Gene
Penetrance
Risk category
References
Breast genes
ATM
Moderate
Higher than general population
Easton DF
BARD1
Moderate
Higher than general population
Couch FJ
CDH1
High
High
Pharoah PD
CHEK2
Moderate
Higher than general population
Easton DF
NBN
Moderate
Higher than general population
Easton DF
PALB2
Moderate
High
Easton DF
PTEN
High
High
Tan MH
STK11
High
High
Hearle N
TP53
High
High
Easton DF
Other genes
CDKN2A
Moderate
Higher than general population
de Snoo FA
CDK4
Moderate
Higher than general population
APC
Moderate
Higher than general population
Goldgar DE
BMPR1A
Moderate
Higher than general population
EPCAM
High
High
Win AK
MLH1
High
High
Win AK
MSH2
High
High
Win AK
MSH6
High
High
Tung N
MUTYH
Higher than general population
Out AA
PMS2
High
High
Tung N
Eccles and colleagues studied 591 patients with HER2+ BC diagnosed at ≤40 years and found that overall the incidence of predisposing mutations was low in the absence of a family history. Deleterious TP53 mutations in these patients were more frequent than BRCA mutations, suggesting that clinicians should consider this possibility in this specific population, particularly when BRCA testing is negative [17]. TP53 germline mutations may also be identified among individuals without the «typical» Li-Fraumeni syndrome (LFS) family histories due to de novo mutations [18], mosaicism, incomplete family history information or variable penetrance of TP53 germline mutations. Based on these data, the addition of TP53 to BRCA testing should be considered in young BC patients [19].
Genetic counselling and testing can change both primary surgical management (mastectomy vs. breast conserving; see below) and subsequent follow-up (radiologic surveillance, risk-reducing surgery). Risk-reducing mastectomy (RRM) and salpingo-oophorectomy (RRSO) have been shown to be effective preventive measures in healthy mutation carriers, and the NCCN 2017 guidelines recommend in particular to start discussing RRSO in BRCA1 carriers between 35 and 40 years of age, upon completion of childbearing [20, 21].The PROSE multicentre prospective cohort study evaluated the effect of RRSO on mortality in 2482 BRCA carriers: the surgical cohort had a lower all-cause, BC-specific and ovarian cancer-specific mortality [22]. Overall, RRSO can decrease BC risk by 40–70%, ovarian or fallopian tube cancer risk by 80%–90% and overall mortality [23]. No data are available in BRCA-mutated BC patients: new studies are needed to evaluate the impact of RRSO in this setting and properly counsel patients (see ► Chap. 7, for more detailed discussion).
Another important consequence of genetic testing in the clinical management of young BC patients derives from development of poly ADP-ribose polymerase inhibitors (PARPi) in BRCA carriers and the recent FDA approval in metastatic ovarian cancer [24]. This new class of drugs is under active development also in BRCA-mutated BC patients, and phase III studies are ongoing both in the metastatic and early disease settings [25]. The identification of somatic (i.e. in tumour tissue) BRCA mutations in sporadic BC can further expand the indications of PARPi beyond germline mutation carriers. Meric-Bernstam and colleagues reported that the relative frequency of somatic versus germline variants differs according to the involved gene (pathogenic TP53 mutations are more likely somatic; pathogenic somatic BRCA1 and BRCA2 mutations are relatively rare) [26]. Recently, olaparib has been approved in Europe in relapsed ovarian cancer patients with BRCA1 and BRCA2 germline or somatic mutations [27]. In BC, given the technical challenges of somatic genetic testing, the uncertain clinical consequences of such findings and the lack of available therapeutic consequences, clear-cut patient information and consent procedures need to be implemented and investigated before its routine application.
40.4 Diagnostic Procedures, Staging and Follow-Up
Breast density in young women often limits the diagnostic accuracy of mammography in asymptomatic patients, but additional routine screening with tomosynthesis or ultrasound has not yet been proven to be beneficial: the preliminary results of the ASTOUND trial in 3.231 women suggest that ultrasound has better incremental BC detection than tomosynthesis [28], but these results might be overtaken by the forthcoming introduction of tomosynthesis as a routine screening modality in many countries. Digital breast tomosynthesis was approved by the FDA in 2011 based on its improved detection of breast lesions especially in women with non-fatty breasts. The additional benefit of breast MRI is still controversial, but the lack of evidence on improved outcomes speaks against its routine use, irrespective of age [29]. MRI is indicated as an adjunct to mammography in the setting of equivocal mammography/ultrasound, in pathologic axillary nodes with an unknown primary tumour and in monitoring the response to neoadjuvant chemotherapy [30, 31] (see ► Chap. 21 ‘Breast Surgery After Primary Systemic Therapy’).
MRI in addition to mammograpy is recommended in high-risk women, in particular BRCA carriers, and annual MRI screening is indicated in women with history of chest radiation for Hodgkin disease [32].
Systemic staging in young BC patients is not substantially different than in older patients. For patients with stage I/II disease, routine systemic imaging is not recommended in the absence of signs or symptoms suggestive of metastatic disease; in patients with T3 N1–3 or symptomatic disease, additional testing should be considered. Post-therapy follow-up should include regular physical examination every 4–6 months and annual breast imaging for at least the first 5 years. In the absence of clinical signs-symptoms suggestive of recurrent disease, additional investigations are not recommended as no impact on survival or ability to palliate recurrent disease has been described [1, 33].
40.5 Treatments
40.5.1 Treatment for Early Breast Cancer
Neoadjuvant Treatment
BC in young women tends to be diagnosed more often at locally advanced stages than in older individuals [8], and consequently young patients often require and benefit from preoperative systemic therapies [34]. A pooled analysis of individual data from eight randomized trials of the German Breast Group showed that young women (n = 1.453) were more likely to achieve a pCR after neoadjuvant chemotherapy, especially in HR+/HER2- and triple-negative disease (20.9% in women under 40 vs. 17.7% in women 40–49 years vs. 13.7% in women ≥50 years; p < 0.001) [35, 36]. Age was also independently prognostic for survival in HR+/HER2- disease, with women <40 years without a pCR having a worse disease-free survival (DFS) compared to their counterparts achieving a pCR. Despite a higher local relapse rate after neoadjuvant chemotherapy and breast conservation, no long-term significant adverse survival impact in young women has been demonstrated [37] as prognosis in patients most likely to benefit from neoadjuvant strategies (triple-negative and HER2+) is mainly driven by systemic recurrences [38].
The indications for (inoperable or locally advanced tumours especially in BC subtypes associated with high chances of response) and strategy of preoperative therapy need to be discussed and planned in a multidisciplinary team and do not differ according to age [39].
The preoperative therapeutic options include chemotherapy (the same regimens as recommended in the adjuvant setting) and HER2-targeted therapy (dual anti-HER2 blockade with trastuzumab and pertuzumab combined with chemotherapy, if available) [40]. Improved pCR rates among triple-negative disease were shown with the incorporation of platinum agents [41], especially in BRCA mutation carriers [42]. No long-term survival data are yet available, and therefore platinum agents should not be routinely incorporated into neoadjuvant regimens. Limited data are available for neoadjuvant endocrine therapy (ovarian function suppression (OFS) plus tamoxifen or aromatase inhibitors (AIs)) in young patients. An International Breast Cancer Study Group (IBCSG) randomized phase II trial (IBCSG 41–13 TREND) is evaluating the efficacy of the luteinizing hormone-releasing hormone (LHRH) antagonist degarelix versus triptorelin as neoadjuvant treatment in premenopausal patients receiving letrozole. In the absence of definitive data, neoadjuvant endocrine therapy should not be routinely recommended for young women outside of clinical trials however.
The management of patients with significant residual disease after preoperative treatment is challenging. Clinical trials with novel agents/approaches are needed to define the best loco-regional and ≪adjuvant≫ medical treatments in this difficult disease setting.
Adequate time for genetic counselling and testing should be allocated during the preoperative period.
Surgery
Young patients with early BC routinely undergo primary breast (breast conserving or mastectomy) and axillary surgery (sentinel node biopsy or axillary dissection) with or without loco-regional radiotherapy. Margin assessment is particularly relevant for young patients undergoing breast conservation as young age is a well-known independent risk factor for increased local recurrence (LR) [43]. In 2,233 consecutive BC patients who underwent BCT, the risk of LR was associated with younger age (HR for age > 50 = 0.56; p = 0.01), non-luminal A subtype (HR for luminal B = 2.64, HER2 = 5.42, TNBC = 4.32; p < 0.001 each) and higher regional nodal involvement, the latter both representing the most frequent biology and staging characteristics in young women [44]. In the recently published POSH prospective cohort study of 3,024 BC patients aged 18–40 years, the LR rate was higher in patients treated with BCS as compared to mastectomy at 5 and 10 years (5.3% vs. 2.6%, p < 0.001, and 11.7% vs. 4.9%, p < 0.001, respectively), but distant metastases and deaths were lower for BCS when adequate postsurgical radiotherapy, stage at diagnosis and margin width were considered [45]. In addition, a recent registry-based study [46] and meta-analysis confirmed no difference in overall survival (OS) among young BC patients undergoing mastectomy or breast conservation [47]. When mastectomy is indicated or desired by a patient, limited data are available on the survival impact of contralateral RRM, even in mutation carriers. A recent meta-analysis found neither an absolute reduction in the risk of distant metastases nor an improvement in OS associated with contralateral RRM in patients at increased genetic risk, despite the decrease in contralateral BC incidence [48] (for additional details, see ► Chap. 7, ‘Risk-Reducing Surgery’).
The indications for and techniques of axillary and breast/oncoplastic surgery are identical to other age groups (see ► Chaps. 17, 18, 19, 23 and 24). Patients who have one or two pathologically involved sentinel nodes may not require a complete axillary dissection [49].
When mastectomy is indicated, skin- and nipple-sparing techniques with immediate breast reconstruction should be preferred if technically possible, oncologically appropriate and desired by the patient [50]. The indications for postmastectomy loco-regional radiotherapy should not discourage immediate reconstruction when modern radiotherapy techniques are applied, but women should be made aware of the risks of adverse outcomes and inferior long-term cosmesis in such cases.
Radiotherapy
Given the higher local recurrence risk seen in younger women, a boost to the site of excision has to be routinely delivered in young patients treated with breast conservation, and partial breast irradiation is contraindicated outside a clinical trial. Postmastectomy radiotherapy and internal mammary chain irradiation should continue to be discussed on an individual basis, as in older women, and based on initial staging if neoadjuvant treatment is given. Hypo-fractionated abbreviated regimens with higher doses/fractions are becoming more widely used in premenopausal women with satisfactory safety and cosmetic outcomes [51]. While waiting for long-term data, this approach should be proposed on a case-by-case basis.
40.5.2 Adjuvant Systemic Treatments
Adjuvant treatment recommendations are based on the individual risk of relapse, the predicted sensitivity to a specific therapy and the patient’s preferences. Treatments have to be tailored, irrespective of age, according to tumour subtype (defined by grade, vascular invasion, proliferation rate, HR and HER2 expression) and stage. The genomic profile of the tumour (luminal A and B, basal-like, HER2+) [52] may add prognostic information to the classic clinico-pathologic factors, helping in selecting patients likely to benefit from different adjuvant treatments. Young women are nevertheless under-represented in the retrospective studies evaluating the clinical value of these tests. Prospective definitive data from randomized trials (TAILORx, XPonder, PlanB) are awaited to assess in particular the benefit of chemotherapy according to individual tumour gene profiling. In the preliminary reports of the TAILORx [53] and in the GEICAM 9906 trial [54], premenopausal patients represented ~5% of the total. The recent results of the MINDACT study suggest that in patients at high clinical risk of BC recurrence, defined by the traditional clinical and pathological factors, the addition of the 70-gene signature adds relevant information for selecting which patient might benefit from adjuvant chemotherapy over endocrine therapy alone. The authors concluded that chemotherapy could be avoided in patients with high clinical risk but low genomic risk [55]. Patients aged 35–50 years nevertheless represented only 28% of the entire population (women <35 years were <1% of the total). Overall, the evidence is limited in this age group due to the rarity of the disease, and thus these tests should be used with caution in young women.
In view of the longer life expectancy of young women, side effects of adjuvant treatments need to be carefully balanced against benefits, and long-term morbidity should be monitored (e.g. cardiovascular, bone health, cognitive impairment and premature menopause). In particular, the impact on fertility and the available preservation measures should always be discussed with patients as early as possible when planning a treatment strategy (see Fertility and Birth Control below).
Endocrine Therapy
Oophorectomy was the first endocrine treatment used in young women with advanced BC in 1896 [56]. From 1995 tamoxifen, a selective estrogen receptor modulator (SERM), was recognized as an effective adjuvant treatment for patients with HR+ BC. The Early Breast Cancer Trialist’ Collaborative Group (EBCTCG) meta-analysis of all randomized trials of adjuvant tamoxifen showed that 2 to 5 years of treatment have similar efficacy in all age groups, including patients <40 years [57]. However, young HR+ patients showed a constantly high risk of long-term relapse and a worse 10-year BC-specific survival, independent of nodal status, compared to older HR+ patients in a series of 111,993 patients, diagnosed between 1990 and 2003 and included in the SEER database [58]. The sustained reduction in BC mortality beyond year 10, achieved by 5 years of tamoxifen, is therefore of particular interest in younger women. Extended endocrine therapy has not been specifically studied in young patients. The ATLAS [59] and aTTom [60] trials show that continuing tamoxifen to 10 years provides a further reduction in both disease recurrence and mortality. Premenopausal patients constituted only approximately 9% of the ATLAS population, and statistical significance was not reached in this subgroup, likely because of the smaller number of events: nevertheless, these results provide the only available evidence of benefit of extended endocrine therapy in premenopausal patients and should be discussed on an individual basis, especially in patients at high risk of recurrence. The role of aromatase inhibitor (in combination with OFS) as extended endocrine therapy has been investigated in a phase II single-arm trial (2 years of OFS in combination with letrozole after at least 4.5 years of tamoxifen). The study closed after only 16 patients enrolled over 3.5 years, suggesting young women may not be motivated to undergo extended therapies and challenging the feasibility of future studies.
Tamoxifen can induce amenorrhoea and ovarian cysts and increase estradiol secretion, but ovarian hyperstimulation does not appear to impact on DFS [61, 62].
The benefit of OFS (either temporary by LHRHa or permanent by bilateral salpingo-oophorectomy (BSO)) has been long debated.
The results of the IBCSG SOFT and TEXT trials [63] (◘ Fig. 40.1) helped to identify the population of patients most likely to benefit from adjuvant OFS. In the non-chemotherapy cohort of the SOFT trial (949 patients) (mainly older premenopausal patients at low risk of relapse defined as lower-grade, node-negative, low-proliferating, smaller tumours), >95% of patients remained free from BC at 5 years irrespective of treatment received (tamoxifen alone, OFS plus tamoxifen or the AI exemestane). In women at higher risk of recurrence remaining premenopausal after chemotherapy (1,084 patients), in particular if <35 years, a significant improvement in the 5-year BC-free interval (BCFI) was observed with OFS plus exemestane (85.7%) compared to OFS plus tamoxifen (82.5%) or tamoxifen alone (78%). In alignment with the SOFT low-risk cohort data, the Eastern Cooperative Oncology Group (ECOG) trial 3193 (E-3193) conducted in 345 low-risk patients (node negative, tumours <3 cm, no adjuvant chemotherapy) showed no significant DFS or OS difference, at a median follow-up of 9.9 years, between tamoxifen and tamoxifen plus OFS, with OS >95% in both patients’ groups [64].
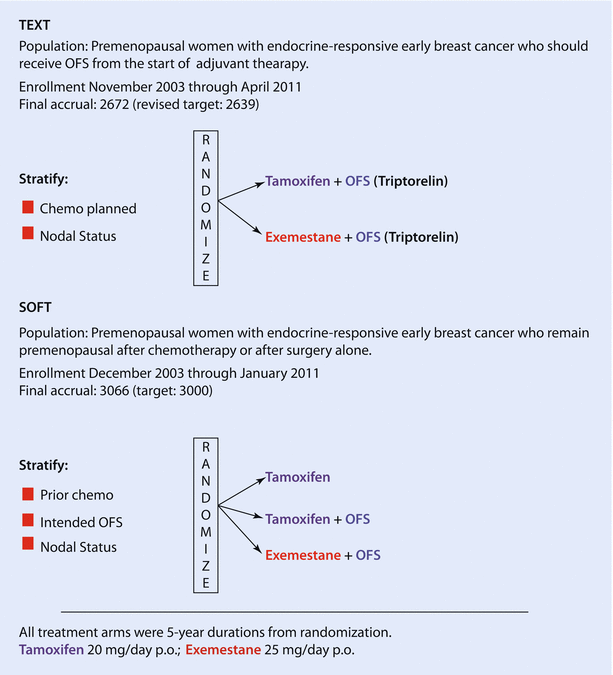

Fig. 40.1
SOFT and TEXT designs
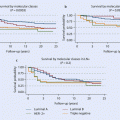
In the combined SOFT-TEXT analysis (4,690 patients), OFS plus exemestane significantly improved the 5-year DFS compared to OFS plus tamoxifen (91.1% vs. 87.3%, HR 0.72; p < 0.001) [65], with an absolute gain comparable with the benefit of AIs in postmenopausal women (◘ Fig. 40.2). The Austrian Breast and Colorectal Cancer Study Group (ABCSG) 12 trial also compared OFS plus tamoxifen or the AI anastrozole in premenopausal patients with early HR+ BC [66]. The trial did not show any difference in DFS between arms, but a significantly higher risk of death for anastrozole-treated patients was reported at 94.4 months of median follow-up. This latter finding may at least in part be explained by both the imbalance in post-relapse AI treatment between arms (61% vs. 41% of patients in the tamoxifen and anastrozole arms, respectively) and differences in outcome between normal-weight and obese patients (overweight patients treated with anastrozole had more than a doubling in the risk of death compared with normal-weight patients). Overall, the different outcomes between ABCSG 12 and SOFT-TEXT may be explained by differences in study design and power: in particular, in the Austrian trial the statistical power was lower (half the number of events), and the 3-year treatment duration is no longer standard for oral endocrine therapy.
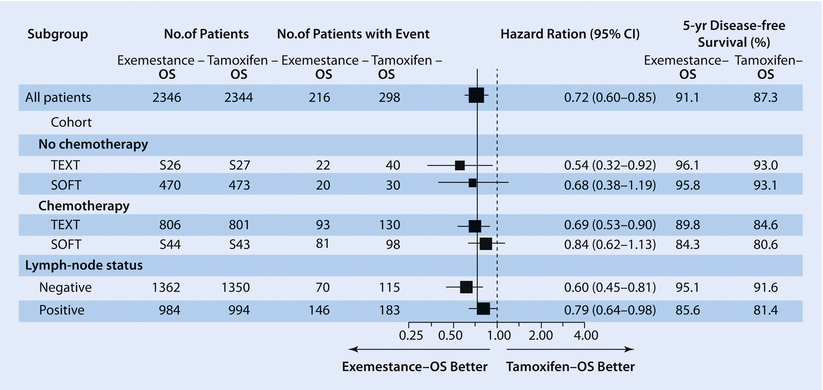

Fig. 40.2
Disease-free survival according to treatment group and patients’ characteristics (From: Pagani et al. [65]. Copyright © [2014] Massachusetts Medical Society. Reprinted with permission. ► http://www.nejm.org/doi/full/10.1056/nejmoa1404037#t=article. ► http://www.nejm.org/doi/full/10.1056/NEJMoa1404037)
All these data contributed to the most recent treatment guidelines (ASCO, St. Gallen, BCY2, NCCN, ESMO) [1, 39, 67, 68], which state higher-risk patients should receive OFS in addition to oral endocrine therapy and lower-risk patients should not. The accurate definition of risk is therefore of paramount importance to tailor treatment in the individual patient. A continuous, composite measure of recurrence risk in HER2-negative patients treated in the TEXT-SOFT trials (4,891 patients) (incorporating age, nodal status, tumour size and grade, estrogen and progesterone receptor and Ki-67 expression levels) allows better weighing of the relative benefits of OFS, tamoxifen and exemestane. The absolute improvement in the 5-year BCFI with exemestane plus OFS versus tamoxifen alone can range from 10–15% in patients with high recurrence risk to at least 5% in women at intermediate risk while being minimal for those at lowest risk [69].
The toxicity profile in TEXT-SOFT was consistent with the given therapy. In TEXT, musculoskeletal symptoms, sexual complaints (vaginal dryness, decreased libido and dyspareunia), osteoporosis and fractures were more frequent in patients taking exemestane than tamoxifen where a higher incidence of sweating, hot flushing and urinary incontinence was reported [65]. In the SOFT trial, grade ≥ 3 adverse events were more frequent in patients receiving OFS compared to those taking tamoxifen alone. Overall, no significant differences in quality of life (QoL) were observed between treatments, but potential toxicity needs to be incorporated in individual treatment planning [70, 71]. Young age is a well-known risk factor for non-adherence/nonpersistence to endocrine therapy [72]: effective strategies to identify and manage non-adhering patients are crucial to ensure the best survival opportunities.
Chemotherapy
The impact of adjuvant chemotherapy in premenopausal women with HR+ disease has been debated and questioned since the 2007 EBCTCG meta-analysis which showed a benefit of OFS only in the absence of chemotherapy [73]. This observation raised the hypothesis that temporary or permanent OFS might partly or fully explain the chemotherapy benefits reported in premenopausal women. Less than 50% of women <40 years develop amenorrhoea with CMF: modern anthracycline-taxane-based regimens are unlikely to cause permanent amenorrhoea in most women <40 years (and almost none in women <35 years). A joint retrospective analysis of 9,864 patients treated within trials of chemotherapy alone from four international cooperative groups (IBCSG-NSABP-ECOG-SWOG) showed the relative recurrence risk was substantially higher for women <35 years with HR+ disease compared with older patients. This finding was not observed for patients with HR- tumours, suggesting the endocrine effects of chemotherapy alone are insufficient for the younger age group [74, 75]. All randomized trials studying the additional benefit of chemotherapy added to endocrine therapy in premenopausal women failed to accrue: the available indirect evidence [64, 66, 76, 77] of excellent 5- and 10-year outcomes in HR+ premenopausal women (even with node-positive disease) treated with combined endocrine therapy alone argues against the universal need for adjuvant chemotherapy in all premenopausal patients.
When chemotherapy is needed (HR- or high-risk HR+ disease), the choice of regimens (taxane-anthracycline based) and total duration (4–8 cycles) are the same in pre- and postmenopausal patients (see ► Chap. 36 Adjuvant Chemotherapy, Curigliano) as the proportional risk reductions with modern drug combinations are little affected by age [78]. In the absence of any data favouring one approach over the other, sequential regimens have at least equal efficacy over combination regimens and may be better tolerated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree