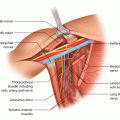
Fig. 60.1
Screenshot of FRAX assessment tool (© Centre for Metabolic Bone Diseases, University of Sheffield, UK. Used with permission from the University of Sheffield)
60.1.2 How Bone Health Is Monitored and Assessed
DEXA Scans
The standard for bone health monitoring is the use of DEXA scans to assess bone mineral density. The principle behind the DEXA scan is the measurement of difference between penetrations of two photon beams of different energies through the body. This allows the inference of the density of two tissues (bone and soft tissue) and a real (not true volumetric) density to be estimated. Advantages of DEXA scans are low doses of ionising radiation, good precision, short scan times and stable calibration. The major disadvantage is that changes in BMD often take many months or years to be assessable by DEXA scan.
Bone Turnover Markers (BTMs)
BTMs can be divided into two groups, formation and resorption markers. Formation markers reflect the activity of osteoblasts and include bone-specific alkaline phosphatase (BALP) and procollagen type 1 amino-terminal propeptide (P1NP). Resorption markers reflect the activity of osteoclasts and include type 1 collagen C-terminal telopeptide (CTX) and type 1 collagen amino-terminal telopeptide (NTX).
BTM monitoring may allow for earlier identification of patients with accelerated bone resorption and therefore future BMD loss and may potentially provide a more dynamic, non-invasive and cheaper assessment of skeletal metabolism [7, 8]. In an exploratory subset analysis of the patients who had BTM assessment in the Z-FAST trial (Zoledronic acid-Letrozole Adjuvant Synergy Trial), an early increases in NTX and BALP were predictive of clinically relevant long-term bone loss [9]. However, further studies are needed and BTMs are not routinely used in clinical practice.
60.1.3 Factors Affecting Bone Health in Breast Cancer Survivors
Bone health can be affected by cancer treatment irrespective of menopausal status. In premenopausal women there is a risk of accelerated bone loss due to oestrogen suppression from adjuvant treatments including chemotherapy, aromatase inhibitors and ovarian suppression or due to premature ovarian failure [10]. In postmenopausal women the rate of BMD loss is doubled in patients administered aromatase inhibitors in the adjuvant setting [11]. Decrease in BMD related to cancer treatment is usually described as treatment-induced bone loss (TIBL).
60.1.4 Premature Ovarian Failure
Cytotoxic Chemotherapy
Combination cytotoxic chemotherapy is administered perioperatively to prevent disease recurrence and improve breast cancer-related mortality. In premenopausal patients, the use of such treatments can result in either temporary or permanent ovarian failure. Approximately 68% of patients, ranging from 20–100% depending on age, type and cumulative dose of cytotoxic agent, will experience chemotherapy-induced ovarian failure and amenorrhea [10, 12, 13]. This results in rapid decrease in BMD of up to 7% within 1 year [14]. Bone loss does not appear to be clinically significant in those that retain their menses following treatment.
Ovarian Suppression/Ablation
Interruption of the hormonal axis, through the use of drugs affecting the hypothalamic-pituitary-gonadal axis (e.g., GnRH/LHRH analogues), results in loss of menses and potentially reversible ovarian suppression. Rapid bone loss has been seen for the duration of amenorrhoea. Recent data has suggested a decrease in disease-specific recurrence with the addition of adjuvant ovarian suppression to either tamoxifen or exemestane in higher-risk patients who remain premenopausal after chemotherapy [15]. In view of this, the use of ovarian suppression and tamoxifen or exemestane may play an important role in high-risk patients who have premenopausal levels of oestradiol following chemotherapy. In premenopausal breast cancer patients, a Phase 3 trial (ABCSG-12) randomised 1803 patients with hormone receptor-positive breast cancer to receive endocrine treatment (goserelin and tamoxifen or anastrozole), each with or without zoledronic acid every 6 months for 3 years [16, 17]. Data from the bone sub-study (n = 404) showed that in patients who did not receive bone protective therapy with zoledronic acid, there was a significant reduction in BMD at 3 years (trochanter, 7.3%; lumbar spine, 11.3%), with a larger detrimental effect in those patients receiving anastrazole. At 5 years, there was only partial recovery with BMD levels remaining less than baseline (trochanter, 4.1%; lumbar spine, 6.3%).
Tamoxifen
Tamoxifen is a selective oestrogen receptor modulator and is one of the most commonly used treatments in patients with ER-positive breast cancer. In the premenopausal setting, it has a predominantly antioestrogenic effect resulting in a small (1–2%) increased loss of BMD. This is not clinically significant and no bone protection is recommended in this setting. In the postmenopausal setting, tamoxifen has been shown to increase BMD of the spine and hip.
Aromatase Inhibitors
In the postmenopausal setting, patients with ER-positive breast cancer are increasingly treated with an aromatase inhibitor (AI), and the most recent meta-analysis showed that 5 years of treatment with an AI leads to 15% relative reduction in the 10-year breast cancer mortality rates when compared with 5 years of tamoxifen [18]. After the menopause, circulating oestrogen results from the conversion of androgens to oestrogen in the peripheral tissue by the enzyme aromatase. Inhibition of aromatase, either by reversible non-steroidal inhibitors (anastrozole/letrozole) or the irreversible steroidal inhibitor (exemestane), results in almost undetectable levels of circulating oestrogen. However, BMD loss with an AI is double the normal physiological rate [11] resulting increased fracture risk.
The bone sub-study in the «Arimidex, Tamoxifen alone, or in combination» (ATAC) trial [19], reported the longer-term effects on BMD following hormone treatment for 5 years in patients with early breast cancer. A total of 308 women had baseline lumbar and hip BMD assessed by DEXA and then on treatment at 1, 2, and 5 years. Following treatment, 50 patients treated with anastrozole alone had further assessment at years 6–7. Patients treated with anastrozole alone showed a median decrease in BMD of 6.1% and 7.2% in the lumbar spine and hip, respectively, compared to an increase of 2.77% and 0.74% in the lumbar spine and hip, respectively, in patients receiving tamoxifen. Of note, women who had normal BMD at baseline did not develop osteoporosis. DEXA measurements at 6 and 7 years showed increases in BMD by 2.35% and 4.02% at the lumbar spine and 0.71% and 0.5% at the hip suggesting that treatment-related bone loss does not continue beyond treatment [20]. These results were replicated in the Intergroup Exemestane Study [21].
Although BMD loss appears reversible after stopping AI treatment, fracture risk increases throughout the duration of AI use when compared to tamoxifen. At a median follow-up of 100 months in the ATAC study, the incidence of fracture during active treatment in the anastrozole arm was 12% compared to 7.5% in patients receiving tamoxifen with annual rates of 2.93% and 1.9%, respectively [22]. However, the difference in fracture rates between the two arms resolved after AI treatment was discontinued, potentially explained in part by the increase in BMD observed when patients were off anastrozole treatment [22]. In the BIG 1–98 study, 4895 patients were randomised to receive 5 years of letrozole or tamoxifen, and at a median follow-up of 5 years, the fracture incidence was 9.3% and 6.5% in patients receiving letrozole and tamoxifen, respectively [23]. Recognition and treatment of patients at particular risk of fracture will therefore help to select a patient group who would benefit from bone-directed therapy.
60.1.5 Pharmacology of Bone-Directed Therapy
Bisphosphonates
Bisphosphonates are the first line for treatment for patients with established osteoporosis of any cause. Bisphosphonates are stable synthetic analogues of pyrophosphate and have a P-C-P backbone that acts as a bone hook. Following either oral or intravenous administration, they accumulate in the bone and are selectively internalised by osteoclasts during bone reabsorption. Osteoclast apoptosis is induced by the metabolism of nonnitrogen-containing bisphosphonates to ATP analogues [24] or inhibition of farnesyl diphosphate synthase in the mevalonate pathway by nitrogen-containing bisphosphonates which disrupts the prenylation of important signalling GTPases [25]. Bisphosphonates can be administered orally or intravenously. Intravenous bisphosphonates must be used with care in patients with renal insufficiency with dose reductions as per the manufacturer’s guidelines.
Denosumab
Denosumab is a fully humanised IgG2 monoclonal antibody administered subcutaneously that binds to RANK ligand (Receptor Activator of Nuclear Receptor ĸ B) and prevents activation of the RANK receptor on osteoclasts and their precursors and ultimately inhibits osteoclast formation, function and survival [26]. It does not require dose reduction in renal or hepatic impairment and does not accumulate in the bone.
Side Effects of Bone-Directed Therapy
Both bisphosphonates and denosumab are generally well tolerated, and side effects are related to mode of administration. Oral bisphosphonates can cause gastrointestinal complications including gastrointestinal bleeding. Intravenous bisphosphonates are associated with infusion reactions, metabolic effects (hypocalcaemia) and renal toxicity. Subcutaneous denosumab may cause local skin reactions and hypocalcaemia. Due to the metabolic effects, all patients must have adequate vitamin D levels and receive calcium supplementations. A rare but serious side effect of bisphosphonate therapy and denosumab is the development of osteonecrosis of the jaw [27]. The risk of developing this with zoledronic acid is 0.12–0.7% if used biannually [28]. The pathogenesis of this is unclear and may be largely avoided with patient education and pretreatment dental evaluation. A further rare but serious side effect are atypical fractures. At present there are no consensus guidelines for the management of such patients, and each case should be reviewed by a specialist bone team.
Use of Bisphosphonates in TIBL
Both intravenous and oral bisphosphonates have been evaluated for the treatment of AI-related TIBL [4]. The most extensively studied bisphosphonate is zoledronic acid. In three parallel-designed international trials (Z-FAST [9, 29], ZO-FAST [30] and E-ZO-FAST [31]), and a fourth trial N03CC [32], approximately 2750 postmenopausal women with hormone receptor-positive breast cancer receiving 5 years of adjuvant letrozole were randomised to receive either upfront or delayed zoledronic acid (both at a dose of 4 mg every 6 months). Delayed zoledronic acid was initiated due to accelerated bone loss (T-score < −2.0) or fracture. The primary end point for these trials was lumbar spine bone mineral density change at 12 months. In all these trials, upfront zoledronic acid effectively prevented letrozole-induced bone loss with an increase of mean percentage change of bone mineral density at the lumbar spine of 4.3–6.19% in the patients treated with upfront zolendronic acid versus a decrease in bone mineral density of 2–5.4% in those who received delayed treatment; this improvement was sustained at ongoing follow-up of over 60 months.
A handful of smaller trials have shown efficacy of oral bisphosphonates including the SABRE [33] and ARIBON [34, 35] studies. In the SABRE study, 154 patients with a moderate risk of fracture received either risedronate 35 mg or placebo once a week alongside treatment with anastrazole. The mean percentage change of bone mineral density was 2.2% at the lumbar spine and 1.6% at the hip compared to decreases of 1.8% and 1.1%, respectively, in the placebo group. The ARIBON study enrolled 131 postmenopausal women of which 13 patients had osteoporosis, and 50 patients had evidence of osteopenia. All patients with osteoporosis received ibandronate, and those with osteopenia were randomised to receive ibandronate 150 mg every 28 days or placebo in addition to anastrazole. At 24 months of follow-up, patients in the bisphosphonate group showed a mean increase in bone mineral density of 2.98% at the lumbar spine, compared to a decreased of 3.22% in the placebo group. These trials do suggest that oral bisphosphonates given in osteoporotic dosing regimens demonstrate efficacy in AI-related TIBL; however follow-up was shorter, and there are concerns about compliance with oral bisphosphonates.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree