There have been several important advances not only in the treatment of breast cancer but also in our understanding of breast cancer biology since the last edition of this text was published in 2008. There have been direct clinical applications of translational research in the understanding of breast cancer molecular subtypes and gene profiling. This has resulted in the recognition of the “triple negative” (estrogen receptor [ER], PR, and HER2 neg) subtype and its clinical behavior and also in a better understanding of the therapy of HER2 positive disease. Also, there seems to be evolving several “trends” in breast cancer therapy; some without a scientific basis. There is a trend away from breast-conserving therapy (BCT) despite 30 years of data confirming the equivalence of BCT with mastectomy in selected cases. There is an even more disturbing trend toward contralateral prophylactic mastectomy despite a lack of data supporting benefit from the procedure.
Another trend that does indeed have a sound rationale is in the use of anthracycline in the adjuvant setting both in HER2 positive and HER2 negative breast cancer. However, this is especially important in HER2 positive cases because of the increased cardiotoxicity risk associated with trastuzumab plus an anthracycline.
There is a trend away from the use of tamoxifen or raloxifine in breast cancer risk reduction. Women at high risk of developing breast cancer are seldom treated with these agents despite their proven clinical efficacy.
Since the last edition of this text, targeted biologic therapy has had a checkered track record in breast cancer. We have observed the rise and possibly the fall of bevacizumab in combination with chemotherapy in the treatment of metastatic breast cancer. Adjuvant trials of bevacizumab in early breast cancer are still in progress, but similar trials in early colon cancer have been disappointing.
However, poly (ADP-ribose) polymerase (PARP) inhibitors, which block excisional DNA repair process by poly (adenosine diphosphate-ribose) polymerase or PARP, appeared to show promise in the treatment of metastatic triple negative and BRCA-1 positive breast cancer, but even this is being questioned.
1Each of these “advances” as well as the “trends” is discussed in more detail in subsequent sections of this chapter.
MOLECULAR SUBTYPING OF BREAST CANCER
It was originally proposed by Perou et al.
2 in the year 2000 that the phenotypic diversity of breast tumors might be accompanied by a corresponding diversity in gene expression patterns. They noted that there are distinct types of epithelial cells in the human mammary gland:
basal (myoepithelial) and
luminal. Many genes are expressed by these two cell lineages, but not by the other. ER+ tumors had a high expression of the genes expressed by breast luminal cells. ER negative tumors often had basal-like epithelium. Breast tumors that overpressed HER2 were associated with a high expression of a specific subset of genes. They proposed four groups of breast cancer: ER+ or luminal-like, basal-like, HER2 positive, and normal-breast-like.
This preliminary work has been amplified by other investigations such as Sotiriou and Pusztai,
3 Parker et al.,
4 Loi et al.,
5 Cheang et al.,
6 and Nguyen et al.,
7 to mention a few. With more refinement, the luminal ER+ subgroup has been divided into two groups: luminal A and luminal B. The normal-like subgroup has been dropped as a probable artifact, but the basal-like and HER2 overexpression groups have been retained.
The distribution of molecular subtypes varies with race and age. The good prognosis luminal A tumors are significantly less common in premenopausal as well as in African American women, and the latter group has a greater incidence of the poor prognosis “triple negative” tumors. In general, luminal breast cancers represent 67% (two-thirds) of all breast cancers, of which 57%-61% are luminal A, 31%-33% are luminal B, and 9% are luminal HER2.
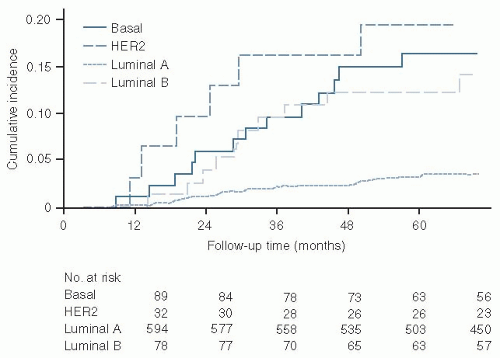
The cumulative incidence of distant metastases by molecular subtype is illustrated by
Figure 32.1. The characteristics of the luminal A subtype are illustrated in
Table 32-1, of the luminal B in
Table 32-2, of the basal type in
Table 32-3, and the HER2 positive subtype in
Table 32-4.
Given that molecular subtyping is a major advance in our understanding of breast cancer biology, has this new data been transferred into clinical trials? The answer is both yes and no.
Yes in ER+ node negative breast cancer. The
TAILORx study coordinated by the Eastern Cooperative Oncology Group (ECOG), which evaluated the 21-gene the 21-gene recurrence score assay (Oncotye Dx) in ER+ node negative breast cancer therapy.
8,9 Their accrual goal was 10,500 cases. The other large clinical trial evaluating ER+ node negative breast cancer is the MINDACT (microarray in node-negative disease may avoid chemotherapy) trail being conducted in Europe.
10 This is evaluating the 70-gene Mammaprint assay. The accrual goal is 6,000 patients.
No in ER+ node + HER2 neg breast cancer. This is a very fertile area in which the differential sensitivities of luminal A versus luminal B cases need to be evaluated in terms of hormonal sensitivity and chemosensitivity. No clinical trials are currently available evaluating these patients.
Yes in HER2 positive breast cancer. Several adjuvant trials have been completed which contain the benefit of combining trastuzumab
with chemotherapy in both the adjuvant and the neoadjuvant setting.
11,12,13,14 No in “triple negative” breast cancer in the adjuvant setting despite their well-recognized poorer prognosis.
Practical considerations. How can the average clinician take advantage of breast cancer molecular subtyping without subjecting the patient to expensive genetic profiling? There are a series of
standard prognostic criteria available on all new breast cancer patients (
Table 32-5). Given these standard criteria, one can identify two of the four subtypes easily. These are HER2 positive and triple negative. One can identify the ER+ luminal subtypes, but cannot differentiate luminal A from luminal B. This differentiation is of increasing importance because of the poorer prognosis of luminal B cases, their relative tamoxifen resistance, and possible greater chemosensitivity. The differentiations between luminal A and B can be approximated by clinical means (
Tables 32-1 and
32-2). Luminal A tends to be both ER+ and PR+. The primary tumor tends to be small (almost 2/3 are T1), and they are always HER2 neg. Luminal B is often ER+ and PR-. The primary tumors are larger (>50% T2), they occur at a younger age, are associated with higher grade, and 30% are HER2 positive. If a Ki67 index is done, luminal A are <14% positive, whereas luminal B are >14% positive. Therefore, the clinician can have a pretty good idea as to which patients may need additional therapy based on the standard prognostic criteria.
THE ALTERNATIVE HYPOTHESIS
With a better understanding of breast cancer biology, systemic therapy has been integrated into the multidisciplinary treatment of newly diagnosed disease. The recognition that breast cancer is frequently a systemic disease at diagnosis is a major conceptual change that has revolutionized our approach to this common malignancy. This conceptual change has provided the theoretic framework not only for systemic adjuvant therapy but also for breast-conserving surgery (BCS). Appropriately administered systemic therapy, in conjunction with conservative surgery and radiation, has improved the survival and decreased the morbidity of breast cancer patients compared with radical surgery alone.
Henri François Le Dran (1685 to 1770), a French surgeon, proposed that breast cancer was a localized disease that spread to regional lymph nodes (RLNs) and that the only hope for cure was early surgery.
15 Le Dran concluded that once a drop of cancerous lymph passed the adjacent lymph nodes, it contaminated the entire system. The concept that breast cancer was a localized disease that spread in an orderly manner dominated cancer theory for the next 200 years.
The hypothesis that breast cancer is a localized disease was carried to its logic conclusion by William Stewart Halsted, who developed radical mastectomy in 1890. The theoretic rationale for radical mastectomy was based on an orderliness of tumor spread from the primary lesion in the breast to RLNs, followed ultimately by systemic metastases. To Halsted, the proper cancer operation consisted of removal of the primary tumor, RLNs, and pectoral muscles en bloc. Radical cancer surgery based on anatomic considerations remained unchallenged for >75 years.
16The major driving force behind challenging the fundamental principles of radical surgery through establishment of new concepts of breast cancer biology has been Dr. Bernard Fisher of the University of Pittsburgh.
17 Fisher noted that the Halstedian theory of en bloc resection was based on Virchow’s proposal that RLNs are effective filters and barriers to tumor spread. In collaboration with his brother, Edwin Fisher
18,19 was able to demonstrate in the laboratory that RLNs were ineffective barriers to the passage of tumor cells and that hematogenous and lymphatic dissemination of tumor cells was of equal importance. Fisher then proposed an alternative hypothesis of breast cancer biology (
Table 32-6) and proceeded to verify the hypothesis through the clinical trials of the National Surgical Adjuvant Breast and Bowel Project (NSABP).
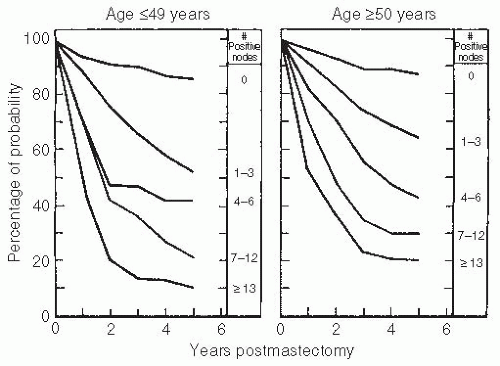
20,21,22,23,24,25Early NSABP trials confirmed the prognostic value of RLN involvement. These data and subsequent follow-up data confirmed that the natural history of breast cancer was directly related to the number of axillary nodes involved and that axillary nodal involvement was the single most important prognostic variable
26,27 (
Table 32-7;
Fig. 32.2).
Between April 1976 and January 1984, the NSABP randomized 1,855 women to modified radical mastectomy (MRM) or segmental mastectomy (lumpectomy) with an axillary dissection.
28 Data from this trial confirmed that women with primary breast tumors of 4 cm or less who were treated with lumpectomy, axillary dissection, and primary breast irradiation had disease-free (DFS) and overall survivals (OS) equivalent to those of women treated with MRM. Therefore, the surgical principles of the alternative hypothesis are sound.
29,30Numerous trials of systemic adjuvant chemotherapy (AdC) and endocrine therapy have confirmed these principles by demonstrating improved DFS and absolute survival in systemically treated patients.
25,31,32,33,34 The adjuvant therapy of breast cancer are discussed in more detail in a subsequent section.
ENDOCRINE THERAPY
Hormonal therapy for metastatic breast cancer has been in use for >100 years. This is the first form of targeted therapy ever used for the treatment of malignant disease to date. Hormonal therapy of breast cancer remains a powerful tool.
In hormone-responsive patients with advanced breast cancer, endocrine therapy is the mainstay of effective, well-tolerated treatment. Metastatic and inoperable breast cancers are essentially incurable, and the therapeutic challenge is in providing effective palliative therapy while maintaining a good quality of life. Endocrine therapy is the treatment of choice for patients with metastatic disease that is not immediately life-threatening, such as soft tissue or bone metastases.
There was a resurgence resurgence of interest in endocrine therapy from the 1940s through the 1960s with several major breakthroughs (1) the full range of ablative therapy (oophorectomy, adrenalectomy, and hypophysectomy) was developed and (2) additive hormonal therapy was initiated with high-dose estrogens, androgens, and progestins.
With the introduction of combination chemotherapy in the late 1960s and 1970s, endocrine therapy of breast cancer briefly fell out of favor.
35 Endocrine therapy was unreliable, yielding only a 20% to 30% objective response and at least 6 to 8 weeks were required for the response to occur. With chemotherapy, objective responses occurred in 60% to 70% of patients treated, and the onset of action was relatively rapid.
In the late 1970s, however, there was a strong resurgence of interest in endocrine therapy of breast cancer. Receptors for estrogen and progesterone and their role in predicting response to hormonal manipulation were documented (
Table 32-8). Endocrine therapy could now be more specific, and higher response rates in selected cases could be anticipated. The introduction of tamoxifen, megestrol acetate, and the aromatase inhibitors (AIs) relegated the more toxic androgens and estrogens to tertiary and quaternary roles. The major surgical ablative procedures of adrenalectomy and hypophysectomy are now of historic interest only. Luteinizing hormone-releasing hormone (LHRH) agonists have been synthesized and have been introduced into clinical practice.
36 The era of hormonal therapy that was spawned in the 1980s continues to mature in the new century.
37The pioneering work of Jensen et al.,
38 McGuire,
39 Wittliff,
40,41 and DeSombre et al.
42 has established the relationship between responsiveness to additive or ablative forms of hormonal manipulation and the presence of the ER (
Table 32-9). By restricting hormonal manipulation of patients whose tumors are ER+, response rates to endocrine therapy can be increased from 25% in unselected cases to 55%. Interestingly, the response rates to additive (56%) and ablative (55%) hormonal therapy in ER+ cases are equivalent. This reflects the fact that the most commonly used forms of hormonal therapy basically do the same thing: block the production or the action of estrogen.
Horwitz et al.
43 noted that the synthesis of PR depends on an intact cellular hormonal system. It was postulated that the presence of PR in addition to ER would further predict hormonal responsiveness. This has, indeed, proved to be the case; ER+ PR+ tumors yield a 78% rate of response to hormonal therapy
41 (
Table 32-8). The relationship between PR and ER appears to be more complex than originally thought. ER+ PR- tumors express higher levels of HER1 and HER2 and are more clinically aggressive than ER+ PR+ tumors. The lack of PR expression in ER+ tumors appears to be a marker of aberrant growth factor signaling. ER+ PR- tumors also tend to be relatively tamoxifen resistant. ER+ PR- tumors tended to be larger than ER+ PR+ tumors and to have higher proliferation rates.
44,45 However, ER+ PR- tumors remain responsive to AIs which implies which the ER pathway remains intact.
46,47,48,49The ER and PR content of a breast cancer is an important prognostic indicator as well as an indicator of response to endocrine therapy.
50,51,52,53 Receptors correlate with cellular turnover rates, nuclear grade, and degree of histologic differentiation.
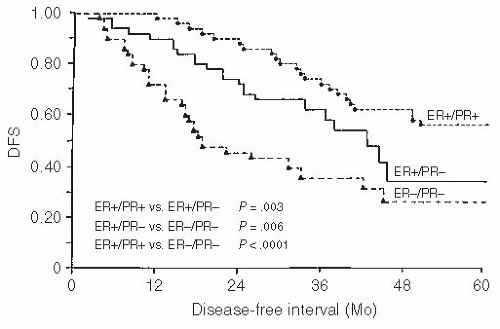
54,55 They also correlate with disease-free interval
(the time from diagnosis to documented recurrence), with receptor-positive patients having a significantly longer disease-free interval than receptor-negative patients (
Fig. 32.3). This correlates well with the earlier clinical observation that patients with disease-free intervals of 2 years or longer are more likely to respond to hormonal therapy than are patients with shorter disease-free intervals.
It has now been demonstrated that hormone receptor assays for ER and PR performed by immunohistochemical (IHC) assay are superior to the previously standard ligand-binding assays. IHC assays more accurately predict response to endocrine therapy and have now largely replaced other techniques for determination of ER and PR.
56,57,58,59 This is especially important because these assays can be performed on paraffinembedded tissue and on small pieces of tissue obtained by core needle biopsy.
Tamoxifen
Tamoxifen was the gold standard in breast cancer therapy for 30 years. Several recent studies,
60 however, have confirmed the superiority of the third-generation AIs in the adjuvant setting as well as in the treatment of receptor-positive metastatic breast cancer in postmenopausal women. Tamoxifen remains the hormonal therapy of choice in premenopausal women because the AIs have no activity in this subgroup of patients. Tamoxifen, a nonsteroidal antiestrogen that is structurally related to diethylstilbestrol (DES), is weakly estrogenic in castrated rats.
61 This weak estrogenic activity is helpful, but is also potentially harmful. Tamoxifen appears to protect against the development of osteoporosis in postmenopausal women. In premenopausal women who continue to menstruate, it may promote bone loss,
62,63 but tamoxifen decreases bone loss in premenopausal women with chemotherapy-induced amenorrhea.
64Tamoxifen binds reversibly with the estrogen receptor forming an inert complex that blocks estrogen-mediated protein synthesis. Tamoxifen also has non-ER-dependent tumor-suppressive activity by enhancing the production of the inhibitory growth factor, transforming growth factor-β (TGF-β) and blocking the production of the enhancing growth factors, insulin-like growth factor I (IGF-I), and TGF-α.
65,66,67,68,69 Tamoxifen is cytostatic rather than cytocidal and acts as a cell cycle inhibitor, with cells accumulating in the G0 and G1 phases.
70,71,72 In studies of rat mammary carcinoma, normal cell cycling returns when the drug has been cleared from the system. The cytostatic action of tamoxifen and the potential reversibility of its effect have prompted prolonged use of this drug, especially in the adjuvant setting.
71,73The toxicity of tamoxifen is usually minimal, but headaches,
hot flashes, or both may occur. Hot flashes have been particularly annoying and difficult to control. Treatments include vitamin E, 800 units a day, although the benefit has been marginal in controlled studies
74; soy phytoestrogens, which have been proven ineffective in controlled studies
75; Bellergal-S, which is the only U.S. Food and Drug Administration (FDA)-approved agent for hot flashes, but it has been taken off the market; and megestrol acetate, 20 mg twice a day, which is effective but may alter tamoxifen metabolism.
76,77 The selective serotonin reuptake inhibitors (SSRIs) venlafaxine,
78 fluoxetin, and paroxetine are effective in decreasing hot flashes by approximately 50%. The recommended dose of venlafaxine is 37.5 mg daily and of paroxetine 10 mg daily.
79,80,81 However, the SSRIs may interfere with the metabolism of tamoxifen, and it appears that the efficacy of tamoxifen is decreased by the SSRIs by inhibiting the isoenzyme CYP2D6.
82,83 The Mayo North Central Group has reported that medroxyprogesterone acetate (MPA), a long-acting progestational agent, in a single dose of 400 mg intramuscularly (IM) is more effective than venlafaxine and that the combination of venlafaxine plus MPA is no more effective than MPA alone in controlling hot flashes.
84 The management of tamoxifen-induced or tamoxifen-aggravated hot flashes remains a problem.
There was a definite increase in thromboembolic events in tamoxifen-treated participants as compared with those given placebo in the NSABP Breast Cancer Prevention Trials (BCPT).
85 Deep-vein thromboses developed in more women treated with tamoxifen, and pulmonary emboli were observed in almost three times as many women in the tamoxifen group. The increased risk of thromboembolic events was greater in women older than 50 years. A minor increase in the number of strokes also occurred in the tamoxifen-treated group.
Of more concern have been the reports of endometrial cancer in women who received long-term tamoxifen.
86,87 More than 200 cases of endometrial cancer have been reported in women who are taking or have taken tamoxifen.
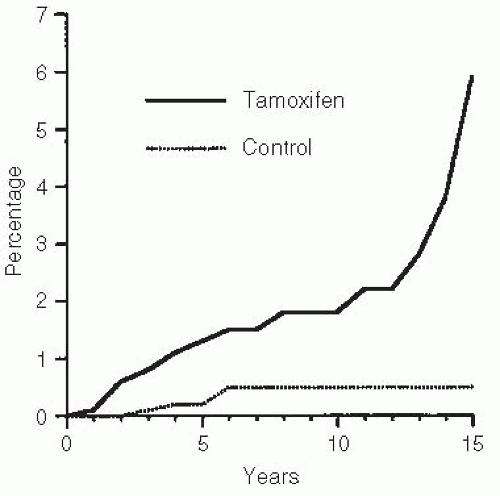
88,89,90 The relative risk of developing endometrial cancer is from 2.5 to 6.0 times the baseline, that is, an increased risk from 1 case per 1,000 women per year to 2.5 to 6.0 cases per 1,000 per year in women who received long-term tamoxifen
91 (
Fig. 32.4). This increased incidence of endometrial cancer was also observed in the NSABP BCPT.
92 These tumors tended to be low grade, low stage, and surgically curable. In fact, the only death from endometrial cancer in the BCPT occurred in the placebo group.
93,94,95
Tamoxifen in Premenopausal Women
For decades, oophorectomy was considered the treatment of choice for hormonally responsive breast cancer in premenopausal women.
96,97 Tamoxifen, however, has successfully
challenged the traditional role of oophorectomy as primary therapy in this group of patients.
98,99 In randomized-controlled trials that involved >200 patients, the response rates and durations of response associated with oophorectomy and tamoxifen are equivalent. In addition, prior response to tamoxifen may be a good predictor of response to subsequent oophorectomy.
100 This implies that tamoxifen should be used as primary therapy and oophorectomy restricted to patients who have responded previously to tamoxifen. Tamoxifen has emerged as the primary treatment of choice in premenopausal women.
Tamoxifen Flare
Tamoxifen flare occurs in approximately 10% of cases with metastatic disease.
101 This curious phenomenon is characterized by increased bone or soft tissue pain and occasionally hypercalcemia.
102 When a flare occurs, it develops in the first few weeks of therapy. Contrary to its manifestations, which may mimic progression, a flare generally heralds a response to treatment. Flares should be treated with analgesics or other symptomatic therapy, and full-dose tamoxifen should be continued. Brooks and Lippman
103 have proposed that the flare occurs because it requires several weeks for tamoxifen to reach therapeutic levels, and at lower concentrations, the drug may be estrogenic and stimulatory. Legha et al.
104 noted hypercalcemia in 9 of 470 patients with metastatic breast cancer who were treated with tamoxifen. Mild hypercalcemia was not considered to be a reason for stopping tamoxifen and was treated with saline diuresis and oral phosphates. If hypercalcemia was severe (15 mg per dl), a brief interruption of tamoxifen usually helped to control the hypercalcemia. Tamoxifen could be reinstituted within a few days. If hypercalcemia recurred, a small dose of prednisone, 10 to 30 mg per day, was found to be helpful.
Hormonal flares are by no means unique to tamoxifen and have been described by Hall et al 105 in 1963 with higher dose DEX (inducing hypercalcemia). It was noted that hormonally induced noted that hormonally induced hypercalcemia could indicate that the tumor has retained its hormonal responsiveness and that hormonal treatment should be continued. Hypercalcemia and pain flares have also been reported with megestrol acetate and, again, may herald a response.
106
Tamoxifen as Primary Therapy
Tamoxifen has been evaluated as primary therapy for breast cancer in elderly women.
107,108,109 A 27% complete remission (CR) rate and a 61% total objective response rate can be anticipated. OS is the same as in elderly women treated with mastectomy; however, the local recurrence rate in tamoxifen-treated patients is higher. Surgery should be performed if at all possible, despite the age of the patient; however, in elderly patients who are poor surgical candidates because of intercurrent medical disease, tamoxifen alone may provide worthwhile palliation.
110,111,112,113,114
Aromatase Inhibitors
As ovarian function declines, the relative proportion of estrogens synthesized in extragonadal sites increases. The synthesis of estrogen in peripheral tissues occurs by the aromatization of androgenic precursors of adrenal origin. The enzyme aromatase converts androstenedione to estrone, which is subsequently converted to estradiol.
115,116 Aromatase is present in adipose tissue, liver, muscle, and notably in the epithelial and stromal components of breast tissue. In the 1980s, the only commercially available AI was aminoglutethimide (AG).
117,118,119 However, AG was never a widely used drug because of a series of undesirable side effects. AG blocked adrenal steroidogenesis, and hydrocortisone replacement was required with standard-dose AG. In addition, in a number of patients who were treated with AG, lethargy, skin rash, ataxia, and even pancytopenia developed.
Several new AIs are now available.
120,121,122 These new compounds are largely devoid of the undesirable side effects of AG. They are selective AIs that do not block adrenal steroidogenesis. The two major classes of AIs are (1) the nonsteroidal triazole inhibitors, such as anastrazole, letrozole, vorozole, and fadrozole, and (2) the steroidal AIs formestane and exemestane. Of these, anastrazole, letrozole, and exemestane are now commercially available in the United States.
The steroidal AIs bind irreversibly to aromatase, whereas the triazoles are reversible inhibitors. The clinical relevance of this difference in the mechanism of action has not been established because they all suppress aromatase activity by >95%. However, exemestane may have activity in patients in whom a nonsteroidal AI has failed.
123Because of their efficacy and lack of toxicity, these new AIs have emerged as the clear choice for hormonal therapy in postmenopausal women with metastatic breast cancer. AIs have no activity in premenopausal women. However, by making a premenopausal woman chemically postmenopausal with an LHRH agonist such as goseralin 3.6 mg subcutaneously monthly, her response to letrozole becomes equivalent to that of a postmenopausal woman.
124 The dose of anastrazole
(Arimidex) is 1 mg by mouth (PO) once a day; for letrozole (Femara), 2.5 mg PO once a day; and for exemestane (Aromasin), 25 mg PO once a day.
The incidence of thromboembolic events and vaginal bleeding with AIs is less than that observed with tamoxifen. AIs do not induce endometrial cancer. The most annoying adverse effects of the AIs are myalgias and arthralgias, which may be severe enough to require discontinuation of the drug. When severe myalgias occur, the patient may tolerate one of the other AIs better and AI therapy may not need to be discontinued.
125The nonsteroidal AIs letrozole and anastrozole accelerate postmenopausal bone loss and are associated with an increased fracture risk. The steroidal AI exemestane appears to have only a modest enhancement of bone loss. In any case, patients on an AI should have their bone mineral density (BMD) monitored, especially if they are on long-term therapy. At a minimum, they should take adequate vitamin D and calcium. An oral bisphosphonate should be prescribed if the patient has a significant decrease in BMD.
126,127,128,129,130,131,132
Fulvestrant
Fulvestrant (Faslodex, AstraZeneca) is a selective estrogen receptor downregulator (SERD) with pure estrogen antagonist activity and no estrogen agonist effect. Fulvestrant binds to ER with an affinity similar to estradiol and produces a loss of ER (ER downregulation or SERD).
133 Fulvestrant reduces both ER and PR concentrations in a dose-dependent manner.
134,135 Fulvestrant is at least as active as anastrozole in tamoxifen-resistant breast cancer.
136,137 It is also active in breast cancers that have progressed following therapy with a third-generation AI. Although all of the reported studies on the efficacy of fulvestrant have been in postmenopausal women with metastatic breast cancer, one would expect the drug to have equal efficacy in premenopausal women. The standard dose of fulvestrant is 500 mg IM on day 0, then 500 mg IM on days 14 and 28, and then every 28 days.
138,139 The parenteral mode of administration has the potential benefit of enhanced patient compliance. Because fulvestrant binds irreversibility with the ER, it was anticipated that it should be superior to tamoxifen and anastrozole. However, randomized trials have shown equivalence.
140 Recent studies of fulvestrant in a dose of 500 mg have confirmed a statistically significant increase in progression-free survival (PFS) when compared with the 250-mg dose.
139As AIs come into more widespread use in first-line therapy for advanced disease, as well as in the adjuvant setting in operable breast cancer, fulvestrant will play an increasing role in the treatment of metastatic disease.
Progestins
The progestational agents megestrol acetate (Megace) and MPA (Provera) have activity in advanced, hormonally responsive breast cancer in postmenopausal women.
141,142,143 Progestins are generally relegated to a tertiary role. Progestins have a direct cytotoxic action on human breast cancer cells in long-term tissue culture, but probably act in vivo as antiestrogenic compounds that inhibit estrogen-induced protein synthesis.
144 Progestins may also act by the inhibition of autocrine growth factors.
144 The side effects of progestins are greater than those of tamoxifen or the AIs. Progestins have a mild glucocorticoid action, and weight gain is a frequent problem. Johnson et al.
145 noted a 5% or greater increase in weight in 23% of breast cancer patients who were treated with progestins. The appetite-stimulating effect of progestins has been exploited for the treatment of cancer anorexia and cachexia.
146 The usual recommended dose of megestrol for this purpose is 800 mg per day, but doses as low as 160 mg per day may stimulate appetite.
The standard dosage for megestrol acetate for the treatment of advanced breast cancer is 160 mg per day as a single or divided dose or 500 mg three times a week for MPA.
147 Progestins had been thought to have a steep dose-response curve. MPA in doses of 1,000 mg per day were reported to yield a higher response than 500 mg three times a week.
148 The definitive dose-response trial of megestrol in metastatic breast cancer was performed by the Cancer and Leukemia Group B (CALGB).
149,150 A group of 366 women with metastatic breast cancer was randomized to receive 160, 800, or 1,600 mg per day. Response rates were identical at the three dose levels (23%, 27%, and 27%, respectively). However, the median response duration of the highest dose level was significantly shorter than that of the two lower dose levels (7.8 vs. 13.9 and 14.2 months). Overall survival and time to disease progression were not statistically different between the three arms. The incidence of serious vascular complications, arterial thrombi, and pulmonary emboli was higher at the higher dose level. It can be safely concluded that there is no advantage to the high dose over the standard dose of 160 mg per day.
Estrogens
Thirty years ago, DES, a synthetic estrogen, was the hormonal treatment of choice in postmenopausal women with advanced breast cancer.
151,152 The response rate to DES in patients with ER+ tumors is 63%. In general, the median duration of response to estrogen is 12 to 18 months, but responses of longer than 5 years have been documented.
The mechanism by which estrogens act on metastatic breast cancer is unknown. Tumor cells that contain ER bind estrogens with greater affinity and specificity. High-dose estrogen acts as an antagonist to endogenous estrogen.
The most commonly used estrogen was DES in the dosage of 5 mg three times a day, but this is no longer commercially available. Other estrogen preparations that have been used are ethinyl estradiol, 3 mg per day, and conjugated equine estrogens, 30 mg per day.
Breakthrough or withdrawal uterine bleeding in postmenopausal women on estrogen therapy occurs in 40% of patients. This is usually of little clinical significance and responds to cessation of treatment or abates spontaneously with continued therapy, but if it persists, it may require further investigation. Persistent uterine bleeding associated with estrogen therapy may signal the presence of an endometrial carcinoma.
Patients who respond to estrogen therapy initially but in whom the disease progresses later may respond to the sudden withdrawal of estrogens. Estrogen rebound regression was originally described by Escher
153 in 1949 and occurs in up to 32% of estrogen responders.
154 The duration of rebound regression
is usually 3 to 10 months, but Nesto et al.
155 reported a median duration in excess of 18 months. Tamoxifen withdrawal responses have also been reported.
156
Androgens
Androgens were the first additive hormonal agents to prove useful in the treatment of metastatic breast cancer.
157,158 They exert an antiestrogenic effect by complex interactions with three receptors: ER, PR, and androgen receptor (AR). Androgens compete with estradiol for the ER and can bind to PR. Because the dosage of androgen that is required to give an antiestrogenic effect is in the range to saturate the AR but is too low to saturate the ER, it has been postulated that the therapeutic effect is mediated through the AR. This is verified, at least to some degree, by the observation that in tissue culture of human breast cancer, antiandrogens such as cyproterone inhibit the antiestrogenic effect of androgens.
159Androgens exert desirable subjective hematopoietic and anabolic effects.
152 Patients who are treated with androgens may experience an increased sense of well-being, pain relief, increased appetite, and weight gain.
The side effects of androgens are predominantly those associated with the physiologic effects of male hormones, that is, virilization with frontal baldness, plethora, acne, hirsutism, fluid retention, and less commonly, an increased libido and clitoral hypertrophy. The virilizing effects vary with the androgenic preparation used. They occur in >50% of patients treated with testosterone propionate and in 35% to 40% of patients treated with fluoxymesterone. The virilizing and therapeutic effects of androgens appear to be inseparable. Androgens with a 17α-methyl substitution, such as fluoxymesterone and methyltestosterone, can cause reversible cholestatic jaundice and, rarely, a multifocal hepatocellular necrosis termed
peliosis hepatis.
160,161 Large areas of cystic hemorrhagic necrosis of peliosis hepatis may cause an abnormal liver scan that can be confused with metastases. Patients with breast cancer may need to reduce their dosage of thyroid replacement medication during androgen therapy.
162 Fluoxymesterone (Halotestin) is the androgen of choice. The dosage for fluoxymesterone is 20 to 30 mg per day PO
Combination Hormonal Therapy
Numerous combinations of hormonal agents have been evaluated in the treatment of metastatic breast cancer: Fluoxymesterone plus ethinyl estradiol, DES plus testosterone propionate, tamoxifen plus megestrol acetate, tamoxifen plus MPA, tamoxifen plus DES, tamoxifen plus prednisone, tamoxifen plus AG, tamoxifen plus fluoxymesterone, MPA plus AG, and ethinyl estradiol plus MPA. The combinations failed to demonstrate an advantage over the use of single-agent hormonal therapy. This is what would be anticipated, because the mechanism of action of each of these agents is basically the same inhibition of the synthesis or action of estrogen.
LHRH Agonists
Treatment with an LHRH agonist effectively induces a reversible suppression of ovarian function. In animals, chronic treatment with supraphysiologic doses of LHRH agonists causes (1) a decrease in gonadotropin (follicle-stimulating hormone [FSH] and luteinizing hormone [LH]) excretion, (2) a decrease in prolactin excretion, (3) a decrease in plasma sex steroid concentration, (4) a reduction in the weight of secondary sexual organs, and (5) an inhibition of the actions of the sex steroids at their target organs.
163 LHRH analogs, therefore, act directly or indirectly on the pituitary, the gonads, and the target organs of the sex steroids. Currently, the LHRH analogs, buserelin, goserelin, decapeptyl, and leuprolide, are being evaluated in clinical trials in advanced breast cancer.
Goserelin (Zoladex), an LHRH agonist, was reported to yield a 45% objective response rate (10% complete responses plus 35% partial responses) in 134 premenopausal women with metastatic breast cancer.
164 The highest response rates were seen in patients with local-regional metastases (62.5%), followed by osseous (46.7%), visceral (45%), and multiple sites (35.1%). Side effects included amenorrhea, vaginal spotting, and, infrequently, headache and sleep disturbances. Leuprolide was tested in 26 premenopausal women, with similar results.
165 Attempts at total estrogen suppression with goserelin plus tamoxifen have been evaluated in premenopausal women with metastatic breast cancer. The combination of an LHRH agonist and tamoxifen appears to be more active than either agent alone.
166,167The ECOG has conducted a phase II study of goserelin in postmenopausal women.
168 In ER+ patients, the response rate was only 11% (4 of 36 patients), and 16 ER- patients had no responses. Goserelin has now been approved for the treatment of metastatic breast cancer in ER+ premenopausal women at a dose of 3.6 mg subcutaneously once a month. In metastatic breast cancer in premenopausal women, goserelin has a response rate equivalent to that of oophorectomy.
169 There is increasing data that the combination of goserelin plus anastrazole
170 or letrozole
124 has substantial activity in the treatment of premenopausal women with hormone receptor positive metastatic breast cancer.
CYTOTOXIC CHEMOTHERAPY IN METASTATIC
BREAST CANCER
Although one of the primary roles of the medical oncologist is the treatment of breast cancer in adjuvant setting of early disease in women at risk of recurrence; the principles of cytotoxic chemotherapy in advanced disease will be discussed first for two reasons:
The adjuvant therapy for early breast cancer evolved from the treatment of metastatic disease; and
If the drugs are not active in the advanced disease setting, they do not appear to have activity in the adjuvant setting.
A major philosophical issue needs to be addressed at this point and that is you have one chance to cure a breast cancer; that is, the first time—since when and if a breast cancer recurs the chance of cure is minimal and almost all patients will succumb within 24 to 36 months. Many of us believe therefore, that you should “fire
your best guns up front” the problem then is how do you manage the patients who recur despite your best adjuvant therapy. If the patient is hormone receptor positive, you have the safety net of hormonal manipulation. If she is hormone receptor negative, she will require further chemotherapy but with what? Currently, our approach is serial single agents unless the patient has immediately life-threatening disease such as multiple liver metastases when we would use a doublet (two drugs) combination. Which single agents and what doublet is dictated by the prior therapy?
Historical Aspects. The excitement generated by Richard Cooper’s report
171 in 1969 to the American Association of Cancer Research was tremendous. He reported an 88% response (53 of 60 patients) to combination chemotherapy in hormoneresistant breast cancer. Cooper’s regimen was cyclophosphamide, methotrexate, 5-fluorouracil, vincristine and prednisone (CMFVP). After this report, clinical trials testing CMFVP and multiple variants of CMFVP were conducted. Although none of these trials had a response rate of 88%, it was confirmed that in patients with metastatic breast cancer, combinations of cytotoxic drugs could produce an objective response rate in the range of 60%, with 10% to 15% complete responses and a response duration of 8 to 12 months or more.
172,173,174,175,176,177,178,179
Combination chemotherapy was rapidly adopted as the treatment of choice for metastatic breast cancer because of its predictably high response rate and rapid onset of action. Conversely, hormonal therapy was relegated to a secondary or tertiary role, because response rates were low and unpredictable and the onset of response would take 6 to 8 weeks. By the late 1970s, it was believed that with more fine-tuning of the pharmacokinetics of chemotherapeutic agents and the integration of drug pharmacokinetics with cell cycle kinetics, the potential for cytotoxic agents in breast cancer was almost limitless. The 1980s were foreseen as a period of great progress for breast cancer chemotherapy, but this did not happen. Promising new agents did not materialize, cell cycle kinetics proved to be of less importance clinically than in the laboratory, and new combinations of existing agents failed to produce an increasing response rate or duration of response. In short, the cytotoxic chemotherapy phase of breast cancer plateaued. With the introduction of the taxanes, gemcitabine, carboplatin, capecitabine, and vinorelbine, the chemotherapeutic treatment of breast cancer is again moving forward, especially in the adjuvant arena.
Single Agents
Breast cancer is responsive to all major classes of cytotoxic drugs: alkylating agents, antimetabolites, mitotic inhibitors, and the antitumor antibiotics. Phase II data of single-agent chemotherapy in advanced breast cancer are presented in
Table 32-10. These data have been compiled from multiple phase II studies and should be interpreted as indicating the approximate response rate rather than absolute values. Presenting data in this manner presents many problems, because dosage levels or dosage schedules (infusion vs. intravenous [IV] push, single-day treatment vs. 5-day treatment schedules) may vary. More importantly, prior therapy and response criteria may not be specified.
180,181,182As can be seen in
Table 32-10, patients who have been treated previously with chemotherapy and those who have never been treated often show a marked difference in response rate for the same agent: 6% versus 38% for cisplatin, 28% versus 52% for doxorubicin, and 13% versus 31% for mitoxantrone. This tremendous discrepancy in responses may well mean that an active drug might be overlooked if it is tested only in previously treated patients. For this reason, and because none of the chemotherapeutic regimens is curative, it is perfectly justifiable to test new phase II agents in chemotherapy-naive patients with advanced metastatic disease.
Doxorubicin (Adriamycin)
Before the introduction of the taxanes, doxorubicin was the most active single agent in the treatment of breast cancer. Doxorubicin produces a 52% objective response rate in previously untreated patients and 28% in patients who have had prior chemotherapy (
Table 32-5). Doxorubicin has undesirable side effects, such as cardiac toxicity, almost universal alopecia, and marked corrosiveness if the drug infiltrates the skin. This has prompted the search for a less toxic, equipotent analog and for methods to reduce the cardiac toxicity of doxorubicin.
Using the standard dosage schedule for doxorubicin of 50 to 75 mg per m2 IV every 3 weeks, the incidence of cardiomyopathy increases dramatically once a cumulative dose of 450 mg per m2 is exceeded. Three approaches have been used to modify the development of doxorubicin-induced cardiomyopathy: changing to a weekly dosage schedule, using a continuous-infusion technique, or the addition of bispiperazinedione (dexrazoxane, Zinecard).
The effect of weekly doxorubicin has been studied extensively by Torti et al.
183 Endomyocardial biopsies were performed in 98 patients who received 60 mg per m
2 every 3 weeks and in 27 patients who received 20 mg per m
2 doxorubicin once a week. At equivalent cumulative doses of doxorubicin, the weekly schedule was associated with significantly less anthracyclineinduced cardiac damage, as confirmed by biopsy (
P = .002). The response to weekly doxorubicin is equivalent to the every 3-week dose schedule.
184Continuous-infusion doxorubicin in advanced breast cancer has been associated with less cardiotoxicity. Investigators at the M.D. Anderson Cancer Center
185 compared 48-hour (79 patients) and 96-hour (62 patients) continuous infusion with bolus IV administration (133 patients). No difference in response rate was observed. At cumulative doses of 450 mg per m
2 or higher, the frequency of clinical congestive heart failure (CHF) decreased 75% in continuous-infusion groups (
P = .004). According to Legha et al.,
186 when doxorubicin is administered by 96-hour infusion, the risk of cardiac toxicity is almost negligible up to a cumulative dose level of 800 mg per m
2.
Dexrazoxane has been reported to protect against doxorubicin-induced cardiac toxicity in women with advanced breast cancer.
187 Cardiac toxicity was evaluated by clinical examination, by left ventricular ejection fraction measured by multigated nuclear scans, and by endomyocardial biopsy. A group of 92 women was randomized to receive 5-fluorouracil (5-FU) plus doxorubicin plus cyclophosphamide (FAC) or FAC plus dexrazoxane. At equivalent cumulative doses of doxorubicin, the group that received dexrazoxane had significantly less cardiac
toxicity (
P = .001) and no alteration in antitumor effect. With the concurrent use of dexrazoxane, doxorubicin doses of up to 700 mg per m
2 appear to be well tolerated.
188,189
Liposomal Doxorubicin
The incorporation of doxorubicin into liposomal particles has been studied extensively.
190,191 Liposomal doxorubicin (Doxil) has several potential advantages over standard doxorubicin: longer circulatory half-life, more specific delivery to tumor tissue, and reduced cardiac toxicity. The exact role of these compounds in the treatment of advanced breast cancer is yet to be defined.
192
Epirubicin
In previously untreated patients with breast cancer, epirubicin has a response rate of 65%, which is equivalent to that of doxorubicin.
193,194 Epirubicin is less cardiotoxic than doxorubicin at equimolar concentrations but not at equimyelosuppressive doses.
195 The recommended dose of epirubicin is 100 to 120 mg per m
2 to a maximum cumulative dose of 900 mg per m
2. In a meta-analysis of anthracyclines in relation to cardiovascular side effects, the risk of clinical cardiotoxicity was significantly lower with epirubicin when compared with doxorubicin (OR 0.39 95% confidence interval [CI], 0.20 to 0.78) but no statistical heterogeneity was noted.
196
Mitoxantrone
Mitoxantrone is a synthetic hydroxyquinone that is related structurally to doxorubicin. It is an active drug in breast cancer, as well as in acute leukemia and lymphoma.
197 Clinically, significant cardiac toxicity occurs in approximately 3% of patients who receive cumulative doses of 175 to 250 mg per m
2. Alopecia occurs much less frequently in patients who are treated with mitoxantrone than in those who are given doxorubicin and occurs in fewer than 10% of cases. Nausea and vomiting are uncommon. Mitoxantrone appears not to be a vesicant if it is infiltrated. The dose-limiting toxicity is myelosuppression, which may be prolonged. The current consensus is that mitoxantrone is an active drug in breast cancer and is less toxic than doxorubicin, but its response rate is approximately 10% less than that of doxorubicin.
198,199,200 Mitoxantrone probably has a role in the treatment of frail elderly patients or patients with breast cancer who have an intercurrent medical illness.
201 The dosage of mitoxantrone as a single agent is 10 to 14 mg per m
2 IV every 3 weeks.
Taxanes
The most active exciting class of anticancer agents in the treatment of breast cancer are the taxanes.
202 The prototype drug, paclitaxel (Taxol, Bristol-Myers Squibb [BMS]), was first extracted from the bark of the Pacific yew tree
Taxus brevifolia in 1971. Early drug development was hampered by supply and concern over the high incidence of hypersensitivity reactions. Docetaxel (Taxotere, Sanofi Aventis) is a semisynthetic taxane produced from the needles of the European yew tree
Taxus baccata. Both taxanes have shown impressive activity in patients with previously treated and untreated metastatic breast cancer.
Paclitaxel and docetaxel have similar mechanisms of action. Both taxanes inhibit microtubule depolymerization.
203 Cells exposed to taxanes cannot form a normal mitotic spindle, and cells become blocked in the G2 and M phases of the cell cycle. The taxanes are also potent inhibitors of tumor-induced endothelial cell angiogenesis.
204The clinical development of the taxanes is a prime example of efficient drug development. In less than a decade, paclitaxel and docetaxel moved from second-line therapy for anthracycline-resistant breast cancer to first-line therapy with both drugs being incorporated into the adjuvant setting.
Paclitaxel
Holmes et al.
205 first reported the activity of paclitaxel in metastatic breast cancer in 1991. In 25 patients treated with 250 mg per m
2 over 24 hours, there were 3 complete and 11 partial responses, for an overall response rate of 56%. Several other investigators have verified the activity of paclitaxel in metastatic breast cancer, with responses of 45% to 60% in previously untreated cases and 30% to 40% in anthracycline-resistant cases.
206,207,208The cytotoxicity of paclitaxel is dose and schedule dependent. Extending the time of exposure (infusion) enhances the cytotoxicity.
209 The NSABP in protocol B26 demonstrated that paclitaxel, 250 mg per m
2, administered as a continuous 24-hour infusion, resulted in a higher response rate in advanced breast cancer than when the same dose is given as a 3-hour infusion.
210 However, no difference in survival was seen. The complexities associated with a 24-hour infusion, the similarity in response rate (54% vs. 44%), and the lack of survival benefit have made the 3-hour infusion the standard of practice. The usual recommended dosage of paclitaxel is 175 mg per m
2 over 3 hours every 3 weeks. Higher doses of paclitaxel, 210 to 250 mg per m
2, have not improved the response rate.
211 To block the hypersensitivity reactions, the patient must be premedicated with dexamethasone, 20 mg PO 12 hours and again 6 hours before the paclitaxel infusion. Paclitaxel is now commonly used on a weekly 80 mg per m
2 dose schedule. Weekly therapy is well tolerated with mild hematologic toxicity but equivalent neurotoxicity.
212Other than alopecia and granulocytopenia, the most troublesome side effect of paclitaxel is neurosensory toxicity. In the NSABP study,
210 neurosensory toxicity of at least grade 1 was experienced in 66% of patients in the 3-hour group and 64% in the 24-hour group. In addition, there was an 80% incidence of grade 1 or higher myalgias and arthralgias with paclitaxel. The neurosensory toxicity is particularly troublesome, and many patients have discontinued treatment as a result. The neuro-muscular toxicity is considerably less common with docetaxel, making it the drug of choice in breast cancer.
Docetaxel
Docetaxel is highly effective in the first-line treatment of metastatic breast cancer, achieving an objective response rate of 52% to 69%.
213,214 This response rate is seen in patients with poor prognostic factors such as liver metastases and multiple organ involvement. In previously treated metastatic disease, docetaxel
is highly active, with an overall objective response rate of 41%.
215,216 Docetaxel is the most active agent available for the treatment of advanced breast cancer.
217 Docetaxel may have some activity in paclitaxel-resistant breast cancer.
218Other than dose-related neutropenia and alopecia, the most troublesome side effect of docetaxel is fluid retention, which may occur in up to 80% of patients. This fluid retention may be manifest as peripheral edema, weight gain, and even pleural effusions. The fluid retention may be severe enough to require discontinuation of therapy. Fortunately, the fluid retention can be blocked by premedicating the patient with dexamethasone, 8 mg PO twice a day for 3 days starting the day before the docetaxel infusion. The standard dose of docetaxel is 100 mg per m
2 over 1 hour every 3 weeks, but heavily pretreated patients may have difficulty tolerating >75 mg per m
2.
219 Docetaxel, 40 mg per m
2 per week given weekly × 6 with a 2-week break, is tolerated well and gives an equivalent response to 75 to 100 mg per m
2 every 3 weeks. Myelosuppression is mild, but fatigue, fluid retention, tearing, and conjunctivitis are more common. The major disadvantage of a weekly schedule is just that it must be given weekly, which can be a significant problem for patients who must travel any distance for treatment.
Abraxane
Abraxane (nanoparticle albumin-bound paclitaxel) was developed to avoid the toxicities of polyethylated castor oil (Cremophor). The taxanes are highly hydrophobic and require synthetic solvents to enable parenteral administration. Paclitaxel is dissolved in polyethylated castor oil and ethanol; docetaxel is dissolved in polysorbate 80 and ethanol. These solvents contribute directly to the toxicity of docetaxel and paclitaxel. Solvent-related toxicities include hypersensitivity reactions, which may be severe and peripheral neuropathy which may be irreversible.
220,221,222Abraxane is administered as a colloidal suspension of 130 nm particles. It does not require steroid or antihistamine pre-medication and can be administered by more rapid intervenous infusions (30 minutes as opposed to 3 hours). Despite the absence of premedication and the rapid infusion time, hypersensitivity reactions are rare.
The dose-limiting toxicity is sensory neuropathy, which is generally reversible because Abraxane does not cause axonal degeneration and demyelination as does Cremophor. Myelosuppression also appears less severe.
Single-agent Abraxane appears to have equivalent to superior efficacy to single-agent paclitaxel and to single-agent docetaxol with a superior safety profile.
223Albumin receptors facilitate drug entry into tumor cells. Abraxane has a significantly higher response rate and longer time to tumor progression than standard paclitaxel in patients with metastatic breast cancer. The recommended dose of Abraxane is 260 mg per m2 every 3 weeks.
Epothilone
The epothilones are a new class of microtubule stabilizing drug. These drugs do not appear to be cross-resistant with the taxanes. The epothilones are active in metastatic breast cancer. Four epothilones are in clinical trials: BMS 247550, BMS 310705, EPO 906, and KOS 862. Of these, BMS 247550 (Ixabepilone) is best studied, and it has a 22% response rate in patients previously treated with a taxane.
224,225 Ixabepilone has been evaluated in previously untreated metastatic breast cancer in a dose of 6 mg/m
2/day IV days 1 through 5 every 3 weeks. The overall response rate was 57%. There was minimal hematologic toxicity, and the response duration was 5.6 months.
226 Ixabepilone appears to have an increasing role in breast cancer therapy
Eribulin
Eribulin mesialte is a nontaxane microtubule inhibitor and is active on cancer cells resistant to other antimicrotubule agents. In the EMBRACE trial, eribulin was administered IV 1·4 mg per m
2 over 2 to 5 minutes on days 1 and 8 of a 21-day cycle in patients who were previously treated for metastaic breast cancer or locally recurrent breast cancer. This was compared with physicians treatment of choice. The OS favored eribulin (median 13.1 months) compared with physicians choice of chemotherapy (10.6 months). Asthenia or fatigue noted in 54%, neutropenia 52%, and peripheral neuropathy 5% of the patients. Peripheral neuropathy was the cause of discontinuation in patients receiving eribulin.
227
Vinorelbine
Vinorelbine (Navelbine, Burroughs Wellcome) is a vinca alkaloid that differs from vinblastine by virtue of a modification of the cantheranthine moiety.
228 Vinorelbine is highly active as a single agent in previously untreated patients with advanced breast cancer. Fumoleau et al.
181 reported a response rate of 41% in 145 previously untreated patients at a dose of 30 mg per m
2 per week, with 7% complete responses. Other studies using single-agent vinorelbine as initial therapy
222,229 have given similar results.
Combination therapy using vinorelbine with other drugs that are active against breast cancer has also been explored. The combination of vinorelbine and doxorubicin was given as initial therapy for advanced disease in 89 evaluable patients by Spielmann et al.
230 Vinorelbine was given at 25 mg per m
2 on days 1 and 8, whereas doxorubicin was administered at 50 mg per m
2 on day 1 of each 3-week cycle. The overall response rate was 74%, with 21% complete responses. Neutropenia was the major toxicity, with 41% of patients experiencing grade 3 or 4 neutropenia; however, only 3% of cycles required hospitalization for febrile neutropenia. The combination of vinorelbine and doxorubicin gave an overall response rate of 54% in a US multicenter trial by Hochster
231 and 74% in a study by Blajman et al.
232 Combinations of vinorelbine with mitoxantrone or epirubicin also appear to be potentially useful.
233,234Substitution of vinorelbine for cyclophosphamide in the classic CMF-type regimen yielded a 44% to 55% response rate as first-line therapy.
235 Combinations of vinorelbine with docetaxel,
236 paclitaxel,
237 or cisplatin
238,239,240 appear to be quite active with response rates of 45% to 65%, even in patients with prior anthracycline and taxane therapy.
Vinorelbine plus mitomycin C has also been used in several different schemas.
241 Objective responses of 35% to 44% have been found, with activity seen in anthracycline-refractory patients.
242The combination of vinorelbine with gemcitabine is an active combination. Other than hematologic toxicity, this regimen is well tolerated, even in elderly populations
243 and can be given on a biweekly
244 or on a day 1 and 8 schedule.
245The lack of significant toxicity, other than self-limited and brief neutropenia and phlebitis, makes vinorelbine an attractive option for salvage therapy in patients with advanced breast cancer. This is especially true with the increasing use of anthracyclines and taxanes in the adjuvant setting.
Several trials have investigated the use of combination therapy with vinorelbine in the neoadjuvant setting. Phase II studies with vinorelbine plus epirubicin,
246 vinorelbine plus epirubicin and paclitaxel,
247 or docetaxel plus navelbine,
248 have yielded pathologic CR rates of 4% to 20%.
Vinorelbine plus epirubicin was found to be as effective as Adriamycin plus cyclophosphamide as preoperative therapy in a randomized trial of 451 patients with operable breast cancers 3 cm or greater. Pathologic CR was seen in 12% of both groups.
249
5-Fluorouracil
5-FU was synthesized in 1957 as an antitumor agent by Heidelberger et al.
250 at the University of Wisconsin and was rapidly introduced into clinical practice. As a single agent, 5-FU became the most commonly used nonhormonal drug in the treatment of advanced breast cancer.
251 With the introduction of combination chemotherapy in the 1970s, 5-FU was incorporated into many commonly used regimens, including Cooper’s original CMFVP.
Continuous infusion of 5-fluorouracil
The continuous infusion of 5-FU was introduced by Lokich et al.
252 and appears to be useful in previously treated patients with advanced breast cancer. Jabboury et al.
253 evaluated 5-FU, 250 mg per m
2 per day, given by continuous infusion. The median duration of the infusion was 65 days, with a range of 19 to 508 days. Five of 32 patients responded. The main toxicities were stomatitis in 13 patients, the hand-foot syndrome (HFS) (palmar-plantar erythrodysesthesia) in 6 patients, and Coombs-positive hemolytic anemia in 2 patients. Myelosuppression was uncommon. Using similar regimens, Hatfield et al.
254 reported a 28% response (7 of 25 patients), and Huan et al.
255 reported a 53% response (15 of 28 patients). 5-FU given by continuous infusion is feasible with ambulatory infusion pumps and is worth considering as a tertiary regimen in patients with metastatic breast cancer who retain a good performance status.
256
Capecitabine
Capecitabine (Xeloda, Roche Laboratories) is an orally active fluoropyrimidine that delivers 5-FU selectively to the tumor.
257 This conversion to 5-FU is promoted by cytidine deaminase and thymidine phosphorylase, enzymes that are in high concentration in neoplastic tissue. Capecitabine produces minimal bone marrow suppression, but can cause a troublesome HFS and, less frequently diarrhea, nausea, and stomatitis.
Capecitabine offers the efficacy of infusional 5-FU without the inconvenience of drug pumps and venous access devices and has largely replaced infusional 5-FU in the treatment of metastatic breast cancer.
258,259 Capecitabine is active in taxane and anthracycline-resistant breast cancer with an objective response rate of 20% to 30%. The initial recommended dose of capecitabine was 2,500 mg per m
2 per day in two divided doses on days 1 through 14, followed by a 7-day rest period. This dose is often poorly tolerated and doses of 1,500 mg to 1,750 mg per m
2 appear to be equally effective with considerably fewer side effects.
Gemcitabine
Gemcitabine is an antimetabolite with activity in several types of cancer, including breast cancer. In chemotherapy-naive patients who receive single-agent gemcitabine, response rates of up to 37% have been observed, and previously treated patients have shown an overall response rate as high as 28%.
260,261 The recommended dose schedule is 1,000 to 1,250 mg per m
2 IV weekly for 3 weeks of a 4-week cycle. Gemcitabine is generally associated with low toxicity, but has been linked to myelosuppression, nausea, and fatigue. Because of its unique mechanism of action and favorable toxicity profile, gemcitabine has been evaluated in combination with a variety of other cytoxic agents: the taxanes, vinorelbine, carboplatin, the anthracyclines, and capecitabine.
262 Most of these two drug combinations have demonstrated higher efficacy than either single agent alone, particularly in previously treated patients. The role of doublets and even triplets in the treatment of metastatic breast cancer will be discussed in the next section.
Combination Chemotherapy in HER2 Negative Metastatic Breast Cancer
Approximately 20% of patients initially diagnosed with regional disease will recur. Once recurrence develops, the median survival is from 15 to 26 months. Metastatic breast cancer is responsive to a variety of therapeutic agents, but the primary goal of treatment is palliation and improvement in survival.
263,264Hormonal agents constitute the first approach to the patient with metastatic breast cancer because of their efficacy and relative lack of side effects. However, at some point, all patients become hormonally refractory, and receptor-negative patients are not hormonally responsive initially. In these patients, palliation is the goal and should be accomplished with minimal toxicity.
It has been recognized for years that combination chemotherapy is associated with increased response rates over single agents. This principle is now the basis for the adjuvant therapy of operable breast cancer where cure is the objective. As a result, many, if not most, patients who develop metastatic breast cancer have already been treated with first-line combination chemotherapy including anthracyclines and taxanes.
In the metastatic setting, it is still true that two drug combinations (doublets) offer higher response rates and longer time to progression than single agents. But it is not at all clear that the sequential use of single agents is not equally effective with less toxicity and superior quality of life.
The prognostic factors in patients with metastatic breast cancer are outlined in
Table 32-11.
265 Patients presenting with favorable metastases are good candidates for hormonal therapy provided they are receptor positive. Patients presenting with unfavorable metastases are best treated directly with cytotoxic chemotherapy.
Should a new metastasis be biopsied? As a rule, the first metastasis should be biopsied for two reasons. Treatment changes from a curative mode to palliative mode. Secondly, there is a substantial discordance between receptor status, the primary tumor and the metastases, which may lead to altered management in up to 20% of cases.
266
Taxane Combinations
Paclitaxel and docetaxel, either alone or in combination, are emerging as the treatment of choice for previously untreated, hormonally refractory metastatic breast cancer. As single agents, these drugs will achieve a 50% to 60% objective response rate in previously untreated patients, which is greater than what would be expected with the older CMF or CAF combinations.
Paclitaxel and docetaxel have been studied in combination with a variety of other agents, such as doxorubicin, vinorelbine, mitoxantrone, epirubicin, gemcitabine, capecitabine, and carboplatin.
267,268,269,270,271,272,273,274,275,276 The combination of docetaxel or paclitaxel with doxorubicin has yielded 80% response rates with up to 20% complete responses. A note of caution must be mentioned because the paclitaxel-doxorubicin combination has been associated with an unacceptably high incidence of CHF (20%). This can be reduced by the concurrent use of the cardioprotective agent dexrazoxane or restricting the cumulative dose of doxorubicin to 360 mg per m
2.
277 An increased incidence of cardiomyopathy has not been observed when docetaxel is combined with doxorubicin.
278 The results of various trials of taxanes in combination with other agents are summarized in
Table 32-12.
267,268,269,270,271,272,273,274,275,279,280,281,282,283 Taxane combinations are clearly very active in metastatic breast cancer, yielding not only a high overall response rate but a very high complete response rate. Taxanes and taxane combinations are clearly the regimens of choice in metastatic breast cancer. However, these drugs are being used with increasing frequency in the adjuvant setting, leaving fewer and fewer drugs available for salvage therapy after recurrence.
Platinum-based Combinations
The NCCTG evaluated carboplatin plus paclitaxel as first-line chemotherapy for metastatic breast cancer. Paclitaxel, 200 mg per m
2 over 3 hours, and carboplatin area under the curve (AUC), 6 mg/ml/minute, were administered to 53 patients. The overall response rate was 62%, with 16% of the patients achieving a complete response. Median time to progression was 7.3 months.
256 Clearly, this is a very active combination, and carboplatin deserves further study in advanced breast cancer. Platinum-based combinations are active in metastatic breast cancer and have become increasingly important.
284
CHEMOHORMONAL THERAPY
Several clinical trials have evaluated combination chemotherapy with and without tamoxifen. In the largest of these trials, conducted by the CALGB,
287,288 patients were stratified by ER status, dominant site of metastatic disease, menopausal status, and prior AdC. They were then randomized to CAF chemotherapy, with or without tamoxifen, 10 mg orally twice a day. A total of 474 patients were entered; fewer than 5% were ineligible or inevaluable. The addition of tamoxifen conferred no significant advantage in response rate, response duration, time to treatment failure, or survival over CAF alone. The NCCTG and the ECOG did similar trials.
289,290 In the ECOG trial, there was improvement in time to treatment failure for the chemohormonal group but no prolongation of survival. These trials have confirmed that the mere addition of a hormonal agent (e.g., tamoxifen) to combination chemotherapy (e.g., CAF or CFP) adds little to the response rate or response duration. This confirms in vivo results that chemotherapy kills breast cancer cells indiscriminately, regardless of receptor status. It also confirms that chemotherapy and hormonal therapy compete for the same pool of ER+ cells.