Acute lymphoblastic leukemia (ALL), the most common type of cancer in children, is a heterogeneous disease in which many genetic lesions result in the development of multiple biologic subtypes. Today, with intensive multiagent chemotherapy, most children who have ALL are cured. The many national or institutional ALL therapy protocols in use tend to stratify patients in a multitude of different ways to tailor treatment to the rate of relapse. This article discusses the factors used in risk stratification and the treatment of pediatric ALL.
Acute lymphoblastic leukemia (ALL), the most common type of cancer in children, is a heterogeneous disease in which many genetic lesions result in the development of multiple biologic subtypes. The etiology of ALL is characterized by the acquisition of multiple consecutive genetic alterations in the (pre)leukemic cells. In the most common genetic subtypes of ALL, the first hit occurs in utero, as evidenced, for example, by the presence of the TEL/AML1 gene fusion or hyperdiploidy in neonatal blood spots on Guthrie cards. These first genetic abnormalities are, in fact, initiating preleukemic cells, not leukemic ones, because most children whose neonatal blood spots show a genetic defect typically associated with leukemia never develop leukemia. Also, such preleukemic cells harbor additional genetic abnormalities. T-cell acute lymphoblastic leukemia (T-ALL) is an exception, because the majority of genetic lesions described in T-ALL seem not to occur in the neonatal blood spots.
Today, with intensive multiagent chemotherapy, most children who have ALL are cured. The factors that account for the dramatic improvement in survival during the past 40 years include the identification of effective drugs and combination chemotherapy through large, randomized clinical trials, the recognition of sanctuary sites and the integration of presymptomatic central nervous system (CNS) prophylaxis, intensification of treatment using existing drugs, and risk-based stratification of treatment. The many national or institutional ALL therapy protocols in use tend to stratify patients in a multitude of different ways. Treatment results often are not published for the overall patient group but rather are reported only for selected subsets of patients. This limitation hampers the comparison of outcomes in protocols. In 2000, the results of ALL trials run in the early 1990s by the major study groups were presented in a uniform way. The 5-year event-free survival (EFS) rates seemed not to vary widely, ranging from 71% to 83% ( Table 1 ). Overall remission rates usually were 98% or higher.
| Study Group | Years of Study | Patient Number | Overall 5-year Event-free Survival (%) | B-lineage ALL 5-year Event-free Survival (%) | T-lineage ALL 5-year Event-free Survival (%) |
|---|---|---|---|---|---|
| DFCI-91-01 | 1991–1995 | 377 | 83 | 84 | 79 |
| BFM-90 | 1990–1995 | 2178 | 78 | 80 | 61 |
| NOPHO-ALL92 | 1992–1998 | 1143 | 78 | 79 | 61 |
| COALL-92 | 1992–1997 | 538 | 77 | 78 | 71 |
| SJCRH-13A | 1991–1994 | 167 | 77 | 80 | 61 |
| CCG-1800 | 1989–1995 | 5121 | 75 | 75 | 73 |
| DCOG-ALL8 | 1991–1996 | 467 | 73 | 73 | 71 |
| EORTC-58881 | 1989–1998 | 2065 | 71 | 72 | 64 |
| AIEOP-91 | 1991–1995 | 1194 | 71 | 75 | 40 |
| UKALL-XI | 1990–1997 | 2090 | 63 | 63 | 59 |
Risk-based stratification allows the tailoring of treatment according to the predicted risk of relapse. Children who have high-risk features receive aggressive treatment to prevent disease recurrence, and patients who have a good prognosis receive effective therapy but are not exposed to unnecessary treatment with associated short- and long-term side effects. Clinical factors that predict outcome and are used for stratification of patients into treatment arms are age, gender, and white blood cell count at presentation. Biologic factors with prognostic value are the immunophenotype and genotype of the leukemia cells. Another predictive factor is the rapidity of response to early therapy, such as the decrease in peripheral blood blast count in response to a week of prednisone or the decrease in bone marrow blasts after 1 to 3 weeks of multiagent chemotherapy. More recently the determination of minimal residual disease (MRD) in the bone marrow during the first months of therapy using flow cytometry or molecular techniques has been shown to have a high prognostic value and therefore is used for stratification in many contemporary trials. The detection of MRD accurately distinguishes very good responders to therapy from those who will respond poorly to therapy, irrespective of the biologic subtype of ALL and the underlying mechanism of this response. In several protocols, MRD is used to stratify patients for reduction of therapy (ie, patients who are MRD negative especially at early time points) or intensification of therapy (ie, patients who are MRD positive at later time points).
Age and immunophenotype
Over the years, age has remained an independent predictor of outcome ( Table 2 ). Children aged 1 to 9 years have the best outcome; children and adolescents aged 10 to 20 years have a slightly worse outcome, which is associated in part with a higher incidence of T-cell leukemia and a lower incidence of favorable genetic abnormalities such as TEL/AML1 and hyperdiploidy. For adults, survival rates decrease further with increasing age. When results are corrected for differences in immunophenotype, ALL cells from older children and adults are more resistant to multiple antileukemic drugs than are cells from children in the first decade of life.
| Factor | Favorable | Unfavorable |
|---|---|---|
| Age at diagnosis | 1–9 years | <1 or >9 years |
| Sex | Female | Male |
| White blood cell count | Low (eg, <50 or <25 × 10 e 9/L) | High (eg, >50 or >25 × 10 e 9/L) |
| Genotype | Hyperdiploidy (>50 chromosomes) | Hypodiploidy (<45 chromosomes) |
| t(12;21) or TEL/AML1 fusion | t(9;22) or BCR/ABL fusion | |
| t(4;11) or MLL/AF4 fusion | ||
| Immunophenotype | Common, preB | ProB, T-lineage |
Infants diagnosed at less than 1 year of age have a relatively poor outcome that is associated with a high incidence of the unfavorable very immature proB-ALL phenotype and especially the presence of MLL gene rearrangements. The poor outcome has led physicians in the United States, Japan, and the International Interfant collaborative group including European and non-European countries and institutes to develop specific protocols to treat infant ALL. Biologic characteristics of infant ALL cells are described later in the paragraph discussing the MLL gene.
T-cell ALL is detected in approximately 15% of childhood ALL. It is characterized by a relative resistance to different classes of drugs when compared with B-lineage ALL. T-cell ALL cells accumulate less methotrexate polyglutamates and less cytarabine triphosphate than precursor B-ALL cells. With risk-adapted therapy the outcome of T-cell ALL now approaches that of B-lineage ALL in many study groups (see Table 1 ).
Approximately 85% of childhood ALL is of B lineage, mainly common or preB ALL. A very immature subtype characterized by the lack of CD10 expression (proB ALL) is associated with a high incidence of MLL gene rearrangements and an unfavorable outcome. Mature B-lineage ALL, defined by the presence of immunoglobulins on the cell surface, has a favorable outcome only when treated with B-non-Hodgkin lymphoma protocols.
Genetics
Hyperdiploidy
Hyperdiploidy (a DNA index > 1.16 or > 50 chromosomes per leukemia cell) is found in approximately 25% of children who have B-lineage ALL. It is associated with a favorable outcome, especially when extra copies of chromosome 4, 10 or 17 are present. Hyperdiploid ALL cells have an increased tendency to undergo apoptosis, accumulate high amounts of methotrexate polyglutamates, and are highly sensitive to antimetabolites and L-asparaginase.
TEL/AML1
The TEL/AML1 fusion, also found in approximately 25% of cases, is mutually exclusive with hyperdiploidy and also is associated with a favorable outcome. It is formed by a fusion of the TEL gene on chromosome 12 encoding for a nuclear phosphoprotein of the ETS family of transcription factors and the AML1 gene on chromosome 21, a transcription factor gene encoding for part of the core-binding factor. The TEL/AML1 fusion probably inhibits the transcription activity of the normal AML1 gene involved in proliferation and differentiation of hematopoietic cells. TEL/AML1 fusion is associated with a high chemosensitivity, especially for L-asparaginase. The mechanism behind this asparaginase sensitivity remains unclear but is not caused by a low asparagines synthetase activity in the leukemic cells. TEL/AML1 -rearranged cells also may be more sensitive to other drugs, especially anthracyclines and etoposide.
Both hyperdiploidy and TEL/AML1 occur mainly in children younger than 10 years of age with common/preB ALL and are rare above this age and in other ALL immunophenotypes.
MLL
Abnormalities of the mixed lineage leukemia ( MLL ) gene on chromosome 11q23 occur in only approximately 2% of children above the age of 1 year, although it is present in approximately 80% of infants who have ALL. All types of MLL gene rearrangements, such as MLL/AF 4 created by t(4;11), MLL/ENL created by t(11;19), and MLL/AF9 created by t(9;11), are associated with a poor outcome in infants who have ALL ; in older children this poor outcome may only hold true for the presence of MLL/AF4 . The MLL/AF9 rearrangement occurs in older infants and is characterized by a more mature pattern of immunoglobulin gene rearrangements, suggesting another pathogenesis.
The precise actions of the fusion products involving MLL are not known, but they are associated with abnormal expression of HOX genes, which may lead to abnormal growth of hematopoietic stem cells. ALL cells with MLL gene abnormalities are highly resistant to glucocorticoids in vitro and in vivo and also to L-asparaginase. These cells, however, show a marked sensitivity to the nucleoside analogues cytarabine and cladribine. This sensitivity is related to a high expression of the membrane nucleoside transporter ENT1. MLL -rearranged ALL cells do not show a defective methotrexate polyglutamation and have no overexpression of multidrug resistance proteins. Methotrexate pharmacokinetics might be different in the youngest infants.
BCR-ABL
The translocation t(9;22) fuses the BCR gene on chromosome 22 to the ABL gene on chromosome 9 causing an abnormal ABL tyrosine kinase activity associated with increased proliferation and decreased apoptosis. The BCR/ABL fusion is found mainly in common and preB ALL. The incidence of BCR/ABL increases with age: it is seen in approximately 3% of children who have ALL but in approximately 25% of adults who have ALL. The presence of BCR/ABL predicts a poor outcome.
Children who have BCR/ABL -rearranged ALL or MLL -rearranged ALL more often show a poor response to prednisone and have high levels of MRD after induction therapy.
Genetics in T-cell Acute Lymphoblastic Leukemia
The prognostic value of genetic abnormalities in T-ALL is less clear. Ectopic expression of TAL-1 is caused by the translocation t(1;14) in only a few percent of T-ALL cases or, more often, by the SIL-TAL fusion transcript. Activation of HOX11 by the translocations t(10;14) and t(7;10) occur in approximately 10% of T-ALL cases. Two recently described abnormalities occur frequently and exclusively in T-ALL. These are the ectopic expression of HOX11L2 , mainly caused by the translocation t(5;14), in approximately 25% of T-ALL cases and activating mutations of the NOTCH1 gene in 50% of T-ALL cases. NOTCH1 mutations are not associated with a poor outcome and may be associated with a favorable outcome.
Others
Many other recurrent genetic and molecular genetic lesions exist in small subsets of childhood ALL such as the translocation t(1;19) leading to a E2A-PBX1 fusion detected in less than 5% of precursor B-ALL, mainly preB ALL. Although in the past this translocation had been associated with a poor prognosis, this is not longer true with contemporary treatment protocols. Two percent of precursor B-lineage ALL cases harbor an intrachromosomal amplification of chromosome 21 that is associated with poor survival. Hypodiploidy (<45 chromosomes) is detected in only 1% of children who have ALL and is associated with poor outcome, particularly in the low-hypodiploid (33–39 chromosomes) or near-haploid cases (23–29 chromosomes) as shown in a recent retrospective international study.
A discussion of all other abnormalities is beyond the scope of this article. It should be mentioned that children who have Down syndrome and ALL do not have a better outcome and perhaps even have a worse outcome than other ALL cases because they lack favorable genetic features.
Genetics
Hyperdiploidy
Hyperdiploidy (a DNA index > 1.16 or > 50 chromosomes per leukemia cell) is found in approximately 25% of children who have B-lineage ALL. It is associated with a favorable outcome, especially when extra copies of chromosome 4, 10 or 17 are present. Hyperdiploid ALL cells have an increased tendency to undergo apoptosis, accumulate high amounts of methotrexate polyglutamates, and are highly sensitive to antimetabolites and L-asparaginase.
TEL/AML1
The TEL/AML1 fusion, also found in approximately 25% of cases, is mutually exclusive with hyperdiploidy and also is associated with a favorable outcome. It is formed by a fusion of the TEL gene on chromosome 12 encoding for a nuclear phosphoprotein of the ETS family of transcription factors and the AML1 gene on chromosome 21, a transcription factor gene encoding for part of the core-binding factor. The TEL/AML1 fusion probably inhibits the transcription activity of the normal AML1 gene involved in proliferation and differentiation of hematopoietic cells. TEL/AML1 fusion is associated with a high chemosensitivity, especially for L-asparaginase. The mechanism behind this asparaginase sensitivity remains unclear but is not caused by a low asparagines synthetase activity in the leukemic cells. TEL/AML1 -rearranged cells also may be more sensitive to other drugs, especially anthracyclines and etoposide.
Both hyperdiploidy and TEL/AML1 occur mainly in children younger than 10 years of age with common/preB ALL and are rare above this age and in other ALL immunophenotypes.
MLL
Abnormalities of the mixed lineage leukemia ( MLL ) gene on chromosome 11q23 occur in only approximately 2% of children above the age of 1 year, although it is present in approximately 80% of infants who have ALL. All types of MLL gene rearrangements, such as MLL/AF 4 created by t(4;11), MLL/ENL created by t(11;19), and MLL/AF9 created by t(9;11), are associated with a poor outcome in infants who have ALL ; in older children this poor outcome may only hold true for the presence of MLL/AF4 . The MLL/AF9 rearrangement occurs in older infants and is characterized by a more mature pattern of immunoglobulin gene rearrangements, suggesting another pathogenesis.
The precise actions of the fusion products involving MLL are not known, but they are associated with abnormal expression of HOX genes, which may lead to abnormal growth of hematopoietic stem cells. ALL cells with MLL gene abnormalities are highly resistant to glucocorticoids in vitro and in vivo and also to L-asparaginase. These cells, however, show a marked sensitivity to the nucleoside analogues cytarabine and cladribine. This sensitivity is related to a high expression of the membrane nucleoside transporter ENT1. MLL -rearranged ALL cells do not show a defective methotrexate polyglutamation and have no overexpression of multidrug resistance proteins. Methotrexate pharmacokinetics might be different in the youngest infants.
BCR-ABL
The translocation t(9;22) fuses the BCR gene on chromosome 22 to the ABL gene on chromosome 9 causing an abnormal ABL tyrosine kinase activity associated with increased proliferation and decreased apoptosis. The BCR/ABL fusion is found mainly in common and preB ALL. The incidence of BCR/ABL increases with age: it is seen in approximately 3% of children who have ALL but in approximately 25% of adults who have ALL. The presence of BCR/ABL predicts a poor outcome.
Children who have BCR/ABL -rearranged ALL or MLL -rearranged ALL more often show a poor response to prednisone and have high levels of MRD after induction therapy.
Genetics in T-cell Acute Lymphoblastic Leukemia
The prognostic value of genetic abnormalities in T-ALL is less clear. Ectopic expression of TAL-1 is caused by the translocation t(1;14) in only a few percent of T-ALL cases or, more often, by the SIL-TAL fusion transcript. Activation of HOX11 by the translocations t(10;14) and t(7;10) occur in approximately 10% of T-ALL cases. Two recently described abnormalities occur frequently and exclusively in T-ALL. These are the ectopic expression of HOX11L2 , mainly caused by the translocation t(5;14), in approximately 25% of T-ALL cases and activating mutations of the NOTCH1 gene in 50% of T-ALL cases. NOTCH1 mutations are not associated with a poor outcome and may be associated with a favorable outcome.
Others
Many other recurrent genetic and molecular genetic lesions exist in small subsets of childhood ALL such as the translocation t(1;19) leading to a E2A-PBX1 fusion detected in less than 5% of precursor B-ALL, mainly preB ALL. Although in the past this translocation had been associated with a poor prognosis, this is not longer true with contemporary treatment protocols. Two percent of precursor B-lineage ALL cases harbor an intrachromosomal amplification of chromosome 21 that is associated with poor survival. Hypodiploidy (<45 chromosomes) is detected in only 1% of children who have ALL and is associated with poor outcome, particularly in the low-hypodiploid (33–39 chromosomes) or near-haploid cases (23–29 chromosomes) as shown in a recent retrospective international study.
A discussion of all other abnormalities is beyond the scope of this article. It should be mentioned that children who have Down syndrome and ALL do not have a better outcome and perhaps even have a worse outcome than other ALL cases because they lack favorable genetic features.
Therapy
The backbone of contemporary multiagent chemotherapeutic regimens is formed by four elements: induction, CNS-directed treatment and consolidation, reinduction, and maintenance.
Induction
The goal of induction therapy is to induce morphologic remission and to restore normal hematopoiesis. Induction therapy contains at least three systemic drugs (ie, a glucocorticoid, vincristine, and L-asparaginase) and intrathecal therapy. The addition of an anthracycline as a fourth drug is matter of debate. In some protocols all patients receive an anthracycline; in other protocols it is reserved for high-risk cases. The induction phase aims to induce complete morphologic remission in 4 to 6 weeks.
Central Nervous System–Directed Treatment and Consolidation
CNS-directed therapy aims to prevent CNS relapses and to reduce the systemic minimal residual leukemia burden. CNS therapy usually is achieved by weekly or biweekly intrathecal therapy along with systemically administered drugs such as high-dose methotrexate (MTX) and 6-mercaptopurine (6-MP). Some groups rely on other drugs (eg, cyclophosphamide, cytarabine) in the consolidation phase to reduce systemic tumor burden further.
Reinduction
Reinduction therapy or delayed (re)intensification most often uses drugs comparable to those used during induction and consolidation therapy and has clearly shown its value by reducing the risk of relapse.
Maintenance
Therapy for ALL is completed by prolonged maintenance therapy for a total treatment duration of 2 years, or even longer in some protocols. Maintenance consists of daily 6-MP and weekly MTX. In some protocols additional pulsed applications of a glucocorticoid and vincristine and intrathecal therapy are administered.
A fifth element, allogeneic stem cell transplantation (SCT), is reserved for only a small number of selected patients in first complete remission. The contribution of specific parts of treatment depends on the total therapy administered to a patient. A few important topics for which new data have been produced recently are discussed in the following sections.
Anthracyclines in Induction?
It is unclear if addition of an anthracycline to a three-drug induction regimen is of benefit. Regimens that do not contain anthracycline are less myelosuppressive. Studies performed by the Children’s Cancer Group, however, showed that selected patients younger than 10 years of age did not benefit from the addition of an anthracycline, whereas selected older children did.
Dexamethasone or Prednisone?
Several recent randomized studies have shown that the substitution of prednisone (approximately 40 mg/m 2 ) by dexamethasone (approximately 6 mg/m 2 ) significantly decreases the risk of bone marrow and CNS relapses when used in what are thought to be equipotent dosages. One Japanese study, however, did not confirm the advantage of using dexamethasone. The benefit of dexamethasone may result from higher free plasma levels and a better CNS penetration or from the fact that the presumed equivalent antileukemic activity for prednisone/dexamethasone is not a 6:1 dose ratio but is higher, as some (but not all) in vitro experiments suggest. At this dose ratio dexamethasone also results in more toxicity than prednisone. In vitro, a strong cross-resistance to prednisone and dexamethasone exists in ALL cells.
Which Dose Intensity of Which Asparaginase?
Randomized studies have revealed that at the same dose schedules, the use of L-asparaginase derived from Escherichia coli resulted in significant better EFS and overall survival (OS) rates than when asparaginase derived from Erwinia chrysanthemi (Erwinase) was used. This difference results from differences in the half-lives of the drugs, and the difference presumably would not be found if Erwinase were given in an adequate dose-intensity schedule. The dose-intensity schedule to achieve complete asparagine depletion is 5000 units/m 2 every 3 days for E coli asparaginase. Erwinase must be scheduled more frequently than E coli asparaginase to achieve the same asparagine depletion. For the pegylated type of E coli asparaginase (PEG-asparaginase), 2500 units/m 2 once every 2 weeks leads to the same pharmacodynamic effects. Lower doses of PEG-asparaginase (1000 units/m 2 ) also lead to complete asparagine depletion in serum but not in the cerebrospinal fluid.
Intensification of asparaginase in induction and reinduction has improved outcomes in different studies. Also, asparaginase intolerance was an important factor predicting an inferior outcome. Allergic reactions usually are responsible for the discontinuation of asparaginase. Allergic reactions occur mainly when the drug is readministered in reinduction several weeks after first exposure during induction. In addition, the presence of asparaginase antibodies may lead to inactivation of the drug. Consequently, many investigators favor the use of the less immunogenic PEG-asparaginase from therapy outset rather than using it only after allergic reactions have occurred. In the light of these data, pharmacodynamic monitoring of asparaginase administration may prove very important for individual children who have ALL.
Which Central Nervous System–directed Therapy?
To clarify the role of different CNS-directed therapies, a meta-analysis was published in 2003. From this analysis it became clear that long-term intrathecal therapy leads to EFS rates comparable with those of radiotherapy. Radiotherapy seemed to be more effective than high-dose MTX in preventing CNS relapse, but intravenous MTX reduced systemic relapses, resulting in comparable EFS rates for high-dose MTX and radiotherapy. It was concluded that radiotherapy can be replaced by multiple intrathecal doses of chemotherapy and that intravenous MTX reduces systemic relapses. It is still unclear whether intrathecal triple therapy (glucocorticoid, MTX, cytarabine) has any advantage over the use of intrathecal MTX as single drug. A recent Children’s Cancer Group study suggested that intrathecal triple therapy prevented CNS relapse but did not improve OS because fewer bone marrow relapses occurred when intrathecal MTX was used as a single agent.
The results of CNS-directed therapy depend on the treatment used. For example, the use of systemic dexamethasone reduces the incidence of CNS relapse. The comparison of different CNS preventive regimens is hampered because results are described for heterogeneous groups of patients. In several protocols, radiotherapy is still given to selected groups of high-risk patients such as those who have T-ALL with high white cell counts and children who have CNS involvement at diagnosis. Cranial radiotherapy is specifically toxic for very young children because of its detrimental effect on cognitive function.
What Type of Reinduction/Intensification and Maintenance?
Maintenance therapy consists of daily oral 6-MP and weekly intravenous or oral MTX. The intravenous administration of MTX may overcome compliance problems, but there is no evidence that it is more effective than oral MTX. Several randomized studies have shown that the use of thioguanine offers no advantage over 6-MP in maintenance therapy. For unknown reasons, 6-MP is more effective when administered in the evening than in the morning. Continuous adaptations of the doses of MTX and 6-MP based on peripheral blood counts are necessary to reduce the risk of relapse, on the one hand, and the risk of infections, on the other. There are large interindividual differences in the doses that are tolerated or needed to reduce cell counts. This variability reflects pharmacogenetic differences, for instance in the status of thiopurine methyltransferase, a key enzyme that inactivates thiopurines. Also, large intraindividual differences in doses occur (eg, because of concurrent viral infections). Recently, the major ALL study groups reached consensus on how to adjust the doses of 6-MP and MTX during maintenance so that the white blood cell count remains between 1.5 and 3.0 × 10 9 /L. Routine measurements of liver function are not necessary in patients who do not have symptoms of liver dysfunction.
A meta-analysis of 42 trials showed that both longer maintenance (3 years versus 2 years) and the use of pulses of vincristine and a glucocorticoid during maintenance result in lower relapse rates but increased death rates. The most important factor that has helped reduce relapses and improve survival is the use of an intensive reinduction course at the start of maintenance therapy. Several randomized studies proved the value of reinduction therapy for childhood ALL. Attempts to omit reinduction led to a significant increase in relapse rate. More than 50% of patients who were treated without reinduction did not relapse, however, illustrating that not all patients really need this intensification element. The question, of course, is how to identify these patients early on. When an intensive reinduction course is given, neither longer maintenance nor the use of vincristine/glucocorticoid pulses may contribute significantly to a better OS.
The results of the meta-analysis do not exclude the possibility that subgroups of patients may benefit from a longer duration of maintenance. Several study groups use longer maintenance therapy for boys than for girls. Reduction of the duration of maintenance below 2 years in a Japanese study led to an increased risk of relapse. This study, however, also demonstrates that not all patients need 2 years of maintenance therapy. Again, the important question is how to identify these patients. It might be that a long maintenance therapy is less effective in high-risk leukemias with a very aggressive behavior, such as MLL gene–rearranged ALL, bcr-abl –positive ALL, and T-ALL, in which relapses occur relatively early; the more smoldering types of ALL, such as hyperdiploid and TEL/AML1 -gene rearranged ALL, might benefit more from maintenance therapy.
A recent large, randomized study did not show a benefit for the use of pulses with vincristine and a glucocorticoid in a selected group of patients treated on a Berlin/Frankfurt/Münster regimen. The benefit of these pulses therefore may be found only in studies that use no or a less intensive reinduction course, such as in the Dutch Childhood Leukemia Study Group-6 study or in studies in which the upfront therapy is relatively mild.
Who Should (not) be Transplanted?
Autologous SCT is not effective in childhood ALL and therefore should not be performed. A collaborative study of several large study groups has shown that BCR/ABL -positive ALL benefits from allogeneic SCT from a matched related donor both in terms of EFS and OS. For other types of donor this benefit was not proven. A comparable analysis for children who had t(4;11) could not detect a beneficial effect of SCT from any type of donor. Recently, a comparison was performed between children who had very high-risk ALL in first remission who were assigned by the availability of a compatible related donor to receive SCT or to receive chemotherapy when no donor was available. “Very high risk” was defined in this study by the presence of one or more of the following criteria: failure to achieve complete remission after 5 weeks’ therapy, t(9;22) or t(4;11) positivity, a poor prednisone response associated with T-cell phenotype, or a white blood cell count higher than 100 × 10 e 9/L. The 5-year disease-free survival rate was better for the patients who received SCT from a matched related donor than for those who received chemotherapy. Only one in six of these high-risk patients had a suitable family donor, however. SCT from alternative donors resulted in an inferior outcome. Therefore the role of allogeneic SCT in first complete remission is limited in these very high-risk patients. Another recent study failed to prove a benefit for allogeneic SCT in very high-risk cases, whereas the Berlin/Frankfurt/Münster study group showed that high-risk T-cell ALL cases may benefit from SCT.
Treatment of Adolescents
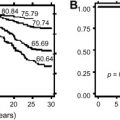
Four recent reports from four different countries show that outcome for adolescents who have ALL is better when these patients are treated on a pediatric rather than an adult protocol. The 5-year EFS of patients aged 15 to 21 years was approximately 30% higher when they were treated according to a pediatric protocol ( Table 3 ). This result could not be explained by differences in immunophenotype and genetic abnormalities, but there seemed to be large differences in the dose intensity used during treatment. The pediatric protocols contained more glucocorticoids, vincristine, L-asparaginase, MTX, and 6-MP. In addition, it is conceivable that the longer delays between different parts of treatment noted in adolescents treated according to the adult protocols might have played a role. It is possible that hematologists have a different approach in managing toxicities because they generally treat older patients who do not tolerate intensive therapy well. Also, the toxicity caused by SCT usually is accepted as part of therapy, whereas adult hematologists have less experience with glucocorticoid- and asparaginase-induced toxicities. In the Dutch study, use of the adult ALL treatment protocol resulted in both a higher relapse rate and in a higher toxic death rate for adolescents.