|
Zoledronic acid |
Denosumab |
Classification |
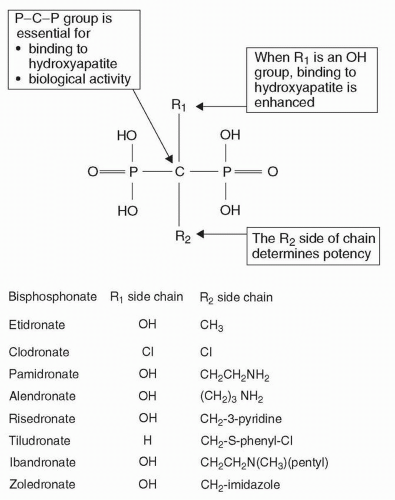
Bisphosphonate |
Fully human monoclonal antibody |
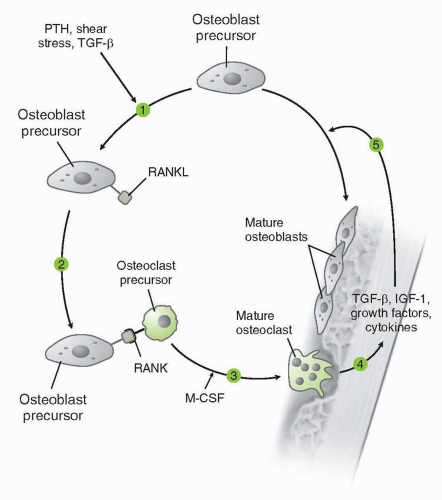
Mechanism of action |
Zoledronic acid is rapidly incorporated into bone where it inhibits osteoclast activity and induces osteoclast apoptosis. |
Monoclonal antibody binds avidly (Kd 3 × 10−12 M) and specifically to RANKL. Binding of RANKL inhibits osteoclast differentiation, activation, and survival. |
Clinical pharmacology |
Bioavailability: Rapidly partitions to bone after intravenous administration. |
Bioavailability: In healthy subjects treated with of doses from 0.03 to 3 mg/kg subcutaneous (SC), bioavailability is 60%-80%. |
|
Pharmacokinetic parameters: Onset of response and time to peak response dependent on the clinical setting. Concentration falls to <1% of Cmax 24 h postinfusion. Plasma area under the curve rises with renal impairment. |
Pharmacokinetic parameters: Single subcutaneous or intravenous dose suppresses markers of bone turnover in a dose-dependent manner. Marker suppression can be observed within 12 h of administration. At doses of 1 and 3 mg/kg, duration of suppression is at least 6 mo in postmenopausal women and 3 mo in most subjects with multiple myeloma or breast cancer with bone metastases. Denosumab did not accumulate appreciably at any of the doses or dosing frequencies investigated for postmenopausal bone loss. Pharmacokinetics did not appear to be altered upon multiple dosing. When given 180 mg every 4 wk, denosumab was observed to have 2.5-fold accumulation by the third dose. |
|
Distribution: Drug binds to bone. Serum protein binding is 28%-53% in vitro. |
Distribution: After intravenous administration, denosumab has a volume of distribution that is similar to plasma volume. After subcutaneous administration, absorption appears to be rapid and prolonged, with detectable concentrations within 1 h after dose and peak concentrations typically observed between 1 and 4 wk. |
|
t1/2 life: 146 h, triphasic |
t1/2 life: 33-46 d at highest doses |
|
Metabolism: Zoledronic acid is not metabolized. |
|
Elimination: Renal elimination within 24 h, 39% ± 16% unchanged. |
Relevant FDA indications |
Bone metastases due to solid tumors
Hypercalcemia of malignancy
Multiple myeloma
Osteoporosis in men
Secondary prophylaxis of osteoporosis after low-trauma hip fracture |
Denosumab is not FDA-approved. A Biologic License Application was submitted to the FDA in December 2008 for:
Treatment and prevention of postmenopausal osteoporosis
Bone loss in patients undergoing hormone therapy for the treatment of prostate or breast cancer |
|
Osteoporosis due to corticosteroids |
|
Paget’s disease |
|
Postmenopausal osteoporosis |
|
Prophylaxis of postmenopausal osteoporosis |
Route of administration |
Intravenous |
Subcutaneous |
Typical dosage |
Bone metastases due to solid tumors: 4 mg IV every 3-4 wk
Hypercalcemia of malignancy: 4 mg IV given as a single dose; may repeat after ≥7 d if serum calcium does not return to normal or remain normal after initial treatment
Multiple myeloma: 4 mg IV every 3-4 wk
Osteoporosis: 5 mg IV every 12 mo
Osteopenia secondary to androgen-deprivation therapy in prostate cancer patients: 4 mg IV every 3-12 mo |
Dosing in phase III trials:
60 mg subcutaneously once every 6 mo for reduction of fragility fracture risk
120 mg subcutaneously once every 4 wk for various applications in the setting of metastatic cancer (e.g., reduction of risk for skeletal-related events) |
Dose adjustment for renal insufficiency |
Always infuse over ≥15 min to avoid nephrotoxicity. If chronic and stable renal insufficiency, start as follows:
3.5 mg if baseline CrCl = 50-60 mL/min
3.3 mg if baseline CrCl = 40-49 mL/min
3.0 mg if baseline CrCl = 30-39 mL/min
Not recommended for CrCl < 5 mL/min |
None required. |
Drug interactions |
Aminoglycosides: Hypocalcemic effects may be enhanced.
Nonsteroidal anti-inflammatories: Gastrointestinal toxicity and nephrotoxicity may be enhanced.
Phosphate supplements: Hypocalcemic effects of phosphate supplements may be enhanced.
Thalidomide: Toxic effects may be enhanced. |
None described. |
Common toxicities |
Self-limited acute phase reaction, incidence reduced with acetaminophen.
Asymptomatic hypocalcemia is common.
Common toxicities include peripheral edema, gastrointestinal upset, arthralgia, asthenia, fatigue, headache, and others.
Additional potential serious adverse effects possible; please see text. |
Generally well tolerated. Most commonly reported adverse events in trials to date have included headache, back pain, upper respiratory tract infection, arthralgia, and nasopharyngitis. Hypocalcemia observed after administration of denosumab has been transient, mild, and within the first 2 wk after administration. |
Warnings and precautions |
Administer with an oral calcium supplement of 500 mg and a multiple vitamin containing 400 International Units of vitamin D unless in the setting of hypercalcemia of malignancy.
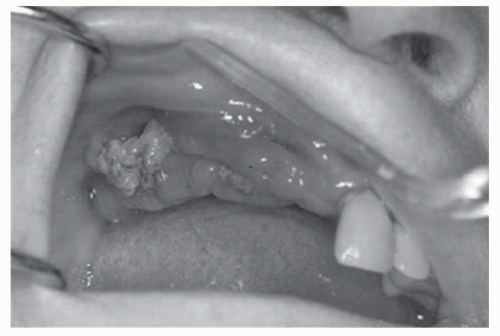
Observe recommended guidelines for dental care.
Contraindicated in hypocalcemia or known hypersensitivity.
FDA pregnancy class D. |
Denosumab has never been tested in combination with other osteoclast-targeted therapies. In a preliminary report involving women with breast cancer, rates of ONJ were similar with zoledronic acid and with denosumab. Dental precautions should be observed as with bisphosphonate treatment. |