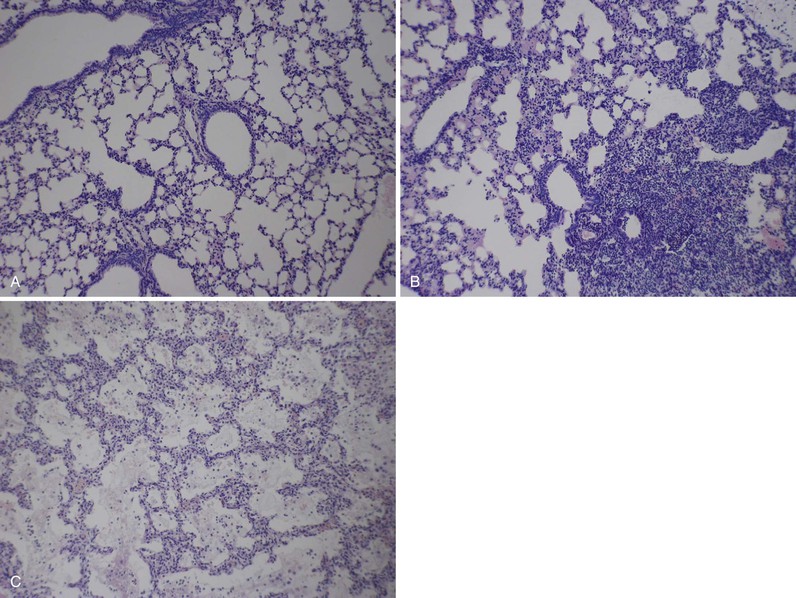
Valerie Waters, Scott A. Halperin The first epidemic of whooping cough was described in 1578 by DeBaillou, who wrote the following: “The lung is so irritated that, in its attempt by every effort to cast forth the cause of the trouble, it can neither admit breath nor easily give it forth again. The sick person seems to swell up, and, as if about to strangle, holds his breath clinging in the midst of his jaws. …”1 This vivid clinical description of whooping cough holds true to this day. In 1679, Sydenham2 gave this respiratory illness the name pertussis, meaning a violent cough of any type. The organism that causes whooping cough was discovered in 1900 by Bordet and Gengou.3 They described a new gram-negative bacillus (subsequently named Bordetella pertussis, after Bordet) that they had found in the sputum of a 6-month-old infant with whooping cough. By 1906, they had developed a culture medium to support the growth of the organism and described in detail its morphology and virulence characteristics. In 1943, Joseph Lapin,1 a pediatrician who worked in the whooping cough clinic at the Bronx Hospital in New York City, wrote an extensive monograph on the subject of pertussis. Bordetella pertussis is the pathogen that causes whooping cough or pertussis.4 It is one of 10 known Bordetella species, namely, B. pertussis, B. parapertussis, B. bronchiseptica, ovine-adapted B. parapertussis, B. avium, B. hinzii, B. holmesii, B. trematum, B. petrii, and B. ansorpii. B. pertussis and B. parapertussis are the most common Bordetella species causing respiratory illnesses in humans. With improved molecular testing methods, polymerase chain reaction (PCR) assays can now distinguish between B. pertussis and B. holmesii and have detected B. holmesii in 0.1% to 20% of patients with pertussis-like symptoms.5,6,7 Although B. pertussis strictly affects humans and has no known animal reservoir,8 many of the other Bordetella species are recognized primarily for the diseases they cause in animals. B. bronchiseptica causes kennel cough in dogs and cats, and human infections occur primarily in immunocompromised patients, often after exposure to animals.9–11 Ovine-adapted B. parapertussis causes respiratory tract infections in sheep.12 B. avium is a pathogen of poultry13 but has been isolated from the ear culture of a patient with chronic otitis media.14 Similarly, B. hinzii also colonizes the respiratory tract of poultry and has been isolated from the sputum of cystic fibrosis patients.15 It has been reported to cause bacteremia in immunocompromised16,17 as well as immunocompetent patients18 and has been described as a cause of chronic cholangitis.19 B. trematum has been isolated from patients with wounds or otitis media.20,21 B. petrii, originally identified from an environmental source,22 has been recently isolated from patients with chronic infections.23–25 Finally, in 2005, a novel species of Bordetella, Bordetella ansorpii, was described after the isolation of a gram-negative bacillus from the purulent exudate of an epidermal cyst.26 16S ribosomal RNA (rRNA) gene sequencing has revealed that this bacterium belongs to the Bordetella genus but is distinct from other Bordetella species. This species was subsequently isolated from an immunocompromised patient in the United Kingdom.27 Bordetella species are small gram-negative coccobacilli.28 Some species are motile and, except for B. petrii, are strictly aerobic. All species possess catalase activity and oxidize amino acids but do not ferment carbohydrates. Bordetella organisms grow optimally at 35° to 37° C. Bordetella species are fastidious because their growth can be inhibited by components commonly found in laboratory media. In addition, their rate of growth is inversely related to their degree of fastidiousness. B. pertussis is the most fastidious and slowest growing of the Bordetella species. Its growth is inhibited by fatty acids, metal ions, sulfides, and peroxides. Isolation of B. pertussis requires a medium containing charcoal, blood, or starch. Traditionally, Bordet-Gengou (BG) medium has been used and consists of a potato-starch base. Charcoal medium (Regan-Lowe [RL] medium), supplemented with glycerol, peptones, and horse or sheep blood, can also be used and may provide better isolation of B. pertussis than the BG medium. B. pertussis infection and disease occur after four important steps: (1) attachment, (2) evasion of host defenses, (3) local damage, and (4) systemic manifestations.4 Filamentous hemagglutinin (FHA) and fimbriae (FIM) are two major adhesins and virulence determinants for B. pertussis. FHA is a 220-kDa surface-associated and secreted protein, and FIM is a filamentous cell surface structure. They are required for tracheal colonization, are highly immunogenic, and are components of certain acellular pertussis vaccines.4 However, there is likely redundancy in the adhesion role of B. pertussis proteins, and it has been suggested that in the absence of FHA, virulence factors such as pertactin (PRN) may mediate attachment.29 Pertussis toxin (PT) also acts as an adhesin30 and has specific recognition domains for human cilia.31 Evasion of host defenses occurs primarily through adenylate cyclase toxin (ACT) and PT.32 ACT is a toxin secreted by B. pertussis that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP), which inhibits the migration and activation of phagocytes. It has also recently been shown to suppress T-lymphocyte activation and chemotaxis.33 PT, one of the most important virulence factors of B. pertussis, also targets the innate immune system of the lung by inactivating or suppressing G protein–coupled signaling pathways. PT has two components, the A subunit and the B subunit. The B (binding) subunit binds to the cell surface to enable adenosine diphosphate (ADP)-ribosylation of G proteins by the A (active) subunit, thereby altering the cell.32 Through this mechanism of action, PT delays the recruitment of neutrophils to the respiratory tract and targets airway macrophages to promote B. pertussis infection.34 The virulence factors of B. pertussis, such as PT, are encoded by the bvg (or vir) gene. The bvg operon is composed of bvgA and bvgS, members of a two-component signal transduction system that controls the genetic state, or phase, of B. pertussis.35 There are virulent and avirulent phases, and their expression is regulated by environmental factors.36 Original reports by Lapin described the local tissue damage caused by pertussis in the lung.1 The initial pulmonary lesion is a lymphoid hyperplasia of peribronchial and tracheobronchial lymph nodes. Necrosis and desquamation of the bronchial epithelium follow, with diffuse infiltrations by macrophages (Fig. 232-1). Most of the damage to the ciliated epithelial cells is caused by tracheal cytotoxin (TCT). TCT is a disaccharide tetrapeptide derived from peptidoglycan, which triggers the production of an inducible nitric oxide (NO) synthase.37 The synthase produces NO, which ultimately kills the tracheal epithelial cells. The induction of the NO synthase is likely caused by the cytokine interleukin-1 (IL-1), generated in response to TCT.38 Dermonecrotic toxin (DNT), a 160-kDa heat-labile secreted toxin that activates intracellular Rho guanosine triphosphate (GTP)ases, may also have a role in local tissue damage.39 DNT was first discovered by Bordet and Gengou and derives its name from the characteristic skin lesion produced when injected into test animals. Unlike other bacterial diseases, there are few systemic manifestations of B. pertussis infection because it does not enter the circulation and disseminate. B. pertussis is relatively sensitive to killing by serum in vitro. However, in vivo, even serum-sensitive strains can efficiently infect mice.40,41 B. pertussis has multiple mechanisms for avoiding antibody-mediated complement killing,42 including the expression of BrkA, a surface-associated protein belonging to the autotransporter secretion system.43 PT is the primary virulence determinant responsible for the systemic manifestations, of which the most prominent is leukocytosis with lymphocytosis.32 Other systemic responses include sensitization to histamine and serotonin and sensitization of the beta-islet cells of the pancreas. This latter effect leads to hyperinsulinemia with resultant hypoglycemia, particularly in young infants.1 Pertussis-associated encephalopathy is rarely observed44; some have suggested that it may be caused by the effect of PT on the central nervous system via monocyte chemoattractant protein-1 (MCP-1) overexpression.45 Fatal pulmonary hypertension has also been associated with pertussis in infants.46 Pathologic studies demonstrate that, in addition to producing pulmonary vasoconstriction resulting from hypoxemia, pulmonary infection with B. pertussis triggers toxin-mediated leukocytosis, causing increased vascular resistance and subsequent refractory pulmonary hypertension.47 In addition to the direct effects of these virulence factors in the lung, some believe that they modulate, in a more global fashion, the immune system itself.48 Studies investigating immunomodulation by B. pertussis have demonstrated skewing of the host immune response toward expansion of the Th17 subset of T lymphocytes, induced by the production of cytokine IL-23.49 The Th17 immune response may be protective against some other gram-negative bacterial respiratory pathogens50 but may also be associated with chronic autoimmune inflammation.51 Some have suggested that the chronic cough seen with B. pertussis infection may be explained by this autoimmune phenomenon, akin to asthma,48 although it has also been hypothesized to be a direct effect of mediators such as bradykinin released in response to tissue damage.52 Despite vaccination, pertussis disease continues to be a problem in the developing and developed world (Fig. 232-2).53 According to the World Health Organization (WHO), an estimated 16 million cases and 195,000 deaths occurred in 2008 because of B. pertussis.54 Case-fatality rates in developing countries may be as high as 3% in infants.55 WHO recommended that a pertussis incidence of less than 1 case/100,000 population be achieved in Europe by 2000. Data from countries represented in the Global Pertussis Initiative (GPI) have indicated that this goal has not yet been achieved.55 In the prevaccine era, pertussis was a major childhood illness and a leading cause of death. Pertussis disease has always been cyclical, with peaks occurring every 3 to 5 years.56 From 1940 to 1948 in the United States, pertussis was responsible for more deaths in the first year of life than measles, scarlet fever, diphtheria, poliomyelitis, and meningitis combined.4 Unlike the current age distribution of pertussis disease, however, pertussis affected children primarily 1 to 10 years of age. From 1918 to 1921 in Massachusetts, for example, more than 80% of pertussis cases occurred in children aged 1 to 9 years, whereas only 10% occurred in infants younger than 1 year of age.57 With the introduction of the whole-cell pertussis vaccine in the 1940s, pertussis rates dropped dramatically. They reached a nadir in the United States in the late 1970s to early 1980s, with a reported 0.5 to 1.0 cases/100,000 population between 1976 and 1982.57 Since then, there has been a gradual increase in pertussis rates over the last 20 years, with peaks of disease continuing to occur every 3 to 5 years.58 The age distribution of pertussis disease has changed as well, with the most cases occurring in unimmunized infants younger than 1 year of age. Data from the National Notifiable Disease Surveillance System and the Nationwide Inpatient Database in the United States have revealed that from 1993 to 2004, 86% of hospitalizations and all deaths caused by pertussis were in infants 3 months of age or younger.59 Similarly, according to Canadian data from 1991 to 1997 from the Immunization Monitoring Program—Active (IMPACT) network, almost 80% of hospitalized cases of pertussis and all deaths secondary to pertussis occurred in children 6 months of age or younger.60 In recent years, there has been a resurgence in pertussis reported in many countries worldwide.7,61–65 The reason for this resurgence is likely to be multifactorial.62 One of the key factors is the finding that neither natural pertussis infection nor immunization produces lifelong immunity to pertussis.66,67 Different pertussis vaccines have had varying rates of success over the years. In the 1990s, Canada experienced a resurgence of pertussis, primarily in young adolescents.68 This was a result of the low effectiveness of the whole-cell pertussis vaccine used between 1985 and 1998 in that country. This resulted in a “marching cohort” effect, or the age of peak incidence increasing each year, by 1 year, revealing the existence of a susceptible cohort (Fig. 232-3).69 This was addressed with universal immunization programs to vaccinate adolescents with a more effective acellular pertussis vaccine.68 However, despite a more effective acellular pertussis vaccine,70 pertussis outbreaks continue to be reported in young adults as well as in young children who recently completed a full pertussis vaccine series.63,71–76 In 2010, a large pertussis outbreak occurred in California, with the highest number of pertussis cases in more than 60 years.76 Second to children younger than 6 months of age, the highest disease rates were observed in fully vaccinated preadolescents (7 to 10 years of age), as observed by others.77 A case control study of children 4 to 12 years of age who were PCR positive for pertussis, compared with PCR negative and matched control subjects, demonstrated that PCR-positive children were more likely to have received the fifth DTaP dose earlier than control subjects, with an odds of acquiring pertussis increasing by an average of 42% per year.78 This suggests that immunity after acellular pertussis vaccination may begin to decline after 4 to 5 years, indicating that a booster dose may be appropriate. Epidemiologic studies have also shown that decreasing antibody levels to pertussis toxin, for example, at a population level, can precede large pertussis epidemics,61 although long-term memory B cells in vaccinated children may persist despite waning antibody levels and provide protection against pertussis disease.79 Additional factors that may have contributed to the resurgence in reported pertussis include increased awareness and subsequent testing for pertussis with very sensitive molecular methods that can detect as little as one organism of B. pertussis, making it difficult to distinguish between colonization and disease (discussed further under “Carrier State”).80 Finally, it is possible that the bacterium itself has evolved and changed over time in response to vaccination practices; additional detail regarding strain variation among B. pertussis is included in “Molecular Diagnosis.” In the past, based on knowledge obtained from traditional culture methods, there was not considered to be a carrier state for B. pertussis in the nasopharynx. However, this may no longer be true, according to studies done with more sensitive PCR methods. In addition to circulating among adults, there may also be transient nasopharyngeal carriage of B. pertussis in immunized children. A case control study63 described a laboratory-confirmed (primarily by PCR assay) outbreak of pertussis occurring in preschool-aged children. This was not a classic pertussis, as evidenced by a lower number of cases meeting a clinical case definition, a very low hospitalization rate of unimmunized infants, and a low secondary attack rate in households. High vaccine rates may have moderated the outbreak, and, with respiratory coinfection in a significant proportion of cases, a positive PCR result may simply have reflected transient nasopharyngeal carriage of B. pertussis in the absence of evidence of seroconversion. There is a spectrum of disease caused by B. pertussis infection, and its presentation will vary according to the patient’s age, degree of immunity, use of antibiotics, and respiratory coinfection.81 Joseph Lapin1 wrote a detailed description of typical or classic pertussis in 1943 that remains true to this day. Pertussis is classically divided into three stages: the catarrhal or prodromal stage, the paroxysmal stage, and the convalescent stage. The catarrhal stage begins after a usual incubation period of 7 to 10 days, with a range of 5 to 21 days. In the catarrhal stage, children will present with signs and symptoms of a common upper respiratory tract infection, including rhinorrhea, nonpurulent conjunctivitis with excessive lacrimation, occasional cough, and low-grade fever. The catarrhal stage typically lasts 1 to 2 weeks and is followed by the paroxysmal stage. As its name suggests, the paroxysmal stage is characterized by paroxysms or fits of coughing. The child will typically have spasms of uncontrollable coughing, often 10 to 15 coughs in a row in a single expiration, the face may turn red or purple and, at the end of the paroxysm, he or she may have an inspiratory whoop. The whoop is caused by inspiring against a partially closed glottis. With the force of the coughing, the child may produce mucous plugs and often have post-tussive vomiting. Paroxysms occur more frequently at night. The paroxysmal stage lasts 1 to 6 weeks. During the end of the first stage and beginning of the second stage, patients may exhibit signs of systemic disease, such as leukocytosis with lymphocytosis, both risk factors for worse clinical outcome.82–84 Hyperinsulinemia may also occur, although it is rarely associated with hypoglycemia.85 Lastly, as symptoms begin to wane, the patient enters the convalescent stage. The length of the cough distinguishes pertussis from other respiratory tract illnesses. In classic pertussis, it usually lasts 1 to 6 weeks, although it can last longer; pertussis is known as the “cough of 100 days” from the Chinese. Most clinical case definitions require a cough of at least 14 days and at least one of the following symptoms: paroxysmal cough, inspiratory whoop, or post-tussive vomiting.81 The mean duration of cough in adults with pertussis is 36 to 48 days.86 For up to 1 year after pertussis infection, it is not uncommon to have recurrences of the paroxysmal cough or inspiratory whoop with other respiratory illnesses. Pertussis can present atypically in adults and infants. Infants are less likely to have the characteristic inspiratory whoop and a significant catarrhal stage and are more likely to present with gagging, gasping, cyanosis, or apnea and have a prolonged convalescent phase.84,85,87 Infants often present with the nonspecific sign of poor feeding and can present with seizures. In adults, paroxysmal coughing is seen in most patients, and several studies have reported cough duration of longer than 21 days.86 There is a wide range in the percentage of adult patients with pertussis who have whooping (8% to 82%) and post-tussive vomiting (17% to 65%, mean 50%). Unlike children, in adults, post-tussive vomiting is strongly suggestive of pertussis. There are several complications associated with pertussis. According to data from Canada and the United States, pneumonia is the most common complication of pertussis in hospitalized patients, especially among newborns younger than 1 month of age (10% to 18%).59,60 Pneumonia can be caused by B. pertussis infection itself (see Fig. 232-1) or coinfection with other respiratory pathogens. Respiratory syncytial virus (RSV) is the best described copathogen with B. pertussis. Rates of RSV coinfection in infants hospitalized with pertussis range from 5% to 33%.59,87 Conversely, 8% to 16% of children with RSV infection may also be positive for B. pertussis.88,89 The case-fatality rate of pertussis is highest in infants; approximately 1% of children younger than 6 months of age will die with pertussis infection.60 In a case-control study done by the IMPACT surveillance network, leukocytosis and pneumonia were independent predictors of death in infants hospitalized with pertussis.82 Encephalopathy is a rare complication of pertussis.44 It occurs more commonly in younger nonimmunized children (in 1.4% of infants younger than 2 months of age)90 but can occur in adults as well.91 It typically presents between the second and fourth week of cough.92 Seizures are the most common clinical manifestation, although paresis and paraplegias, ataxia, aphasia, blindness, deafness, and decerebrate posturing have also been described. Pertussis-specific antigens may cross the blood-brain barrier and directly affect the central nervous system because high cerebrospinal fluid (CSF) antibody titers to PT and filamentous hemagglutinin have been reported in cases of pertussis encephalopathy.44 Complications of pertussis, such as pneumonia and urinary incontinence, are surprisingly high in older patients and significantly more frequent in adults than adolescents, especially in those who smoke or who have asthma.93 Other complications of pertussis caused by the forceful and persistent nature of the cough include subconjunctival hemorrhages, syncope, and rib fractures. The first step in the diagnosis of pertussis is to have the appropriate index of suspicion for pertussis disease. For vaccine trials, WHO defines a case of pertussis as a patient with a paroxysmal cough for 21 days or longer and one or more of the following criteria: positive culture for B. pertussis, significant increases in immunoglobulin G (IgG) and IgA antibody against FHA, agglutinogen (AGG) 2 and 3 or PT, or proven contact with a culture-confirmed case.81 This definition favors specificity of diagnosis rather than sensitivity. According to the Centers for Disease Control and Prevention (CDC) and WHO, for pertussis surveillance, a clinical case is defined as a patient with cough for 14 days or longer and at least one of the following: paroxysmal cough, whoop, or post-tussive vomiting. This is a more sensitive definition, but confirmation requires a positive laboratory finding by culture or PCR assay or a confirmed epidemiologic link. Laboratory confirmation of pertussis has traditionally been made by culture methods.28 Proper collection (including the timing of culture), transport, and storage of specimens are required to enhance the detection of B. pertussis by culture methods. Preferred patient specimens for the diagnosis of pertussis are nasopharyngeal (NP) aspirates and posterior NP swabs. These specimens contain the ciliated respiratory epithelial cells for which B. pertussis has an affinity. The advantage of NP aspirates rather than swabs is an increased culture positivity rate and added sample for any additional tests. If NP swabs are used, swab material should be calcium alginate, Dacron, or rayon because cotton will inhibit the growth of B. pertussis. Specific transport media should be used for NP specimens, including 1% acid-hydrolyzed casein or Amies medium with charcoal. Specimens may be inoculated into enrichment media such as RL transport medium, which contains half-strength charcoal agar and horse blood with cephalexin to suppress normal NP flora growth. For culture testing, specimens should then be inoculated onto BG or RL medium supplemented with glycerol, peptones, and sheep (or, preferably, horse) blood. Cephalexin is added to reduce the growth of normal flora but may inhibit the growth of some strains of B. pertussis. After incubation in ambient air at 35° to 36° C, B. pertussis colonies may become visible after 3 to 4 days, although plates are typically held up to 7 days. B. pertussis colonies are round, domed, mercury silver–colored, and shiny and produce hemolysis on BG agar. Polyclonal or monoclonal antibodies may be used to confirm identity, or B. pertussis can be identified by biochemical tests based on differential phenotypic characteristics. Culture method is the most specific way to diagnose pertussis. The sensitivity of culture for B. pertussis, however, varies widely depending on specimen transport and collection methods, as described earlier, as well as patient factors, such as previous immunization, interval since symptom onset, antibiotic use, and age.81 Different studies have reported culture sensitivity rates for B. pertussis ranging from 15% to 80%.94–96 Many clinical microbiology laboratories now use nucleic acid detection methods, such as PCR assay, as a more sensitive diagnostic test for pertussis. Different PCR assays target different chromosomal regions of B. pertussis, including the PT promoter region, a region upstream of the porin gene, the repetitive insertion sequence IS481, the ACT (cyaA) gene, and a region upstream of the flagellin gene.28 PCR methods for B. pertussis include the standard PCR gel assay and the reportedly more sensitive real-time assay.80 The main advantages of diagnosing pertussis by PCR are its aforementioned increased sensitivity and the rapidity of testing compared with culture methods. Appropriate PCR testing permits the differentiation between B. pertussis and B. holmseii, which can also cause a pertussis syndrome.97 In addition, unlike culture, which is often only positive early in the course of the disease, the PCR will remain positive even after 21 days of antibiotic treatment in more than half of pertussis cases.98 However, one needs to be cautious with the use of PCR testing for pertussis as well. Because the PCR detects genomic material, it will detect both live and dead bacteria. As with any PCR-based method, contamination is always a concern. Although B. pertussis is not an environmental organism, contamination at single collection sites, with positive cultures from environmental surfaces and staff, even from aerosolized B. pertussis vaccine, has been described.99–101 Contamination may also occur in the laboratory and may be responsible for pseudo-outbreaks of pertussis.102 There is no standardized or U.S. Food and Drug Administration (FDA)-approved, commercially available PCR test for B. pertussis, and PCR methodologies and results may vary significantly between laboratories. Depending on where the crossing threshold is set for real-time PCR positivity, the sensitivity and specificity may also vary widely. Studies suggest that PCR may be too sensitive a test to use alone as a screening method for pertussis diagnosis in an outbreak setting71 and, in the absence of clinical, serologic, or culture confirmation, positive results may simply reflect transient nasopharyngeal carriage.63 The advent of newer molecular methods has also furthered our understanding of the genetic variation among strains of B. pertussis. Several investigators have used genotyping methods involving DNA sequencing to look at polymorphisms in the genes encoding for the three major B. pertussis antigens: pertussis toxin (PTX), PRN, and FIM.103,104 Many large molecular epidemiologic studies throughout the world have found that B. pertussis strains have evolved over time, frequently into dominant clones in response to widespread vaccination pressures.105,106 For example, studies in Australia and Europe have noted the emergence of B. pertussis strains carrying a new allele for the pertussis toxin promoter (ptxP3), conferring increased pertussis toxin production.107–109 In the Netherlands, the emergence of ptxP3 strains was temporally associated with increased incidence of hospitalizations and deaths and increased lethality caused by pertussis, suggesting that genetic adaptions in B. pertussis may permit the organism to evade vaccine-induced immunity.110 Pertactin-negative variants were found in Philadelphia.111 These genetic adaptations by the organisms may partially explain the recent resurgence in pertussis disease. However, it is not yet clear what the natural evolution of B. pertussis is over longer periods of time and whether this evolution is directly influenced by vaccination programs worldwide. Hence, continued culturing of B. pertussis isolates from respiratory samples will be necessary to ensure the ongoing genetic characterization of the bacterium.
Bordetella pertussis
History
Description of Pathogen
Pathogenesis
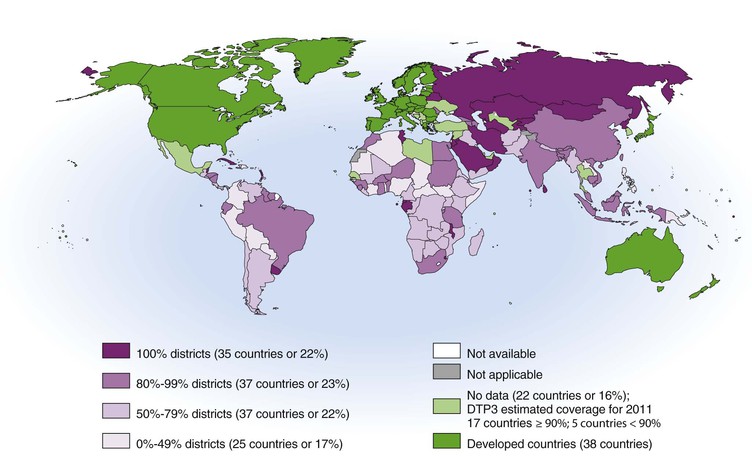
Epidemiology
Prevaccine Era
Vaccine Era
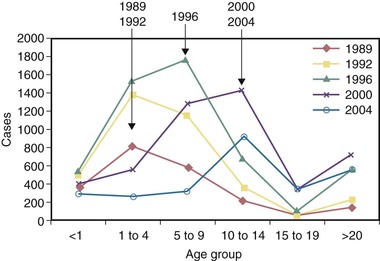
Current Issues Regarding Resurgence of Pertussis
Carrier State
Clinical Presentation
Young Children
Infants and Adults
Complications
Diagnosis
Culture
Molecular Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Bordetella pertussis
232