Fig. 44.1
Data from the UK National Cancer Registry on stage and prognostic factors by age subgroup (Modified from [35])
There is some evidence that if this stage difference could be eliminated, relative to younger cohorts, survival outcomes for older women could be improved [37].
44.4 Screening in Older Women
Most of the trials of breast screening had upper age limits of 65–75 years with very few women in older age groups included, and there is therefore little direct high-level evidence of benefit in this age group and some good theoretical reasons why it may be less effective (increasing risks of death from other causes being the main concern). Detection of breast cancer with mammography is more accurate in older women as breast tissue is less dense and therefore more radiolucent. The sensitivity of mammography in women over the age of 60 is 95% [38]. However, the relative benefit of screening is diluted by the relatively reduced life expectancy and increased comorbidities of this age group. Many will die of other causes before the cancer becomes symptomatic, and therefore rates of overdiagnosis will be higher.
It has been suggested that a woman would need to survive between 5 and 10 years to benefit from screening mammography [40]. The problem is predicting women for whom this is likely and even more problematic, administering such a system at a population rather than an individual level. Whilst it is not possible to use direct evidence from trials to support the use of screening in older women, there are indirect methods based either on the use of surrogate markers (such as reduced stage at diagnosis) or statistical modelling studies [39]. Such studies suggest that screening will have cost-effective benefit up to the age of ~78 years based on UK data (◘ Fig. 44.2). Numerous other studies have suggested that screening is likely to be beneficial beyond current age ranges [41], albeit with reduced relative cost efficacy [42]. However, a note of caution is needed: evidence from screening in the over 70s in the Netherlands has shown a large increase in the diagnosis of early-stage cancers but only a very small reduction in rates of presentation with advanced disease [43]. This implies the benefit will be small and rates of overdiagnosis large.
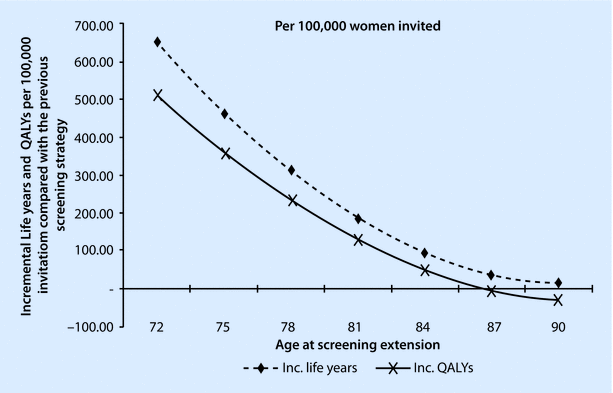

Fig. 44.2
Incremental life years, QALYs, and costs per 100,000 women invited to screening (compared with the previous screening strategy). QALY, quality-adjusted life year (Reproduced from Rafia et al. [39] with permission from Elsevier)
44.5 Tumour Characteristics
44.5.1 Tumour Biology
Breast cancer subtypes vary significantly between older and younger cohorts. The biological characteristics are generally more favourable in older age group, with a strong correlation between expression of the oestrogen receptor (ER) and age [44]. A large study of the US SEER database found that 83% of women younger than 65 years old, 85% of women between 65 and 74 years of age and 91% of women older than 85 years old had ER-positive tumours [36]. There are also higher rates of expression of the progesterone receptor and lower expression of the human epidermal growth factor receptor type 2 (HER-2) which varies from 26% in women under 40 to 12% in women over 80 [35]. There may also be a higher incidence of diploid tumours, a lower proliferative (S-phase) fraction, lower rates of mutation of the tp53 gene and lower rates of lympho-vascular invasion [45]. Little work has been done using the more recently described genomic classifications (luminal A, B, Her-2 enriched and triple negative) [46, 47], but early studies have confirmed that these differences are present with lower rates of Ki67 expression, tp53 mutation and cytokeratin expression rates. It is thought that subtle differences within each subtype according to age (in favour of older women) may play a role in the lack of major variance in relative survival rates between older and younger women, subtype for subtype, despite the relative lack of adjuvant chemotherapy in older women [48].
In modern breast practice, composite multigene arrays are increasingly used to calculate prognosis or recurrence risk (Oncotype DX©, Mammaprint© and many others, overview in ► Chap. 9). In the case of Oncotype DX©, high recurrence scores are more commonly seen in women under age 40 versus women over age 40, but there is little variance between older age subgroups [49]. Whether this carries the same prognostic significance in this age group is yet to be established in prospective studies. There is little data relevant to this age group with the other multigene arrays at this time.
Invasive ductal carcinoma remains the most frequent type of breast cancer regardless of age; however, studies have suggested a higher incidence of mucinous and lobular tumour subtypes in the older age group. Tumour grade may vary by age. UK registry data has shown lower rates of grade 1 cancers in older women ([35] ◘ Fig. 44.1), but this could be a reflection of their later presentation [50] and the lack of screening in this age group which tends to select for slow-growing good prognosis cancers [51].
44.6 Management of Breast Cancer in Older Women
44.6.1 Surgery
Excision of the primary tumour and staging or therapeutic axillary surgery, followed by appropriate combinations of adjuvant therapies, should be the gold standard for patients with breast cancer regardless of their age. Variable surgical practices have been reported when managing an older patient. Assumptions that older patients suffer from multiple comorbidities and are therefore unfit for general anaesthesia and have a lower life expectancy have led to omission of surgery in a significant proportion of older patients with ER-positive cancers. These women may be offered primary endocrine therapy as an alternative to surgery in up to 40% of women over the age of 70 [6, 52, 53]. There have been a number of randomized [54] and non-randomized studies [55] which have compared and evaluated primary endocrine therapy compared to surgery in older women which are reviewed below. Rates of surgery are highly variable in older women [56] reflecting a lack of evidence-based guidelines on best practice in this age group and differing surgeon opinions about the use of surgery or primary endocrine therapy [57]. For those older women who do undergo surgery, most tend to tolerate it well [58]. Mortality from breast surgery is generally low. The UK National Mastectomy and Reconstruction Audit reported an overall mortality from breast surgery of 0.26% but did not break this down by age group [59]. There are several surgical options which are discussed in more detail below in the context of the older patient: breast conservation surgery, mastectomy, axillary surgery and reconstructive surgery.
Mastectomy
The morbidity and mortality rates from mastectomy are relatively low in non-age-specific studies. Mortality rates for patients above the age of 75 can vary from 0.95% to 4.5% [60]. Although not statistically significant, the 90-day mortality rate in patients over 70 is marginally lower for those who undergo breast-conserving surgery compared to those who undergo mastectomy, 0.3% versus 1.4% [61]. Patients undergoing mastectomy are at greater risk of complications than those who have breast-conserving surgery with the common complications of wound infection, bleeding, haematoma and seroma, being more likely to occur in older patients, with the highest number of complications in those over age 85 or those on multiple medications [62]. Rates of mastectomy compared to conservation seem to vary with age. The UK cancer registry shows that the highest rate of conservation surgery is seen in the screened age range (1.8:1, BCS versus Mx, age range 50–70), but in women aged 70 to 79, the ratio drops to 1:1 and to 1:0.7 in the over 80s [35]. It is likely that this partly reflects the lack of screening and larger tumour size in older women, partly a reluctance to undergo radiotherapy and partly patient preference as older women may be less concerned with the cosmetic aspects of such surgery [63, 64].
Breast-Conserving Surgery
Breast conservation surgery, usually followed by radiotherapy, is an oncologically safe option for the majority of women with breast cancer and is generally well tolerated and may be performed under general or local anaesthesia. (The necessity for radiotherapy after BCS in older women is discussed below.) In the past decade, BCS techniques have expanded to include oncoplastic procedures which may involve limited or extensive mobilization of parenchymal flaps and much more extensive surgery. There has been very little published data on outcomes of oncoplastic surgery in older women [65, 66] but that which there is suggests reasonable outcomes very similar to those in younger women. Breast reduction surgery for non-cancer cases in older women is also well tolerated with generally higher satisfaction rates than in younger women [67]. Most of the series have used 60 or 65 years of age as the definition of elderly however and included few women over age 70. Older women tend to present with larger primary cancers and usually have significant levels of breast ptosis which may make them more suitable for type 2 oncoplastic procedures. However the fact that breasts are often fatty, and arteriopathy more prevalent, may increase the risks of fat necrosis if extensive parenchymal mobilization is done injudiciously.
Axillary Surgery
In broad terms the gold standard for management of the axilla is to perform a SLNB for the clinically uninvolved axilla and a clearance for clinically node positive disease (see ► Chaps. 22 and 23).
Sentinel node biopsy is a minor procedure with minimal morbidity which can be performed under local anaesthesia if needed [68]. In the clinically node-negative axilla, in a frail or unfit, older patient, omission of axillary surgery or surgery to the primary only plus adjuvant endocrine therapy is likely to be a valid option.
It is generally accepted that axillary clearance surgery carries only a minimal survival advantage [69–71] and is largely performed to ensure locoregional control, establish prognosis and so guide adjuvant treatment decisions.
Before the introduction of sentinel node biopsy, older patients were less likely to have axillary clearance compared to their younger counterparts [72, 73]. A randomized control trial by Martelli and colleagues (2005) of patients above the age of 65 (median age 70, range 65–80), who underwent surgery with or without axillary node clearance in patients with T1 N0 breast cancer, concluded that axillary dissection was not necessary and did not affect overall survival [74]. The trial findings were updated with 15-year follow-up confirming the lack of difference in overall survival and showing only a small additional number of axillary recurrences (0% versus 6% in the no axillary clearance arm) [75]. Analysis of a cohort of over 400 older women in whom axillary surgery was omitted with 15-year follow-up showed a differential rate of axillary recurrence by stage with 3.7 versus 8.9% in T1 versus T2 disease suggesting that a selective approach should be taken [76]. As many older women have ER-positive cancers, additional local control is provided by endocrine therapy, and in the post Z0011 era [77], it is now known that axillary disease control may be enhanced with low axillary radiotherapy tangents given during whole breast radiotherapy. There is good evidence that even before the publication of the Z0011 trial approximately half of all low-risk women (small ER positive, older age) did not proceed to completion clearance after a positive SLNB [78]. The need for completion clearance may also be guided by the use of predictive axillary nomograms [79]. The AMAROS (After Mapping of the Axilla: Radiotherapy Or Surgery) study suggests that radiotherapy may be a valid treatment option for women with a positive SLN rather than surgical clearance. The trial data have not been examined specifically with relation to older women, but this strategy does provide a good alternative to women for whom a second anaesthetic may carry risks.
As a result of the above, in an older woman with risk factors for anaesthesia, careful consideration should be given to whether completion clearance is necessary, and if there are significant risk factors for surgery, axillary radiotherapy or no further axillary treatment may be appropriate alternatives. Risk-stratified trials in older women regarding the value of axillary clearance are lacking, but there is randomized trial data that this strategy is oncologically safe in women of all age groups.
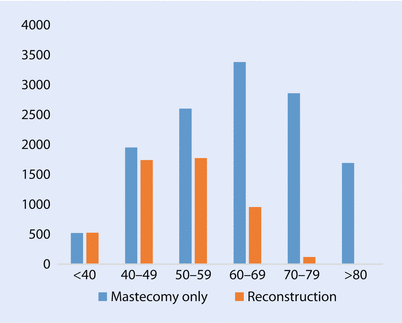
Reconstructive Surgery After Mastectomy
Whilst it is appropriate for older women to be considered for reconstructive surgery after mastectomy if it is oncologically appropriate, consideration must be given to the risks of more major surgery with prolonged anaesthesia in older women with significant comorbidities and frailty. For some women, it may therefore pose an unjustifiable risk. The UK National Mastectomy and Breast Reconstruction Audit showed that older women are significantly less likely to undergo reconstruction (◘ Fig. 44.3), a finding confirmed by many other authors [80]. This probably reflects concerns about the increased risks of surgery, assumptions about body image being less important in this age group and reduced rates of involvement in the decision-making process. The small number of older women who are offered reconstruction generally reports good satisfaction rates and subsequent QoL. Indeed there is some evidence that they may be more satisfied than their younger counterparts [80]. The type of reconstruction varies with age with older women more likely to be offered the simpler and usually quicker implant-based techniques [80]. Rates of complications may vary with age, but the data is far from conclusive. Most series that have compared morbidity rates with younger versus older women have very small older cohorts, and these are usually at the lower end of the age spectrum, usually defined as over 65, rather than over 70, often with very few truly elderly subjects. It is therefore likely that the elderly cohorts are very heavily selected to be at the younger, fitter and more committed end of the spectrum and hence not representative of the average elderly woman. Matters are further complicated in that age is associated with increased comorbidity rates, and whilst some studies have corrected for this in multivariable analysis, it is not always possible to do this fully and none have corrected for frailty. One such study found that complications after implant-based surgery were 2.5-fold higher in older women (over 65, only 5% of the series) [81]. Another study found that age was not an independent risk factor for autologous flap failure when correcting for comorbidities, but once again the series had approximately 5% of patients over age 65. They found no difference in length of stay or rates of medical or surgical complications, just an increased risk of blood transfusion [82].
The level of good-quality evidence of reconstruction safety and outcomes is qualitatively weak but that which there is suggests that in selected women, reconstruction is safe and associated with high levels of satisfaction. Good-quality prospective trials or cohort studies would be very valuable to give more details about QoL, frailty and comorbidity in older reconstruction patients.
In terms of the older woman’s preferences for reconstruction surgery, there is a lower rate of interest. The underlying psychology of body image with ageing is complex. Physical appearance becomes less important with age [64] although body image is age-stable until quite an advanced age. What changes seems to be that age moderates the association between body image and emotional distress [63]. Consequently, older women may be less emotionally upset by changes to their appearance and so less keen to undergo reconstructive surgery, especially when faced with the increased risks and complexity of surgery.
44.7 Systemic Therapy
44.7.1 Adjuvant Hormonal Therapy
There is good evidence to support the benefit for older patients taking adjuvant hormonal therapy if they have ER-positive disease. The EBCTCG review of 5 years of tamoxifen versus placebo found an approximate reduction in breast cancer-specific mortality of one third. Although women over 70 were relatively poorly represented in these trials (~13%), analysis of this cohort found the highest proportionate risk reduction (RR 0.5) of any age group [83]. This is perhaps predictable given the biology of disease in this age group. Overview of the trials comparing AIs with tamoxifen had excellent representation in the over 70 age group, where improved outcomes were seen with AIs, with a trend suggesting greater efficacy in older women (recurrence relative risk of 0.6 in favour of AI) Early Breast Cancer Trialists Collaborative Group (EBCTCG 2015).
Adjuvant AIs also have adverse effects, predominantly joint pains, hot flashes and accelerated bone loss, and although they are generally well tolerated, some toxicities are of increased relevance in older women. One of them is a reduction in bone mineral density (BMD) which may result in an increased rate of bone fractures [84, 85]. This is of particular concern in older women in whom osteopenia and osteoporosis are extremely common. There is evidence that the rate of bone loss on AIs is less in women who are stably into menopause rather than in younger women (◘ Fig. 44.4) [86, 87], but despite this, rates of fracture are still high in this age group (compounded by other age-associated risk factors such as low baseline BMD, fatigue, frailty and sarcopenia). There is good evidence from numerous trials that IV or oral bisphosphonates, when co-administered with an AI, significantly reduce bone loss [86, 88, 89]. The general consensus is that women should be managed according to their risk assessment and a baseline BMD result. Risk factors for fractures include age over 65, smoking, a personal or family history of fragility fractures, a T-score of less than 1.5 and prolonged use of corticosteroids. Older women already have one risk factor due to their age, so all should be advised to take calcium and vitamin D, and many should be advised to take a bisphosphonate and have BMD monitoring at intervals during treatment [90].
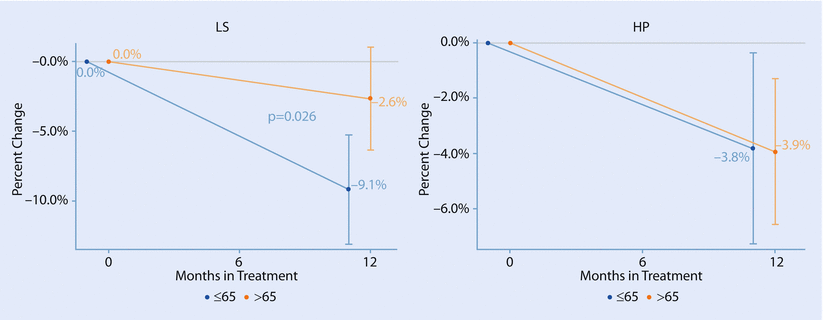

Fig. 44.4
Average bone mineral density change at the lumbar spine and hip by age group in 50 patients with normal BMD at baseline receiving anastrozole (Reproduced from Markopoulos et al. [88] with permission from C Markopoulos)
44.7.2 Adjuvant Chemotherapy
Adjuvant chemotherapy is usually recommended for women with high recurrence risk breast cancer and is a major factor in the improvement in outcomes observed in the past few decades. It is thought to reduce breast cancer-specific mortality by approximately one third across all age groups (on meta-analysis of trials), but few women over 70 have been included in these studies [91], introducing statistical uncertainty over the effect size. The benefit to overall survival is less in older women due to competing risks of death from other causes and increased risks of death and severe morbidity from chemotherapy itself. Mortality rates from chemotherapy in this age group are still low but higher than in younger women.
Consequently, a smaller percentage of older women are offered chemotherapy. A UK study of chemotherapy usage in older women found rates were lower than in younger women and highly age dependent (◘ Fig. 44.5) and also highly variable between breast units. Rate of chemotherapy for all women over age 70 varied between 0 and 29% and for high-risk women between 6 and 60% [92].
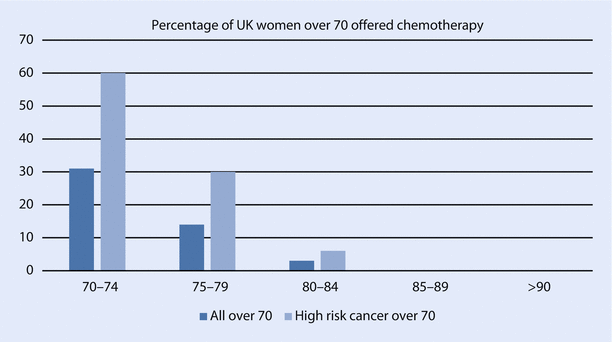

Fig. 44.5
Rates of adjuvant chemotherapy use by age subgroup and cancer recurrence risk (Data modified from Ring et al. [92])
Recent studies suggest that older women with early-stage breast cancer who are active and well with limited comorbidities should be offered the same chemotherapy as younger women [25, 93]. Trials specifically looking at the safety and efficacy of chemotherapy in this age group have been problematic due to small numbers and recruitment problems so data are lacking relative to other age groups to support such a policy. The CASA and ACTION trials, both randomized comparing chemotherapy versus no chemotherapy, were prematurely closed, due to difficulty recruiting patients [94].
The Cancer and Leukaemia Group B (CALGB) trial studied patients over the age of 65. They were randomized either to standard chemotherapy arm treated with cyclophosphamide, methotrexate and fluorouracil (CMF) or capecitabine. Patients who had capecitabine alone were more likely to have relapse of the disease and a higher mortality rate. The patients in the standard arm suffered more often from moderate to severe toxicity from the chemotherapy treatment. Older patients had higher chemotherapy-related deaths [95]. The ELDA trial, a multicentre study, compared the standard chemotherapy regimen (CMF) to weekly docetaxel. The standard chemotherapy regimen was found to be more effective than docetaxel, as QoL and toxicity were noted to be worse in the docetaxel arm [96]. Two agents (nab-paclitaxel and capecitabine) have been reported to have a favourable toxicity profile in older patients [97]. Results from the study by Aapro and colleagues found that weekly nab-paclitaxel was effective and well-tolerated first-line therapy for older patients particularly at the dose of 150 mg/m2 weekly for 3 weeks followed by 1 week of rest [98]. A randomized phase 2 study comparing epirubicin and cyclophosphamide (EC) or CMF versus nab-paclitaxel and capecitabine (NC) in patients above the age of 65 with moderate- to high-risk early breast cancer reported a higher grade of haematological adverse events in the EC/CMF arm compared to the NC arm, but non-haematological toxicities such as diarrhoea and sensory neuropathy were more common in the NC arm [97].
Fitter older patients with HER-2-positive cancer may be offered monoclonal antibody treatment, in the form of trastuzumab, either as monotherapy [99] or in combination with an endocrine therapy or with chemotherapy depending on their level of fitness. Care must be taken to monitor cardiac function however. Levels of efficacy of chemotherapy-free regimens are lower than with chemotherapy-associated administration.
44.7.3 Primary Endocrine Therapy
Primary endocrine therapy is the treatment of oestrogen-sensitive breast cancer solely with endocrine therapy agents, omitting surgery altogether. This was first suggested over 30 years ago [100] and since then has become quite widely used in some countries. Randomized trials comparing surgery and primary endocrine therapy, whilst methodologically flawed, showed that whilst rates of local control are inferior, survival rates are little different except in younger age groups where surgery is advisable [101, 102]. Very long-term follow-up studies have shown that for those patients with a long predicted life expectancy, surgery is beneficial. However, there is no guidance on the age and comorbidity characteristics that predict that surgery is not necessary and the only randomized trial that planned to collect detailed comorbidity data, the ESTEEM trial, closed prematurely due to poor patient participation and is unlikely to ever be replicated [103].
For frail women with reduced life expectancy and for whom surgery will confer little benefit and may impact negatively on QoL, primary endocrine therapy may be appropriate. Older women themselves tolerate this treatment very well [104]. Although there have been no direct RCTs to compare tamoxifen and aromatase inhibitors in the primary endocrine therapy setting, cohort study analysis has demonstrated that aromatase inhibitors are superior [55].
44.7.4 Adjuvant Radiotherapy
Treatment with whole breast radiotherapy is the standard of care after breast-conserving surgery, and chest wall radiotherapy is indicated in high-risk disease after mastectomy. In the post-BCS setting, radiotherapy halves the local recurrence rate and, to a lesser extent and after longer follow-up, reduces the mortality rate in studies of all age groups, including in women over age 70 [105]. However, for an older woman with a reduced predicted life expectancy, especially if she has a low-risk, ER-positive cancer, the benefits of radiotherapy may be small and not outweigh the risks and inconvenience of radiotherapy. Fitter older patients, especially those with higher-risk disease, are still likely to benefit from radiotherapy. Systemic therapy plays a role in local disease control, and women with ER-positive cancers gain significant protection from recurrence from adjuvant anti-oestrogen therapy [105, 106]. The PRIME 2 trial evaluated either radiotherapy or no radiotherapy after breast-conserving study in older women with good prognosis cancer on adjuvant endocrine treatment in a randomized setting. The study demonstrated that post-operative radiotherapy did reduce local recurrence rates at the 5-year median follow-up from 4.1% to 1.3% [107]. The CALGB 9343 study similarly showed a significant effect of radiotherapy on rates of loco-regional recurrence after 10 years of follow-up (10% versus 2%) [108]. However, neither trial showed any difference in overall or breast cancer-specific survival which suggests that omission of radiotherapy may be appropriate in some patients with a reduced life expectancy or after discussion with the patient about the relative risks. The PRIME 2 trial also found that radiotherapy in this age group had no significant impact on long-term overall QoL but did result in an increase in longer-term breast symptoms such as breast oedema [109].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree