher than that usually estimated for patients with one to three positive nodes. Moreover, failure to remove these additional involved axillary lymph nodes would predispose patients to axillary recurrence, which could be avoided by a more complete axillary dissection. Indeed, the most recent update of the Danish studies reported that 43% of all local-regional recurrences included recurrence in the axilla.72 To further investigate this question, the Danish investigators reanalyzed their trial results specifically with regard to the patients who had eight or more lymph nodes resected. For the patients with one to three positive lymph nodes, there continued to be significant improvements in local-regional control (96% vs. 73%) and overall survival (57% vs. 48%) in the patients randomized to receive postmastectomy radiation.74
TABLE 57.4 LOCAL-REGIONAL RECURRENCE, RATES OF DISTANT METASTASIS, AND OVERALL SURVIVAL IN RANDOMIZED TRIALS COMPARING THE USE OF POSTMASTECTOMY RADIATION FOR PATIENTS TREATED WITH MASTECTOMY AND SYSTEMIC THERAPY69–72