Characteristic
Osteosarcoma
Ewing sarcoma
Incidence (cases per 106 < 20 years old)
4.8
2.9
Age peak (years)
12–18
5–25
Cell of origin
Osteoblast
Primitive neuroectodermal cell
Biology
Alteration of tumor supresor genes (TP53, RB1)
Oncogene activation (EWS-FLI1, EWS-ERG, EWS-ATF1)
Bone structure
Metaphysis > diaphysis > flat bones
Flat bones > diaphysis > metaphysis
Primary sites
Lower limbs (78 %)
Central axis (45 %)
Upper limbs (11 %)
Lower limbs (30 %)
Central axis (5 %)
Upper limbs (14 %)
Face/skull (3 %)
Short bones (4 %)
Face/skull (3 %)
Treatment
Chemotherapy
Chemotherapy
Surgery
Surgery
No radiation therapy
Radiation therapy
Osteosarcoma
Epidemiology
Osteosarcoma, a malignant neoplasm derived from primitive mesenchymal cells and characterized by the presence of osteoid-producing spindle cell stroma, is the most common malignant bone tumor in the pediatric age group, accounting for approximately 3 % of all cancers in children [2]. The estimated annual incidence in children in the United States is 3.9 cases per one million population among white children and 4.5 per one million population among African-American children [1]. Most osteosarcomas occur during the first 2 decades of life, a period characterized by rapid skeletal growth [3]. Boys are affected more commonly than girls. Several observations support the association between skeletal growth velocity and osteosarcoma. First, patients with osteosarcoma tend to be taller than their counterparts without this disease. Second, osteosarcoma develops at an earlier age in female patients than in male patients, perhaps because of differences in the timing of onset of puberty and the growth spurt [4]. The lack of population-based cancer registries in low- and middle-income countries (LMIC) limits our understanding of the epidemiology of this bone malignancy. Available data suggests that there is rather little geographic and ethnic variation in the incidence of osteosarcoma; however, lowest rates have been observed in Asian populations, and in the United States the incidence is higher in the black population than in whites [5].
Biology
Unlike osteosarcoma in adults, in whom more than 25 % of tumors are associated with preexisting pathologic osseous conditions such as Paget’s disease or fibrous dysplasia, most pediatric osteosarcomas arise spontaneously in areas of bone without any abnormality. Irradiation is the best-characterized etiologic factor contributing to the development of secondary osteosarcoma. Osteosarcoma as a second malignancy is often associated with retinoblastoma; osteosarcoma is the most common malignancy in survivors of retinoblastoma, both in the irradiated and the non-irradiated areas, and it accounts for 25–40 % of all second neoplasms in this population [6]. Half to two-thirds of osteosarcomas occur in the irradiated fields of the skull and face, one-third of tumors develop in the extremities, and less than 10 % in the trunk. Osteosarcoma is also a very frequent malignancy in individuals with germline TP53 mutations (Li-Fraumeni syndrome) [7] and in patients with REC/helicase-associated disorders (Rothmund-Thomson, Werner, and Bloom syndromes) [8]. Consistent with the association of osteosarcoma with retinoblastoma survivors and Li-Fraumeni syndromes, alterations in components of the cell cycle control system appear to characterize the ontogeny of osteosarcoma. Studies of the retinoblastoma gene (RB1) have shown that alterations affect the RB1 gene in as many as 80 % of cases and that other events, such as CDK4 alterations, also may result in RB1 inactivation [9, 10]. Genetically engineered mice based on osteoblast-restricted deletion of p53 and pRb develop short-latency high-grade osteosarcoma that reproduces many of the defining features of human osteosarcoma, including cytogenetic complexity and comparable gene expression signatures, histology, and metastatic behavior [11].
Pathology
Osteosarcoma is characterized by the presence of spindle cell stroma that produces osteoid. Conventional osteosarcoma can be subdivided histologically into three major groups depending on the predominant cell type. Approximately 50 % of tumors are categorized as osteoblastic, because the predominant extracellular element is osteoid, whereas 25 % are chondroblastic, with a prominent cartilaginous component. Approximately 25 % have a herringbone pattern similar to that observed in fibrosarcoma and are therefore called fibroblastic. No significant differences in outcome are apparent among these three histologic subtypes.
Clinical Manifestations
Pain is the most common symptom in children and adolescents with osteosarcoma. Onset of pain often is insidious and usually involves the area affected by tumor. Severe pain of sudden onset commonly is associated with pathologic fracture. Swelling around the affected bone is the second most common clinical finding. The tumor may be easily palpable when located in areas such as the anterior surface of the femur but may manifest only as leg edema when occurring in difficult-to-appreciate areas such as the popliteal fossa. A painful limp that increases with weight bearing is the third most common symptom. Systemic signs and symptoms such as fever and weight loss are uncommon. Osteosarcoma most commonly involves the long bones, most tumors occurring around the knee. The most frequent sites of involvement are the distal part of the femur, the proximal portion of the tibia, and the proximal part of the humerus. The axial skeleton, including the pelvis, is rarely affected in children (fewer than 10 % of cases) but more frequently is involved in patients older than 60 years [2, 12]. Overt macroscopic metastatic disease occurs in 20 % of cases and carries a grave prognosis [13]. In countries with limited resources, late diagnosis and difficulties in accessing care result in patients presenting with a higher tumor burden, with large tumors and higher frequency of metastatic disease, often in excess of 40 % [14, 15].
Laboratory and Radiologic Evaluation
Laboratory evaluation often is unrevealing. Elevations of serum lactate dehydrogenase (LDH) and alkaline phosphatase levels are the most common laboratory abnormalities. The latter appears to correlate with osteoblastic activity and has therefore proved useful in monitoring response to therapy [16]. Radiologic evaluation of a patient with osteosarcoma must include assessment of the primary site as well as a search for distant metastatic lesions (Table 22.2) [17]. Plain radiography is the most effective method of detection of bone tumors. Characteristic radiologic findings in osteosarcoma commonly include a metaphyseal permeative lesion with periosteal new bone formation and destruction of preexisting cortical bone. A soft-tissue mass is present in more than 90 % of cases. Other radiologic signs commonly associated with osteosarcoma include cumulus cloud-like density and the presence of Codman’s triangle (Fig. 22.1). If available, magnetic resonance imaging (MRI) offers the best estimate of intramedullary tumor extension, joint and vascular involvement, detection of “skip” metastatic lesions, and delineation of the soft-tissue component; this is particularly important if a limb-salvage procedure is considered for local control, but less relevant if an amputation is planned. A baseline chest radiograph should be obtained to search for distant metastatic lesions; however, when possible, a computed tomography (CT) should also be performed at the time of diagnosis for better documentation of metastatic disease. Biopsy of the primary tumor should be done carefully, preferably by the surgeon who will ultimately perform the definitive operation. In performance of biopsy of a suspected bone tumor, the following basic principles should be observed: (1) avoidance of transverse incisions, which can make subsequent surgery difficult; (2) avoidance of contamination of multiple compartments and hematoma formation, because successful limb-sparing procedures can be jeopardized; and (3) if feasible, biopsy of the soft-tissue component only.
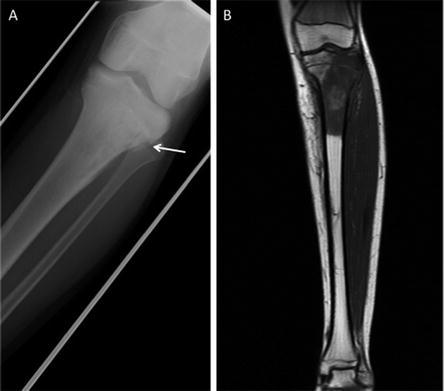
Table 22.2
Diagnostic work-up of bone tumors
Site | Test | Osteosarcoma | Ewing sarcoma |
|---|---|---|---|
Primary tumor | Plain XR | Yes | Yes |
CT/MRI | Yesa | Yesa | |
Biopsy | Yes | Yes | |
Distant disease | Plain XR chest | Yes | Yes |
Chest CT | Yesa | Yesa | |
Bone scan | Nob | Yesa | |
Bone marrow aspirate and biopsy | No | Yes | |
Organ function evaluation | Echocardiogram | Yes | Yes |
Audiogram | Yes | No | |
Renal function | Yes | Yes | |
Liver function | Yes | Yes |

Fig. 22.1
Imaging characteristics of an osteosarcoma of the tibia in a 14-year-old boy. (a) Moth eaten, mixed osteolytic and sclerotic appearance is demonstrated involving the proximal tibial metaphysis with cortical destruction (arrow) and periosteal reaction along the right lateral aspect of the proximal tibial metaphysis. (b) T1-weighted MRI shows low-signal intramedullary lesion extending and destroying the metaphysical cortex
Osteosarcoma Subtypes
In addition to conventional osteosarcoma, a small proportion of patients present with clinical subtypes that are characterized by distinct clinical, radiologic, and histologic characteristics.
1.
Telangiectatic osteosarcoma is characterized microscopically by blood-filled spaces divided by septa containing neoplastic sarcomatous cells. Both radiologically and histologically, it is difficult to differentiate from aneurysmal bone cyst. It accounts for less than 4 % of cases of osteosarcoma. Age and anatomic distributions are similar to those in conventional osteosarcoma. On imaging studies, telangiectatic osteosarcoma manifests as a purely lytic lesion with a permeative destructive growth pattern; it usually disrupts the cortex, but with minimal or no periosteal new bone formation, which often is multilayered, in an onionskin pattern. Histologically, telangiectatic osteosarcoma is very hemorrhagic, similar to the gross appearance of aneurysmal bone cysts. Microscopically, this tumor consists of cyst-like spaces divided by septa, which are composed of highly atypical sarcomatous tissue. With appropriate multimodality therapy, the outcome of telangiectatic osteosarcoma is similar to or better than conventional osteosarcoma [18].
2.
Low–grade intramedullary osteosarcoma is a very rare variant of osteosarcoma, accounting for less than 1 % of cases. Its anatomic distribution is similar to conventional osteosarcoma, with predilection for the distal femur and proximal tibia. In contrast with conventional osteosarcoma, symptoms typically develop over many months or even years before the patient comes to medical attention. Imaging studies usually show a variable pattern of lytic foci and dense areas, poorly demarcated. Periosteal new bone formation is minimal. Histologically, it shows a predominantly differentiated fibroblastic and osseous component, similar to what is seen in parosteal osteosarcoma. The differential diagnosis is with fibrous dysplasia. Treatment includes a complete resection of the lesion. Incomplete resection invariably results in local recurrence, and dedifferentiation increases with each recurrence.
3.
Surface osteosarcomas originate and grow predominantly on the surface of the bone, and include the parosteal, periosteal, and high-grade surface osteosarcomas. Parosteal osteosarcoma is a low-grade tumor that grows predominantly on the surface of long bones, in an exophytic pattern. Because it is derived from the outer layer of the periosteum, it grows without causing elevation of the periosteum or evidence of periosteal new bone formation. They tend to occur in skeletally mature patients, with diagnosis during the third and fourth decades of life. More than 80 % of these tumors are located in the distal portion of the femoral shaft, in its posterior aspect, within the superior popliteal area. Imaging shows a tumor growing on the surface of the bone, with a broad base, in a mushroom-like fashion. The mass typically is densely mineralized and has lobulated outlines. Microscopically, these tumors are characterized by the presence of a spindle cell fibroblastic component, with variable osteoid production, low mitotic rate, and no atypical features. The treatment is surgical, and a complete resection is mandatory; chemotherapy is not necessary. Periosteal osteosarcoma is a low- to intermediate-grade tumor that has a predominantly chondroblastic differentiation. It originates in the deep layer of the periosteum, so its growth is manifested by a separation and elevation of the periosteum from the cortex, causing a prominent periosteal new bone formation. Periosteal osteosarcoma is a tumor of childhood, with a peak incidence during the second decade of life, and it has a female predominance. The usual location is in the long bones of the lower extremity, most commonly the tibia, with an affinity for the diaphysis. The presentation is similar to that with conventional osteosarcoma, with pain and swelling of short duration. Imaging studies show a predominantly fusiform lesion on the surface of a long bone.
Prognostic Factors
The most important adverse prognostic factor in patients with osteosarcoma is the presence of metastatic disease [13]. In addition, primary tumor location is associated with outcome. Children with primary tumors of the tibia and distal femur appear to have a more favorable prognosis than those with axial primary tumors. This finding highlights the importance of complete surgical resection in the management of this malignant disease [13]. For patients with localized disease, factors associated with poor prognosis include measures of tumor burden, such as tumor size, and levels of alkaline phosphatase and LDH [13]. The percentage of tumor necrosis after preoperative chemotherapy is the most consistent and important factor associated with outcome in children and adolescents with localized osteosarcoma. A favorable response (more than 90 % tumor necrosis) correlates with excellent overall survival. Patients who have less than 90 % tumor necrosis are considered poor responders, for whom the prognosis usually is poor [13]. Finally, a proportion of patients with extremity osteosarcoma present with a pathologic fracture, or a pathologic fracture develops after institution of therapy. This has been considered a poor prognostic factor and an indication for immediate amputation. It is possible, however, that with the use of preoperative chemotherapy and judicious use of limb-sparing techniques, a selected group of patients with pathologic fracture may still do well without amputation.
Treatment
Optimal management of osteosarcoma consists of multiple-agent chemotherapy and local control measures, including amputation or limb-sparing surgical procedures. Generally, treatment is initiated with pre-surgery chemotherapy, followed by local control in 6–12 weeks. Before the development of limb-sparing procedures, amputation was the standard surgical method used for curative treatment of osteosarcoma, and it still continues to offer the best local control rates. Limb function after above- or below-the-knee amputation can be reasonably good with an external prosthesis. However, over the past several years, the role of limb-sparing procedures has increased dramatically. As a result of refinements in neoadjuvant chemotherapy, bioengineering, and imaging techniques, it is estimated that as many as 80 % of patients with osteosarcoma in high-income countries (HIC) will eventually be candidates for limb-sparing procedures, which may include vascularized autologous bone grafts, allografts, and endoprostheses. The criteria for limb-sparing procedures include (1) absence of major neurovascular involvement by tumor, (2) feasibility of wide surgical excision to include a normal muscle cuff in all directions and en bloc removal of all biopsy sites, (3) resection of the adjacent joint and capsule, (4) adequate motor reconstruction with regional muscle transfer, and (5) adequate soft-tissue coverage [19]. When these procedures are appropriately performed, the risk of local recurrence is low (less than 5 %) [20]. Long-term functional outcome, however, must be carefully compared with that obtained with amputation alone. Complications of limb-sparing surgery include infection, nonunion, fracture, and unstable joints. In countries with limited resources, options for limb-salvage are limited by the large tumor burden, the lack of infrastructure and trained personnel, and the costs associated with those procedures. However, with the development of centers of excellence and locally grown bioengineering approaches, limb-preserving surgeries are also feasible in LMIC [21–24]. In the development of a limb-salvage program, a very good and careful selection of candidates needs to take place. Local failure rates should be kept under 10 % for a program to be considered effective.
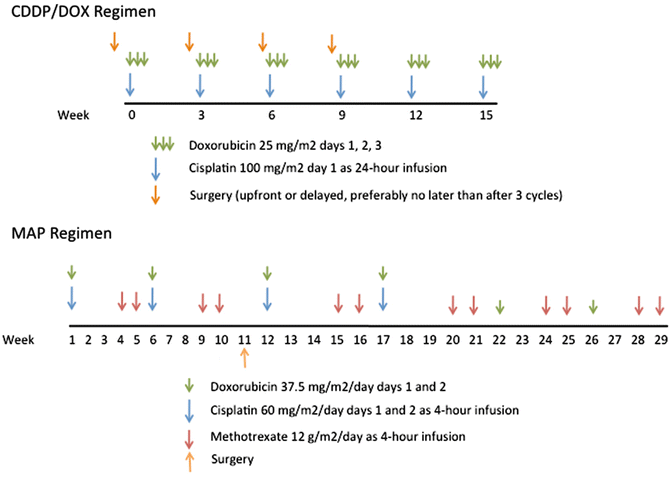
Before the introduction of adjuvant chemotherapy, fatal metastatic disease developed in more than 80 % of patients with osteosarcoma [25]. Currently, the use of systemic chemotherapy in combination with radical surgery of the primary tumor may result in cure rates of 50–70 % for patients with localized disease. Standard treatment includes high-dose methotrexate, Adriamycin (doxorubicin), and cisplatin (MAP regimen) (Fig. 22.2) [26]. One of the major limitations to the implementation of the MAP regimen in LMIC is the use of high-dose methotrexate. Methotrexate was among the first drugs reported to have an antitumor effect in osteosarcoma and it has been a major component of the osteosarcoma treatment ever since [25]. Further, there is evidence that suggests that the pharmacokinetics of this agent may influence outcome, although this influence is minor in the context of more intensive multiagent protocols. However, the role of methotrexate appears to be limited to the administration of high doses (usually 12 g/m2), because the administration of lower doses appears to have a lesser therapeutic effect. The administration of high-dose methotrexate requires close monitoring of serum levels, which cannot be performed in many institutions, particularly those of developing countries, and extensive supportive measures, including hyperhydration, urine alkalinization, and leucovorin rescue, which must be adjusted to the methotrexate serum levels. The development of acute renal failure, mediated by the precipitation of methotrexate and its metabolites in the renal tubules, is a potentially life-threatening complication. Even among patients receiving all available monitoring and supportive care, severe nephrotoxicity develops in 2 % of them, and the mortality for those patients is 4.4 % [27]. This morbidity can be much worse in LMIC and thus the benefit of the use of high doses of methotrexate should be balanced against the elevated risk of severe morbidity. Studies have shown the possibility of obtaining comparable outcomes without the use of methotrexate, using regimens that maximize exposure to cisplatin and anthracyclines [28]; a simple regimen with six cycles of cisplatin and doxorubicin can cure up to 50 % of patients with localized osteosarcoma, and this seems to be a very good regimen for LMIC [29, 30]. One of the most important prognostic factors in osteosarcoma is the histologic response to preoperative chemotherapy; this finding has influenced the development of many strategies that have included the modification of postoperative chemotherapy, usually with the addition of ifosfamide and etoposide (MAP-IE regimen) [31]. The impact of such approach is not yet clear; the EURAMOS protocol is currently investigating this through a randomized study.