Bladder Cancer
The basic management of bladder tumors has remained essentially unchanged for over 50 years, and in spite of its importance in terms of incidence, prognosis, and cost, bladder cancer research remains significantly underfunded.1 Every aspect of the perceptions and management of this disease requires change; departing from the use of the term “superficial” bladder cancer is just the first step,2 because the term is both inaccurate and implies an inappropriate lack of importance. In particular, given the very high costs to health care systems from long-term surveillance and treatment of the disease,3 it is particularly surprising that there has not been more emphasis on the disease from health policymakers and pharmaceutical companies.
In addition, a rethinking of the view that cystectomy is the gold standard for invasive bladder cancer is long overdue. Comparisons of large surgical4 and radiotherapy5 series suggest very similar long-term survival rates, and population-based studies do not appear to show any survival differences linked to the mode of treatment.6 Furthermore, most large surgical series have median ages in the mid-60s,4,7 well below the (rising) disease population median, suggesting the results may not be applicable to many or even most patients with invasive bladder cancer. Use of bladder preservation varies worldwide from around 10% in the United States8 to 25% in Scandinavia9 to around 50% in the United Kingdom.10 Moreover, there is good evidence that older or less fit patients in low-volume centers are less likely to be referred for surgery, despite the likelihood of them being fit for radiotherapy.8,9
In contrast, recent large randomized radiotherapy series from the United Kingdom suggest that radical radiotherapy with sensitization, either with low-dose chemotherapy11 or with hypoxia-targeting agents,12 is effective and well tolerated by elderly patients (median age in both studies was 72 to 73 years). Long-term functional outcomes with radiotherapy are excellent,11–13 making it particularly suitable for less-fit patients who may struggle with major surgery or a urinary diversion.
This chapter outlines the evidence base for the current therapeutic approaches to bladder cancer and in particular will examine the proposition that bladder preservation for muscle-invasive disease is an approach that merits re-examination.
 ANATOMY OF THE BLADDER
ANATOMY OF THE BLADDER
The bladder is a hollow, muscular organ situated in the pelvis when empty but able to extend up into the abdomen when full, particularly in situations where bladder emptying is impeded. At birth, the pelvis is relatively small in comparison to the abdomen, and thus the bladder has a larger abdominal component at birth and becomes more “pelvic” as growth and maturity proceed. By puberty, the bladder has migrated to the confines of the deepened true pelvis.
The bladder is described as having an apex, a superior surface, two inferolateral surfaces, a base or posterior surface, a trigone, and a neck. The apex reaches a short distance cephalad above the pubic bone and ends as a fibrous cord, the remnant of the fetal urachus, which connects the bladder to the allantois. The urachus lies anterior to the peritoneal cavity and is important as tumors can arise in the urachal remnant. The superior surface is covered by the peritoneum, again an important anatomical feature as it means that there is bowel lying superiorly to the bladder, which is potentially a critical site to consider when planning radiotherapy, particularly in men. In women it is associated with the uterus and ileum. The base of the bladder is posterior and is separated from the rectum by the vas deferens, seminal vesicles, and ureters in the male, and by the uterus and vagina in the female. The seminal vesicles form a V-shaped structure at the base of the bladder, with the vas deferens entering the middle of the “V.” The ureters enter into the bladder slightly superior and lateral to the seminal vesicles, with the vas deferens coursing above and in a caudal direction to the ureters. Again these relations are critical as enlarging tumors either at the base or in the prostate can involve the ureters with consequent hydronephrosis. Inferiorly and laterally to the bladder lie the various pelvic bones and muscles: pubis, the levator ani, and obturator internus muscles. Within the pelvis, the lateral parts of the bladder are surrounded by loose connective tissue. Anteriorly the bladder is separated from the pubic bone by the retropubic space. The inferior part of the bladder is described as the neck and is in continuity with the urethra and, very importantly, the prostate gland in males. The neck of the bladder is anchored in the pelvis, and the superior portions distend and expand upward as the bladder fills.
The mucosal lining of the bladder comprises a transitional epithelium that extends from the renal pelvis to the urethra. The most common tumors arising in the urinary system are transition cell (or urothelial) carcinomas (TCC or UC). These tumors can arise from anywhere within the urothelium, so diagnosis, treatment, and surveillance protocols must take account of this important biological feature. As a distensible organ, the macroscopic appearance of the urothelium varies with distension from smooth and flat to folded when empty. A ridge called the interureteral fold lies between the ureteric orifices.
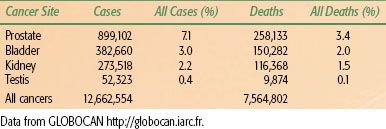
TABLE 64.1 2008 UROLOGICAL CANCER INCIDENCE AND MORTALITY WORLDWIDE
 EPIDEMIOLOGY
EPIDEMIOLOGY
Bladder cancer, with over 385,000 new cases reported worldwide in 2008,14 is a major cause of cancer morbidity and mortality (Table 64.1). Median age at diagnosis is above 70 years and, as the tumor is often smoking related, many patients have significant comorbidity, posing risks for radical surgical approaches. Survival rates are poor, with around 45% of muscle-invasive cancer patients surviving 5 years irrespective of treatment modality.4,5,7 Demographically, the industrializing nations will contribute to a significant rise in the global incidence of bladder UC,15 with particularly large numbers likely in China given the rapid improvement in standards of living and the high prevalence of smoking. However, despite the decreasing incidence in developed nations, there remain specific challenges, mainly due to the aging population and increased life-expectancy. Within two large cohorts separated by 15 years (1991 to 1992 and 2005 to 2010), researchers have recently demonstrated an increase in the median age at presentation of 4 years, with an increase from 13% to 24% in the proportion of patients over 80 years old.16 Overall around 75% to 80% of patients with bladder cancer are male, mostly reflecting historic trends in cigarette smoking.
There are well-known associations of squamous cell bladder carcinomas with bilharzia caused by Schistosoma haematobium infection in Africa, particularly in Egypt.17 Aromatic amines, polycyclic aromatic and chlorinated hydrocarbons, arsenic-laced drinking water, aristolochic acid, cyclophosphamide exposure, and a range of industrial chemicals have been implicated in urothelial carcinogenesis. Importantly, as with most carcinogens, there are variations in individual susceptibility, and the basis of some of these polymorphisms regulating varied detoxification mechanisms has been identified.18 With increasing awareness of these industrial associations, regulation of these processes means that these cases are becoming increasingly rare in the developed world. Their principal importance now is that those with industrially linked tumors may be entitled to compensation payments. In Egypt there have been successful public health approaches to the control of S. haematobium infections, leading to a substantial decline in incidence and mortality from squamous carcinomas of the bladder.19
Within the developed world, the overwhelming majority of bladder tumors are now TCCs, and the main known causative factor is tobacco (particularly cigarette) smoking,20,21–22,23–25 explaining approximately half of the cases in men and one-third of the cases in women in Europe (discussed in detail below). The relation between smoking and other prognostic factors is interesting, as it could give insight into biological mechanisms of disease and, perhaps more importantly, have clinical implications by increasing our ability to identify patients at risk of more malignant disease.
 NATURAL HISTORY
NATURAL HISTORY
Non-muscle-Invasive Bladder Cancer
Most cases (70% to 80%) present with non-muscle-invasive bladder cancer (NMIBC, stage Ta, T1, and carcinoma in situ [Tis]), which is rarely lethal, but shows a high recurrence rate of 50% to 70% after treatment by transurethral resection of the bladder tumor (TURBT).26 In about 10% to 20% of patients with NMIBC, the disease progresses to muscle invasion (≥T2 lesions), which can lead to metastasis and death.26 However, the majority of patients with NMIBC will die of other causes, given the typically advanced age at presentation and the strong association with cigarette smoking,20,21–22,23–25 although it is worth noting that up to 21% of patients with Ta tumors and 49% of patients with T1 tumors will die from bladder cancer.27 For patients with NMIBC, it has been observed that tumor grade and stage, and also tumor number, size, presence of carcinoma in situ (CIS), recurrence rate, and age at diagnosis are risk factors of progression.28–29,30 The risk of both recurrence and progression necessitates lifelong follow-up for patients with bladder tumors. However, there are factors that predict a higher risk of progression (to invasion) as opposed to recurrence in lower risk tumors, and the European Organisation for Research and Treatment of Cancer (EORTC) “bladder cancer calculator” quantifies the risk depending on the tumor characteristics imputed.30
Invasive Disease
Muscle-invasive bladder cancer has a poor prognosis due to a very high rate of occult metastatic disease at the time of diagnosis. Evidence for this comes from the high rate of death from metastasis after apparently successful surgery. Furthermore, reported 5-year survival rates with radiotherapy or surgery are remarkably similar at around 45% to 50%,4,5 despite a higher rate of pelvic recurrence after radiotherapy versus surgery, suggesting that prognosis is driven by the presence or otherwise of metastases at the time of diagnosis, driven by tumor-related factors such as stage and grade.6
Metastatic Disease
A minority of patients (probably <10%) present with metastatic disease; most patients with metastatic disease have had prior treatment for apparently localized disease. Metastatic disease carries a poor prognosis. Overall survival from diagnosis of metastasis is difficult to ascertain as many patients receive only palliative treatment. A minority of patients are fit for systemic chemotherapy, and there are good data on outcomes with chemotherapy. In essence, extensive randomized studies, mostly carried out in the 1980s and 1990s, have demonstrated the superiority of cisplatinum-based combinations over those containing other drugs or cisplatinum alone.31 Of the platinum-based combinations, methotrexate/vinblastine/doxorubicin (Adriamycin)/cisplatinum (MVAC)32 and gemcitabine/cisplatinum (GC)33 have proven to be superior to other combinations and broadly similar in efficacy to each other34,35 (as reviewed by Hussain and James36). Median survival is 12 to 18 months, depending on the extent of disease and fitness of the patient. Intriguingly, however, a small minority of patients do appear to survive long term after chemotherapy for metastatic disease, but this percentage has sadly proved very hard to increase from that originally observed in the MVAC trials.
 ETIOLOGY
ETIOLOGY
The link between occupational exposure and an increased risk of urothelial cancer of the bladder was established more than a century ago when Rehn37 reported on three cases of bladder cancer in a German chemical dye works in 1895.38 During the following 40 years, similar reports appeared from around the world.39 In 1938, Hueper et al.39 demonstrated that when naphthylamine, an industrial arylamine used in the synthetic dye industry, was fed to dogs, it caused bladder carcinomas identical to the human disease. The link between industrial arylamines and bladder cancer was thus established and later confirmed by Case et al.40 in 1954. These authors also identified an excess of bladder cancer in the tire industry, attributable to the use of 2-naphthylamine in the manufacture of rubber.38,40 Around this time the “o-aminophenol hypothesis” of arylamine-induced human bladder cancer was proposed, suggesting that conjugation of aromatic amines by the liver and excretion in the urine with subsequent urinary reactions would liberate the carcinogen o-aminophenol.41 Other workers later added to this hypothesis: arylamines are hydroxylated in the liver and conjugated with glucuronic acid, followed by excretion into urine and reliberalization of the active carcinogenic metabolite into the bladder lumen by urinary glucuronidases.38,42,43 Slow acetylation by N-acetyltransferase, an enzyme involved in the metabolism of arylamines, has been shown to be a contributory risk factor for bladder carcinogenesis.20,44,45
Due to the widespread use of arylamines in textile dyes, hair dyes, and paint pigments, a number of high-risk occupations have been identified, including chemical, dye, textile, and rubber workers and painters and hairstylists.21,46–48 In addition, the presence of various arylamines in tobacco smoke means that a significant proportion of bladder cancer cases can be attributed to cigarette smoking.20,21–22,23–25 In fact, abandonment of the manufacture of many of these arylamines in the latter half of the 20th century means that smoking is currently the single most important cause of urothelial cancer.24,25,49–51 Tobacco (particularly cigarette) smoking now explains approximately half of bladder cancer cases in men and one-third of cases in women in Europe. It has been demonstrated that an increased smoking frequency and duration and a lower age at initiation are associated with an increased risk of bladder cancer, while cessation seems to reduce the risk.49 The relation between smoking and other prognostic factors is interesting, as it could give insight into biological mechanisms of disease and, perhaps more importantly, have clinical implications by increasing the ability to identify patients at risk of more malignant disease. Cigarette smoking also appears to be a risk factor for disease recurrence following a diagnosis of bladder UC,49,52 although due to a lack of conclusive evidence, there is currently a low rate of physicians providing smoking cessation assistance.49
Studies also demonstrate a relation between N-acetyltransferase-2 slow acetylators and cigarette smoking, resulting in a further increase in the risk of bladder cancer, especially in those individuals with a high smoking intensity.53–55 A similar relation has also been demonstrated with arylamine exposure.56,57 A number of other susceptibility loci have also been identified, although such markers do not yet have sufficient discriminatory ability to be utilized for risk prediction in the general population or for prediction or prognostication in patients diagnosed with bladder cancer.54,58,59
 CHRONIC INFLAMMATION AND BLADDER CANCER
CHRONIC INFLAMMATION AND BLADDER CANCER
Squamous metaplasia is considered a precursor of squamous cell carcinoma of the bladder and is a relatively common occurrence, especially on the trigone of the female bladder where a prevalence of up to 50% is reported.60,61 Experimental evidence suggests that this does not occur by direct transformation of the superficial apical umbrella cells of the urothelium or by their dedifferentiation and redifferentiation; it is postulated that basal cells (probable stem cells) are selectively activated.60 The normal urothelium is slowly proliferating, but urothelium undergoing squamous metaplasia becomes hyperplastic, and it may be that the hyperplasia component of urothelial squamous metaplasia is a major contributor to an enhanced risk of cancer formation.60,61
Squamous cell carcinoma and adenocarcinoma of the bladder often occur in the presence of chronic inflammation. In Africa and the Middle East, where these tumors are much more prevalent, the chronic inflammation occurs as a result of infestation with the parasite S. haematobium (bilharziasis), with a bladder carcinoma incidence of 2 to 4 per 100,000 in S. haematobium endemic areas.62,63–67 This infestation can lead to malignancy through local tissue damage, mechanical irritation, bilharzial toxins, secondary bacterial infection, and the production of nitrosamines.62,68,69 With liver involvement and subsequent liver dysfunction, tryptophan metabolism may be disturbed, resulting in excretion of carcinogenic metabolites.62 In S. haematobium infected individuals, the prevalence of squamous metaplasia rises significantly during the first 10 to 15 years of life, with a plateau at roughly constant levels thereafter (30% to 40% in males and 40% to 50% in females) when the active infection may have subsided.61 It is suggested that proliferative changes in the bladder urothelium may become independent of ongoing infection after long periods of chronic S. haematobium–induced inflammation.68,69 Severe metaplasia of the bladder may represent a precancerous transformation in some individuals, but in others it may only represent a marker for the prolonged inflammation that is associated with a high cancer risk.68,69 This sort of proliferative growth combined with the increased excretion or local formation of mutagens in the S. haematobium-inflamed bladder may significantly contribute to the onset of cancer formation, possibly involving mutations of the p53 and CDKN2 tumor-suppressor genes.68
In Europe and North America, the stimulus of chronic bladder inflammation is usually chronic bacterial infection, bladder calculi, or long-term indwelling catheters.70–73 A number of metaplastic conditions (squamous metaplasia, von Brunn’s nests, cystitis cystica, and cystitis glandularis) may occur prior to frank malignant change, although the premalignant nature of some of these lesions is still unclear.70–79
Field Cancerization and Clonality
A fundamental characteristic of neoplasia is monoclonality, in which one transformed cell gives rise to daughter cells that all exhibit the same genetic changes that provided the initial growth advantages to the originally transformed parent cell.80 Further genetic changes accumulate in subsequent daughter cells and provide additional growth advantages.80 However, TCC behaves as a multifocal disease, often with multiple primary tumors and frequent recurrences that can occur anywhere in the urinary tract from the renal pelvis to the urethra. These observations gave rise to the idea of a “field defect” or “field cancerization,” suggesting that the whole urothelium is exposed to the same urinary carcinogens, leading to the transformation of many independent separate urothelial cells and resulting in multiple tumors developing independently in multiple sites. Such tumors are thus genetically unrelated. An alternative explanation is that the multifocality of TCC arises as a result of a single carcinogenic insult to a single cell or group of cells. The progeny or clones of these cells spread throughout the bladder, either through intraepithelial migration or through cell shedding and reimplantation, leading to multiple synchronous and metachronous tumors.80 These tumors are thus topographically distinct but are genetically related. This is the hypothesis of clonality.
Utilizing the relatively rudimentary technique of X-inactivation,80,81–82 the early studies in this field appeared to show that the urothelium is derived from a small number of cells (200 to 300), which subsequently develop into larger patches; each patch is clonally related and possesses different predispositions to tumorigenesis.83 Such stem cell–derived clonal units actively replenish the urothelium during aging.84 Multiple synchronous and metachronous TCCs in the same patient appear to be clonally related when studied by X-inactivation.80,82,85–88 However, these studies only provide a 50% probability that one particular TCC is related to the primary TCC, although that probability improves when multiple TCCs are analyzed. Clonal patch size also needs to be taken into consideration, and because of the large patch size of the urothelium (120 mm2), X-inactivation studies are heavily biased toward demonstrating monoclonality.81 Ideally, these studies should have taken into account the relation of the tumors to patch boundaries.81 In addition, DNA methylation patterns change as a natural consequence of aging.89 Therefore, although X-inactivation studies provided an early insight into the relative importance of the processes of clonality and field cancerization, they cannot be considered as entirely accurate and reliable. Similarly, using immunohistochemistry to study specific p53 and pRb mutations cannot be considered to be wholly reliable for demonstrating monoclonality.
The accuracy of these investigations has been improved by utilizing newer and more sensitive techniques such as comparative genomic hybridization, fluorescence in situ hybridization, and loss of heterozygosity studies. These experiments revealed both monoclonality and oligoclonality in synchronous and metachronous TCCs. More recently, research has suggested that a genetic expression profile is established early in bladder tumor development, and that this profile is stable and maintained in recurring tumors.90–91,92 Majewski et al.91 matched the clonal allelic losses in distinct chromosomal regions to specific phases of bladder neoplasia: these genetic changes mapped to six regions or “forerunner genes” involved in the early phases of bladder cancer development, representing critical hits driving bladder carcinogenesis. It is suggested that the clonal expansion, over vast expanses of the bladder mucosa, of urothelial cell populations containing losses of forerunner genes may represent the earliest molecular change in bladder carcinogenesis. A further wave of genetic “hits” within subregions of these clonally expanded cells leads to the first microscopically recognizable features of dysplasia, and a third and final wave is associated with the fully transformed phenotype of severe dysplasia or CIS.91,93 The genetic changes map to six chromosomal regions that are suggested to represent the critical hits driving the development of bladder cancer.91,93 In addition, Knowles et al.94 have demonstrated that deletions of chromosome 9 occur in over half of bladder tumors of all grades and stages (9p, 51%; 9q, 57%). Loss of heterozygosity also occurs on 17p (32%), 11p (32%), 8p (23%), 4p (22%), and 13q (15%), and loss of heterozygosity of 5p, 8p, and 21q are significantly associated with worse grade and stage.94 Genomic copy number alterations are also frequent in bladder TCC, with the most frequent changes involving complete or partial loss of 4q (83%) and gain of 20q (78%).95 Other frequent losses are of 18q (65%), 8p (65%), 2q (61%), 6q (61%), 3p (56%), 13q (56%), 4p (52%), 6p (52%), 10p (52%), 10q (52%), and 5p (43%).94
Taken together, the studies described above show that multifocal TCCs are frequently monoclonal, whereas others show oligoclonality. The evidence for both theories is compelling (as reviewed by Duggan et al.96), with evidence supporting both the clonality and field cancerization theories. In reality, these theories are equally valid, with both processes seemingly often occurring simultaneously in the same patient.86,87 In addition, many of these studies have demonstrated that deletions on chromosome 9p occur most frequently and early in transitional cell carcinogenesis with 17p13 losses (p53 gene mutations) occurring in more advanced TCCs, shedding some light on the molecular pathology of bladder TCC.
Pathways to Muscle-Invasive and Nonmuscle-Invasive Bladder Cancer
A number of different approaches can be taken to describe the molecular alterations involved in bladder tumorigenesis (TCC). Some authors 97,98 have previously described such pathways in detail based on the six original “hallmarks of cancer” described by Hanahan and Weinberg99 in 2000. In 2011, Hanahan and Weinberg100 updated their original landmark review, describing genome instability and inflammation as underlying these hallmark changes and proposed “reprogramming of energy metabolism” and “evading immune destruction” as two emerging hallmarks with potential for generality. In addition, they reported that tumors exhibit another dimension of complexity by containing a repertoire of recruited, ostensibly normal cells that contribute to the acquisition of hallmark traits by creating the “tumor microenvironment.”100 The particular timing and sequence of hallmark events can vary widely between tumors of the same type and within the same tumor, but ultimately these hallmark capabilities of cancer will be reached.99 In their 2011 update, Hanahan and Weinberg100 also introduce the concept of “cancer stem cells,” a concept that has existed for quite some time for hematopoietic malignancies.101,102 Cancer stem cells are a subset of tumor cells that have the ability to self-renew and to generate all of the heterogeneous cells that comprise a tumor (properties that are analogous to a stem cell, the original cell of an organ, and responsible for organogenesis and organ maintenance).101,103–106 It is proposed that these cells are responsible for tumorigenesis, tumor differentiation, tumor maintenance, tumor spread, and tumor relapse.101,103–106 In the setting of bladder cancer, cancer stem cells appear to play a role in a subset of tumors, but their true significance has yet to be clarified.104
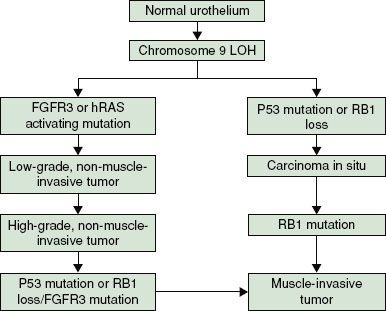
A number of other authors have also reviewed this field in detail,107–112 and there is general consensus on a divergent pathway for the development of Ta/T1 disease and Tcis/T2+ disease, as illustrated in Figure 64.1. Significant contributions to work in this field have been made by Knowles et al.110,113–119 (Leeds, UK); in their 2010 review, Goebell and Knowles113 propose a third hypothetical pathway for the development of high-grade papillary tumors.
A detailed examination of these pathways and related biomarkers is beyond the scope of this chapter. In addition, this is a rapidly changing field and new developments appear frequently with the advent of high-throughput experimental platforms, including “deep sequencing,”120 proteomics,121,122 and metabolomics,123 so readers are directed to the reviews cited above or to the latest work in this field.
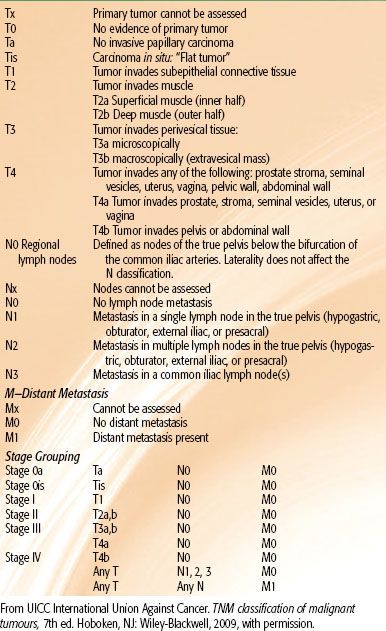
TABLE 64.2 TNM CLASSIFICATION OF TUMORS (2009)
 SYMPTOMS AND SIGNS
SYMPTOMS AND SIGNS
The typical presenting symptoms of bladder cancer include painless, visible hematuria, infection, and storage symptoms. As the first of these is typically transient and the latter two are also attributable to prostate problems, patients often go undiagnosed for considerable periods.27 Given the intermittent nature of the hematuria associated with bladder cancer, patients presenting with a convincing episode of hematuria require urgent assessment. Similar considerations apply to male patients with a urinary tract infection. Female patients present a more difficult problem due to the higher incidence of urinary infection and the lower risk of bladder cancer. Hematuria is also more likely to be misinterpreted in women of childbearing age.
 INVESTIGATION OF PATIENTS WITH BLADDER CANCER
INVESTIGATION OF PATIENTS WITH BLADDER CANCER
This section is divided into the investigation of patients with suspected bladder cancer and the subsequent staging of those with an established diagnosis.
Suspected Bladder Cancer
In developed countries, most patients will be referred to some sort of rapid access hematuria or suspected bladder cancer clinic. A minority may present via other routes (e.g., gynecological clinics) or with metastatic symptoms (<10%). Hematuria clinics will generally include a clinical assessment, full blood count and biochemical profile, prostate-specific antigen (if indicated), urine cytological examination, flexible cystoscopy, and some sort of imaging of the urothelium (e.g., ultrasound, intravenous urogram, or computed tomography [CT] urogram), which will vary with local practice and facilities.124,125 Patients identified as having a bladder tumor on flexible cystoscopy will then require further investigations to stage the disease.
Staging Bladder Cancer
The next stage for most patients will be an examination under anesthetic coupled with TURBT. The presence or absence of a mass after TURBT is also an important prognostic factor as it potentially indicates either unsuccessful clearance of tumor, extravesical extension, or both. This serves as both definitive staging of the bladder lesion as well as a substantial proportion of the initial treatment. Pathological review of the resected specimen will ascertain whether muscle invasion is present or not and whether there is CIS; these are the key determinants of further investigation and treatment. Patients with NMIBC, including CIS, do not usually require detailed further imaging, and treatment and surveillance are primarily by intravesical means. The main exception to this is patients with either extensive CIS or grade 3 lesions for whom additional cross-sectional imaging may be warranted. Patients with muscle-invasive bladder cancer (MIBC) will require detailed cross-sectional staging with CT or magnetic resonance imaging (MRI) of the chest, abdomen, and pelvis. If there are any features suggestive of bone metastasis (e.g., raised alkaline phosphatase, bone pain), an isotope bone scan will be indicated in addition.
A major confounding factor with imaging in bladder cancer is the effect of TURBT on the interpretation of the extent of the primary bladder tumor. Recent TURBT will cause perivesical changes that may be interpreted as extravesical spread. For similar reasons, enlarged pelvic nodes may be related to reactive rather than metastatic effects. On the other hand, changes such as infiltration of adjacent organs or hydronephrosis are likely to be reliable indicators of tumor stage and poor prognosis.
 STAGING SYSTEM
STAGING SYSTEM
The 2010 version of the UICC International Union Against Cancer’s TNM system is the current, internationally used staging system,126 and it is based on the size and extent of the primary tumor (T stage), presence or absence of nodal (N), and metastatic (M) spread (Table 64.2). The TNM stage must be used in conjunction with the pathological assessment of tumor removed at TURBT to decide on optimal therapy. Disappointingly, despite a substantial volume of research, no biomarkers have yet established themselves in clinical practice as either prognostic or predictive markers as has happened, for example, with estrogen-receptor or HER2 status in breast cancer. It is hoped that the wider availability of high throughput systems such as proteomics, in-depth sequencing, and others to be developed will allow the development of such markers in the future.
 PATHOLOGY
PATHOLOGY
In excess of 90% of bladder cancers are transitional cell carcinomas. Of the remainder, around 5% are squamous, although it should be noted that squamous differentiation is often present in poorly differentiated TCCs, so the extent to which these are genuinely distinct is open to question. As already noted, infestation with S. haematobium can lead to squamous cell carcinoma, but this is rapidly decreasing due to eradication programs. Small cell carcinoma is rare but important as it is generally very chemosensitive and treatment tends to follow schedules adapted from treating small cell lung cancer. Other tumor types include melanoma, carcinosarcoma, and adenocarcinoma (particularly in the urachal remnant). Typically bladder TCCs will be graded using the standard TNM system of Gx (cannot be assessed) then G1 through to G3 (well through to poorly differentiated).127 CIS will frequently be found in addition to a tumor mass and critically affects treatment choices.
 TREATMENT OF BLADDER CANCER
TREATMENT OF BLADDER CANCER
Non-muscle-Invasive Bladder Cancer
Around 80% of patients with bladder cancer present with nonmuscle-invasive disease. These are classified as Tis, Ta, and T1 by the TMN classification system. The gold standard for diagnosis is the resection of the lesion with adequate sampling of the detrusor muscle deep to the lesion (TURBT). This will give tissue for accurate staging of the bladder lesion as well as definitive treatment for the lesion. Recurrences after TURBT are found in up to 70% of patients undergoing surveillance, and, more importantly, up to 15% of patients on surveillance will progress to muscle-invasive bladder cancer. At the time of diagnosis, the upper urinary tract also needs assessment. This can be done with intravenous urography, CT urography or ultrasound. The incidence of upper tract tumors at the time of presentation with hematuria is only 1.8%, calling into question the use of contrast imaging for the group as a whole.128 Ultrasound is now being used more frequently, with contrast imaging modalities being used for the higher risk disease.
Prognostication and management strategy are based on accurate initial staging and grading of the disease. There can be variation in the interpretation of pathological specimens, so review of pathology is recommended (European Association of Urology [EAU] guidelines129). If there is uncertainty over the pathology, a further early re-resection is indicated. The risk of residual tumor can be as high as 53% in T1 tumors.130 If the disease is defined as NMIBC, it can be characterized as low, intermediate, or high risk, and this will dictate how the disease is managed (see below). The EORTC bladder cancer calculator provides a valuable online tool for doing this and determining follow-up frequency.131
Following resection of the tumor, there is good evidence that a single dose of mitomycin-C administrated into the bladder for 1 hour within 24 hours of surgery will reduce the relative risk of recurrence by 24.2% but will not impact disease progression and disease survival.132 Recurrences after TURBT are found in up to 70% of patients undergoing surveillance, and up to 15% of patients on surveillance will progress to muscle-invasive bladder cancer. Emerging endoscopic techniques employing photodynamic therapy aimed at improving diagnostic yield and thereby ultimately reducing the rates of recurrence and progression have demonstrated promising results.133
Low-risk tumors are single tumors that are <3 cm in diameter are graded as G1 disease and staged as Ta with no evidence of CIS. These tumors have a 15% probability of recurrence and a 0.2% risk of progression at 1 year.30 These patients should undergo a flexible cystoscopy 3 months after the initial resection, and if this is negative, a flexible cystoscopy should be undertaken 9 months later and then annually thereafter.
Intermediate- and high-risk tumors are defined using a scoring system based on a number of clinical and pathological factors:
a. Number of tumors
b. Tumor size
c. Prior recurrence rate
d. T category
e. Presence of concurrent CIS
f. Tumor grade131
The high-risk tumors should be followed up with 3 monthly flexible cystoscopy for 2 years and 6 monthly for a further 5 years and then annually thereafter. Intermediate risk tumors should be followed up using a surveillance regime somewhere between that used for low- and high-risk disease, which is adapted according to personal and subjective factors. The intermediate-risk tumors have up to a 38% probability of recurrence and a 5% risk of progression at 1 year. The high-risk tumors have a 61% probability of recurrence and a 17% risk of progression at 1 year.30
The use of flexible cystoscopy with urine cytology is the standard of bladder surveillance. There is ongoing research into improving the sensitivity and specificity of flexible cystoscopy using variations in the wavelength of the light source used (e.g., narrow band imaging). There are a wide number of urinary biomarkers available such as NMP22, UroVysion (Abbott Laboratories, Abbott Park, Illinois), and ImmunoCyt (Scimedx, Denville, New Jersey). These agents suffer from high false-positive rates and variable sensitivity and are costly (as reviewed by Vrooman and Witjes134).
The presence of CIS in the bladder carries a 54% risk of disease progression without treatment.135 Patients with high-risk tumors or CIS should be offered intravesical immunotherapy using bacille Calmette-Guérin (BCG) (EAU and American Urology Association guidelines).129,136 There are a variety of treatment schedules in the literature, but the authors recommend an intravesical treatment once a week for 6 weeks followed by a subsequent 3 weeks as an induction treatment. If there is no cystoscopic evidence of recurrence, the patient should then be offered ongoing maintenance BCG with 6-week courses of BCG every 3 to 6 months with regular cystoscopic surveillance. In a recent meta-analysis of trials with BCG maintenance, a 32% reduction in the risk of recurrence was seen for BCG compared with mitomycin-C (P <.0001), whereas there was a 28% increase in the risk of recurrence (P = .006) for patients treated with BCG in the trials without BCG maintenance.137 BCG is a very effective treatment, but not all patients with NMIBC should be treated with BCG due to the risk of toxicity. In a phase III study for NMIBC tumors using maintenance BCG therapy, 20.3% patients stopped BCG due to side effects, mostly local side effects; 68% who stopped due to side effects did so during the first 6 months.138 The choice of treatment depends on the patients’ risk of recurrence and progression based on EORTC subgroups. The use of BCG does not alter the natural course of tumors in the low risk of recurrence subgroup and is therefore considered to be overtreatment. In patients with tumors at high risk of progression, for whom cystectomy is not carried out, BCG including at least 1-year maintenance, is indicated. In patients at intermediate or high risk of recurrence and intermediate risk of progression, BCG with 1-year maintenance is more effective than chemotherapy for prevention of recurrence; however, it has more side effects than chemotherapy. For this reason both BCG with maintenance and intravesical chemotherapy remain options. The final choice should reflect the individual patient’s risk of recurrence and progression and the efficacy and side effects of each treatment modality (EAU guidelines). In treatment refractory disease, the patient should be offered radical treatment for the bladder.
Muscle-Invasive, Nonmetastatic Disease
Relative Roles of Cystectomy and Bladder Preservation
Radiotherapy has been used as the primary treatment for muscle-invasive bladder cancer for many years, but utilization rates vary around the world from around 10% in the United States8 to 25% in Scandinavia139 to in excess of 50% in the United Kingdom.10 There are no prospective randomized trials comparing surgery with radiotherapy, so the data on comparative efficacy can only be inferred indirectly. U.S. authors in particular tend to refer to surgery as the gold standard of care, with bladder preservation with radiotherapy (with or without chemotherapy) being viewed as experimental. However, this opinion seems to be based on custom and practice and not on any hard comparative data. It is thus worth examining in some detail the data that exist on this topic. There is a solitary attempt using modern techniques to compare surgery with bladder preservation combining chemotherapy and radiotherapy. The UK SPARE trial (randomized trial of Selective Bladder Preservation against Radical Excision [cystectomy] in muscle-invasive T2/T3 transitional cell carcinoma of the bladder) was a feasibility study that has now closed due to poor recruitment.140 This phase II and III trial attempted to investigate the potential of using the response to neoadjuvant chemotherapy as a predictive tool for selecting patients for radiotherapy compared with the surgical standard. In the authors’ experience, one of the problems with the trial was that the study used response to neoadjuvant chemotherapy to decide on suitability for bladder preservation. However, once this was explained to the patients in the information sheet, there were patients who had a good response but were reluctant to undergo surgery, particularly if the bladder was free of tumor at the interim cystoscopy.
Large population-based studies suggest that the main determinants of survival after diagnosis of bladder cancer are stage, grade, age, and to some extent social class. For example, Hayter et al.6 studied patterns of care and outcomes in over 20,000 patients with bladder cancer in Ontario. They found significant variations in the use of cystectomy and radiotherapy between different districts but no evidence that these variations led to any differences in long-term outcomes. They concluded that bladder-sparing approaches were equivalent to surgery for invasive bladder cancer.
Another way to compare outcomes between surgery and radiotherapy is to look at large published series. In the United Kingdom, where both of these approaches are routinely employed, it is possible to compare the outcomes in Cancer Registry data. A recent paper from Munro et al.10 examined outcomes in 458 patients with invasive bladder cancer treated in Yorkshire between 1993 and 1996. The ratio of cystectomy to radiotherapy was 1 to 3, reflecting UK practice at the time. Overall 10-year survival was similar between those who underwent radiotherapy (22%) versus radical cystectomy (24%). Prognostic factors for inferior outcome at 10 years were: female versus male, poor performance status, hydronephrosis and increasing T stage; treatment modality was not a factor in the prognosis.
One of the most widely quoted surgical series comes from the University of Southern California and reports the results of 1,054 patients undergoing cystectomy with overall 5- and 10 year survival rates of 60% and 43%, respectively. This series is discussed in detail below. However, this series included patients undergoing surgery for noninvasive tumors and excluded from the denominator 112 patients referred but deemed incurable at operation. If we look at the 5-year survival of those with invasive tumors, the rate drops to around 47% from the quoted 60%. A contemporary series of radiotherapy cases from Rödel et al.5 reports 5- and 10-year survivals of 51% and 31% but included patients deemed inoperable.
Furthermore, if we look at the outcomes in the surgical control arm in the US Intergroup Neoadjuvant MVAC trial, the median survival was 38 months and the 5-year survival was 42%. Other relatively contemporary series quote similar 5-year survivals: for example, Dalbagni et al.141 cite 45% overall and 65% disease-specific survival rates in a series of 300 patients. Furthermore, the only significant prognostic factors in this series were age, T stage, and use or nonuse of neoadjuvant chemotherapy (see the Role of Systemic Therapy section). Data also exist from single institution series with widespread use of both modalities. For example, Kotwal et al.142 report results on 169 patients treated between March 1996 and December 2000 in Leeds, UK. There were no differences in overall, cause-specific, and distant recurrence-free survival at 5 years between the two groups, despite the radiotherapy group being older (median age, 75.3 vs. 68.2 years). There were 31 local bladder recurrences in the radiotherapy group (24 of which were solitary and hence potentially suitable for salvage surgery), but no significant difference in distant recurrence-free survival. In another more recent (2002 to 2006) cohort, the median age of radiotherapy patients but not the cystectomy patients had increased to 78.4 from 75.3 years, respectively, while the age of those undergoing surgery remained similar at 67.9 and 68.2 years for surgery, consistent with the aging trend in new bladder cancer cases. These authors concluded that although the patients undergoing radical cystectomy were significantly younger than the radiotherapy patients, treatment modality did not influence survival. They went on to state that radical radiotherapy is a viable treatment option for these patients, with the advantage of organ preservation. The data thus suggest that bladder preservation gives equivalent long-term survival to surgery when factors such as case selection are accounted for.
A proportion of patients undergoing radiotherapy will relapse within the bladder and go on to salvage cystectomy. An important consideration, therefore, is whether cystectomy after radiotherapy can be carried out safely and whether the delay compromises survival. Again, there are no randomized data on this topic. However, UK surgeons in particular have good practical experience on this topic and have commented that neither prior chemotherapy nor radiotherapy compromises surgical salvage and that long-term results appear similar to primary cystectomy series.143,144
Particularly intriguing in this regard is a comparison of survival rates following primary surgery or salvage surgery following failed radiotherapy from the Christie Hospital in Manchester, UK. The group examined the outcomes in 552 patients who underwent radical cystectomy between 1970 and 2005. Of these, 313 patients underwent primary radical cystectomy and 239 underwent salvage radical cystectomy following radiation failure. The median age was 62.5 years (range, 32.2 to 87.2) for the primary surgical group compared with 65.5 years for the salvage group. Overall 5-year survivals reported were 45.5% for the primary group and 42% for the salvage group, with cause-specific survivals of 51% and 50%, respectively. These differences persisted after stratification for stage, and the authors concluded that a policy of primary radiotherapy with surgical salvage did not compromise the long-term survival chances of patients.143
Clearly there are surgical series with much higher survival rates than this in the literature. However, there are two factors accounting for this. One is case selection, the other is that these series are, to a degree, personal, so to publish results that appear inferior to other major centers is potentially a threat to a center’s (or surgeon’s) reputation and will tend to have a “ratchet” effect on published results. These data also suggest that the predominant prognosis driver in bladder cancer is the presence or absence of distant micrometastasis at diagnosis of invasive disease. The (relatively modest) effect of neoadjuvant chemotherapy tends to bear this out, particularly as the biggest effect in the Medical Research Council (MRC)/EORTC trial was on metastasis-free survival rather than pelvic control rates.145
There is little in the way of randomized data on the efficacy of radiotherapy in either nonmuscle-invasive disease or CIS. The only randomized trial on this topic comes from the United Kingdom and compared radiotherapy with surveillance for patients with a new diagnosis of pT1G3 NXM0 transitional cell carcinoma with unifocal disease and no CIS. Patients with multifocal disease or CIS were randomized between intravesical therapy and radiotherapy. There was no evidence of benefit from radiotherapy in terms of progression-free interval (hazard ratio [HR] 1.07; 95% confidence interval [CI], 0.65 to 1.74; P = .785), progression-free survival (HR 1.35; 95% CI, 0.92 to 1.98; P = .133), or overall survival (HR 1.32; 95% CI, 0.86 to 2.04; P = .193).146 There is thus no indication for radiotherapy in these groups of patients.
In truth, surgery and radiotherapy are not competing, but are complementary approaches to invasive bladder cancer. There are particular groups who appear to do poorly with primary radiotherapy, for example, those with poorly functioning bladders or extensive CIS in addition to their invasive disease. In the former case, radiotherapy is unlikely to improve bladder function; in the latter, the lack of effect of radiotherapy on CIS means that the patient remains at risk of further bladder intervention and ultimately cystectomy.146 Similar considerations apply to patients with pT1G3 disease.146 North American authors will also cite features such as hydronephrosis as a contraindication to radiotherapy.147 However, hydronephrosis is also a poor prognostic factor for surgery and does not help in selecting patients one way or another.6 On the other hand, there are many patients who may benefit from radical therapy but are poor surgical candidates, such as older patients, the obese, diabetics, poor anesthetic risk patients, or those who may struggle with whatever form of neobladder is fashioned. Although large surgical series will include patients over age 80, these will typically comprise only a few percentage of the total, whereas, with a median age at diagnosis of bladder cancer in the middle to late 70s, there are probably many more who do not make it to surgery.
Surgery
Management of Invasive Bladder Cancer
Although the majority of patients present with NMIBC, 20% to 40% will either present with or ultimately develop muscle-invasive disease. Invasive bladder cancer is a lethal malignancy; if untreated over 85% of patients will die of the disease within 2 years of diagnosis.148 Furthermore, a certain percentage of patients with high-grade bladder tumors without involvement of the lamina propria will recur or progress or fail intravesical management, and they may be best treated with an earlier cystectomy when survival outcomes are optimal.149 In these groups of patients, the 5-year survival rates after cystectomy exceed 80%.150,151
The rationale for an aggressive treatment approach employing radical cystectomy for high-grade, invasive bladder cancer is based on several important observations. First, the good long-term survival rates, coupled with the lowest local recurrences, are seen following a definitive surgical approach removing the primary bladder tumor and regional lymph nodes.4,152,153 Although there are no randomized trials comparing radical cystectomy with bladder preserving approaches, surgery remains the preferred treatment option for many clinicians for advanced, localized invasive bladder cancer.154 Second, the morbidity and mortality of radical cystectomy has substantially improved over the past several decades.152,155 Third, advocates say that radical cystectomy provides accurate pathologic staging of the primary bladder tumor (p stage) and regional lymph nodes, thus, selectively determining the need for adjuvant therapy based on precise pathologic evaluation. However, it should be noted that the evidence base for adjuvant (as opposed to neoadjuvant) therapy is rather weak (see on the Role of Systemic Therapy section). For the above-mentioned reasons, radical cystectomy has become a standard form of therapy for high-grade, invasive bladder cancer. Nonetheless, many articles on cystectomy emphasize the need for careful patient selection to achieve optimal results. Although this is undoubtedly true, it begs the question of what should be done with patients who do not meet these stringent selection standards but still need to be treated. This issue is rarely, if ever, addressed in cystectomy series publications.
The evolution and improvements in lower urinary tract reconstruction, particularly orthotopic diversion, have been major components in enhancing the quality of life of patients requiring cystectomy. Currently, most men and women can safely undergo orthotopic lower urinary tract reconstruction to the native, intact urethra following cystectomy,156 although availability varies worldwide. Orthotopic reconstruction aims to mimic the native bladder in location and function, provides a continent means to store urine, and allows volitional voiding per urethra, although patients need to do this by coordinating opening the sphincter with a Valsalva maneuver, which does require training. The orthotopic neobladder eliminates the need for a cutaneous stoma, urostomy appliance, and the need for intermittent catheterization in most cases. These efforts have improved the quality of life of patients who require removal of their bladders and have also stimulated patients and physicians to consider radical cystectomy at an earlier more curable stage for high-grade, invasive bladder cancer.157,158
A dedicated effort has been made to improve the surgical technique of radical cystectomy and to provide an acceptable form of urinary diversion, without compromise of a sound cancer operation.158,159 Timing of cystectomy after the TURBT is important, and it has been demonstrated that a delay of more than 3 months undermines patient survival. This evidence forms the basis to negotiating resource needs with health care providers.160,161 Certain technical issues regarding radical cystectomy and an appropriate lymph node dissection are critical to minimize local recurrence and positive surgical margins and to maximize cancer-specific survival.162 Attention to surgical detail is important in optimizing the successful functional outcomes of orthotopic diversion by preserving the urinary sphincter mechanism and therefore continence. Finally, the observed associations between hospital volume and operative mortality are largely mediated by surgeon volume. Patients can often improve their chances of survival substantially, even at high-volume hospitals, by selecting surgeons who perform the operations frequently, as those centers that have adopted this strategy have demonstrated declining mortality in the past decade.163,164
Radical cystectomy by definition implies the en bloc removal of the pelvic–iliac lymph nodes along with the pelvic organs anterior to the rectum: the bladder, urachus, prostate, seminal vesicles, and visceral peritoneum in men; the bladder, urachus, ovaries, fallopian tubes, uterus, cervix, vaginal cuff, and the anterior pelvic peritoneum in women. An appropriate lymphadenectomy is an important component of radical cystectomy and is related to the clinical outcomes of patients with high-grade, invasive bladder cancer. Evidence suggests that a more extended lymphadenectomy is beneficial in both lymph node–positive and lymph node–negative patients with bladder cancer,165,166,167 although this could be a surrogate for either case selection or surgical skill. Although the exact limits of the lymphadenectomy for patients with bladder cancer undergoing cystectomy are currently debated, the boundaries include initiation at the level of the inferior mesenteric artery (superior limits of dissection), extending laterally over the inferior vena cava or aorta to the genitofemoral nerve (lateral limits of dissection), and distally to the lymph node of Cloquet medially (on Cooper’s ligament) and the circumflex iliac vein laterally. This dissection includes bilaterally all obturator, hypogastric, presciatic, and presacral lymph nodes.168–170 Removal of more than 15 lymph nodes has been postulated to be both sufficient for the evaluation of the lymph node status as well as beneficial for overall survival in retrospective studies.166,171–174 However, potential interindividual differences in the number of pelvic and retroperitoneal lymph nodes and difficulties in processing of the removed tissue by pathologists are issues. Furthermore, the reality is that even in today’s practice the number of lymph nodes retrieved are low (<10) in a majority of patients (75%) and the chance are even lower if the patients are older, Hispanic (in the United States), and managed at low-volume, nonurban centers.170 The true curative value of lymph node dissection and the optimal extent of dissection are both still unknown.
Technical variations from the standard cystectomy have been performed to improve patients’ quality of life, including prostate-sparing cystectomy in order to preserve continence and potency. However, it carries a higher risk of missing unsuspected adenocarcinoma of the prostate. Coexistent prostate cancer and prostatic urothelial cancer are reported in 23% to 54% cases, of which 29% were clinically significant, leading to local recurrence and even metastasis.175,176
TABLE 64.3 CYSTECTOMY OUTCOMES IN SELECTED SINGLE INSTITUTION SERIES
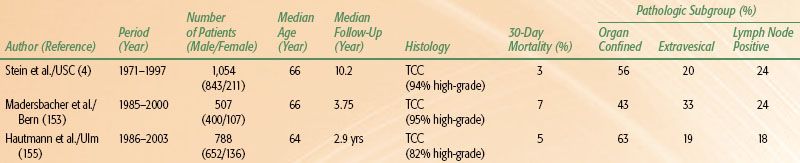
Radical cystectomy is an appropriate standard treatment for patients with high-grade, invasive bladder cancer. The clinical outcomes are presented in Table 64.3. These results should provide a benchmark for outcomes to which other therapies can be compared.
Both laparoscopic and robot-assisted cystectomy have been shown to be feasible and safe, but with a relatively shorter follow-up.177,178–179 Despite an increased materials cost for a robotic-assisted cystectomy, it has been demonstrated to be cost-effective in a subgroup of patients undergoing ileal conduit when the impact of complications are considered in a single institution series.179,180
Morbidity and Mortality of Radical Cystectomy and Lymphadenectomy
The early clinical results and outcomes with regard to the morbidity and mortality of radical cystectomy were disappointing. Lack of universal acceptance of this procedure was attributed to the considerable complication rate and the need for improvements in urinary diversion. Prior to 1970, perioperative complication rates of radical cystectomy were approximately 35%, with a mortality rate of nearly 20%. However, with contemporary medical, surgical, and anaesthetic techniques, along with better patient selection, the mortality and morbidity from radical cystectomy have dramatically decreased (Table 64.3). Importantly, in high-volume centers, the administration of preoperative therapy (radiation or chemotherapy) and the form of urinary diversion performed (continent or incontinent) did not obviously increase the mortality rate of patients undergoing radical cystectomy.150 The issue of the need to select patients for surgery complicates the comparison of bladder-sparing techniques, because frequently those not suitable for surgery will be the ones who appear in the radiotherapy-based series.
The early complication rate following radical cystectomy should not be underestimated in this elderly group of patients. The median age of patients undergoing cystectomy in a University of Southern California series was 66 years.4 Of the 1,054 patients treated, 28% developed an early complication within the first 3 months of surgery. Early complications included all events related to the cystectomy, perioperative care, and urinary diversion. The administration of preoperative therapy (radiation or chemotherapy) and the form of urinary diversion did not significantly increase the early complication rate in these cystectomy patients. Most early complications following radical cystectomy are unrelated to the urinary diversion (85% diversion unrelated) and can be managed conservatively without the need for reoperation in approximately 90% of cases.181 The most common early diversion-unrelated complication is dehydration, while the most common early diversion-related complication following radical cystectomy is prolonged urinary leakage. Overall, the most surgical complications after cystectomy are associated with urinary diversion in relation to the intestinal segments.152,182
Neoadjuvant chemotherapy is discussed in a later section, but it does not seem to increase the perioperative morbidity or mortality.7,183 Preoperative radiation therapy is discussed below.
Radical cystectomy may appropriately be performed in carefully selected elderly patients,184 however, this emphasis in the surgical literature on “careful selection” highlights as much the deficiencies as the efficacy of cystectomy as it emphasizes that the published results are not applicable to the entire population. It is emphasized that physiologic age may be more important than chronologic age when determining appropriate candidacy for radical cystectomy. Proper patient selection, strict attention to perioperative details, along with a dedicated and meticulous team-oriented surgical approach are critical components to minimize the morbidity and mortality of surgery and to ensure the best clinical outcomes in all patients following radical cystectomy.185–187 A recommended approach from the authors’ center previously pioneered by the Danish is adoption of an enhanced recovery pathway protocol (Fig. 64.2).188
FIGURE 64.1. Pathways to development of bladder cancer. (Data from Pollard C, Smith SC, Theodorescu D. Molecular genesis of non-muscle-invasive urothelial carcinoma (NMIUC). Expert Rev Mol Med 2010;12:e10.)