Abstract
The traditional concept of the uterus is that the endometrium is the dynamic tissue, providing an intricate set of functions throughout the menstrual cycle—a process that rarely culminates in implantation and pregnancy. The myometrium has been viewed as an inert tissue, chiefly important during pregnancy and, when abnormal, providing the surgical livelihood of clinical gynecologists. To understand both the physiology of menstruation and the pathophysiology of abnormal uterine bleeding (AUB), both the myometrial and the endometrial layers of the uterus are important. This chapter covers both myometrial disease (adenomyosis and leiomyomas) and endometrial diseases (polyps, AUB, intrauterine adhesions, and dysmenorrhea). The objective is to provide the reader with an understanding of the various clinical presentations as well as the molecular pathophysiology of the disease process and to enlist various therapeutic options for these benign uterine diseases.
Keywords
Uterine fibroids, leiomyoma, myomectomy, uterine artery embolization, magnetic resonance–guided focused ultrasound surgery, adenomyosis, endometrial polyps, abnormal uterine bleeding, intrauterine adhesions, Asherman syndrome, dysmenorrhea
Uterine Leiomyomas
- ◆
Uterine leiomyomas are very common benign clonal smooth muscle cell tumors that have increased prevalence in black women.
- ◆
Gonadal steroids, fibrotic and angiogenic factors, as well as cytogenic and molecular genetic factors play a role in the pathogenesis and growth of uterine leiomyomas.
- ◆
Therapeutic options for uterine leiomyomas can range from observation to medical management, surgical resection as well as the newer interventional radiologic procedures that include uterine artery embolization (UAE) and magnetic resonance–guided focused ultrasound surgery (MRgFUS).
Epidemiology
Uterine leiomyomas, frequently termed myomas or fibroids, are benign clonal smooth muscle cell tumors ranging in size from several millimeters to many centimeters ( Fig. 26.1 ). Clinically, fibroids are appreciated in approximately 25% of all women, and in black women they appear to have a threefold increased incidence and relative risk. Careful pathologic study of surgical specimens suggests that greater than 80% of black and 70% of white women have detectable leiomyomas, which parallels the lifetime incidence of the clinical disease. Thus it appears that there is little occult disease in black women. This suggests that in this group, growth acceleration of transformed myocytes into clinical fibroids may be ubiquitous. Black women are not only significantly more likely than white women to have leiomyomas but also to be younger at the time of diagnosis and hysterectomy. They also have more severe diseases and are 2 to 3 times more likely to have hysterectomy for leiomyomas and sixfold more likely to have a myomectomy ( Table 26.1 ).
| Fibroid Characteristic | Black Versus White Women | Reference Number |
|---|---|---|
| Incidence of uterine fibroids | Threefold increase | |
| Relative risk | Threefold increase | |
| Age at diagnosis | 3–5 years younger | |
| Severity of disease | Fivefold increase | |
| Fibroid growth at older age (≥45 years) | Sevenfold to Eightfold increase | |
| Myomectomy risk | Sixfold increase | |
| Hysterectomy risk | Twofold to threefold increase |
Known risk factors do not adequately explain this racial disparity. Newly discovered genetic polymorphisms that include abnormal transcriptional factors, increased aromatase activity, and signal transduction genes may dictate a more severe phenotype of the disease in black women. Polymorphism of catechol-O-methyltransferase (COMT), an essential enzyme for estrogen metabolism, has been shown to be linked to fibroid formation and is more common in black women. Vitamin D has been shown to inhibit fibroid proliferation via the COMT pathway ; it also reduces fibrosis caused by transforming growth factor-β3 (TGF-β3). The role of vitamin D has been reproduced in the Eker mouse model, where supplementation with 1,25 dihydroxyvitamin D3 at 0.1 and 1 µM for 24 hours led to reduced myoma size compared with controls. Studies in black women correlate fibroid risk with polycystic ovarian syndrome, history of physical and/or sexual abuse, and self-reported experiences of racism. An understanding of the unique genetic and environmental factors leading to an increased risk for black women is a key research agenda for leiomyomas. There are mixed data regarding fibroid risk in Latina women.
Reproductive factors affect the risk of leiomyomas. Numerous studies have shown that parity is associated with decreased fibroid risk. One hypothesis is that the remodeling of the postpartum uterus can clear nascent fibroids. In one of these reports of women who had fibroids at the beginning of pregnancy, 36% had no identifiable lesion on ultrasonography postpartum and 79% of the remaining fibroids had decreased in size. Progestin use in the postpartum period was the only significant risk factor for the limited regression of fibroids postpartum. The fact that this study contrasts with previous reports (i.e., that progestin-only injectable contraceptives are associated with decreased fibroid risk and that these contraceptives are widely used by breastfeeding women) makes it critical that this area be explored further. Although clinical dogma traditionally suggested that oral contraceptives (OCs) were contraindicated for women with myomas, OCs instead appear to protect against clinically evident fibroids, with the caveat that timing of use may be important. Exposure to OCs between ages 13 and 16 for women in the Nurses’ Health Study led to an increased relative risk of leiomyomas, while later OC use was protective in direct proportion to duration of use. Recent work has proposed an increase in vitamin D levels in women who are using OCs as a means of protection against leiomyomas. Postmenopausal hormone replacement therapy (HRT) has been associated with a higher likelihood of having fibroids on pathology after surgery in one report, although this may have been due to bias, since HRT may have inhibited normal postmenopausal fibroid regression. Early menarche is also associated with increased risk of developing fibroids ; this may explain earlier disease in black women, in whom menarche is earlier than in white women.
Environmental and dietary habits also appear to influence the risk of myoma formation. Decreased vegetable and fruit intake, especially citrus fruits, increased dietary long-chain omega-3 fatty acids, specifically marine fatty acids, and significant consumption of red meats or ham were all associated with an increased relative risk of developing fibroids. The consumption of green vegetables was associated with a decreased risk of myomas. Reduced dairy consumption, use of hair relaxer, and increased intake of alcohol, especially beer, appear to increase the risk in black women. An association of fibroid risk with a high alcohol intake has also been reported in Japanese women. No one, however, has demonstrated that dietary intervention leads to changes in the incidence, symptomatology, or regression of fibroids. Major life events and stress as well as a history of abuse in childhood have been linked to the presence of uterine fibroids. In utero exposure to diethylstilbestrol (DES) in animals and humans as well as consumption of soy formula in infancy, low childhood socioeconomic status, early gestational age at birth, and maternal prepregnancy or gestational diabetes have all been associated with a greater risk of fibroid development. Caffeine consumption was not noted to be a risk factor. Smoking is considered protective through an unknown mechanism, although recent work has challenged this and has shown environmental tobacco smoke exposure as a risk factor for the late diagnosis of incident fibroids in women undergoing menopausal transition. Dietary vitamin A from animal sources appears to be protective.
Increased body mass index (BMI) or weight gain since age 18 also appears to influence myoma risk in some cohorts. A high dietary glycemic index and glycemic load and decreased physical activity are also risk factors. Finally, women with leiomyomas appear to be more likely to have hypertensive disease than control women. It is unclear whether this commonality is due to a common underlying mechanism, since leiomyomas have been shown to share pathogenic features with the development of metabolic syndrome.
Finally, uterine infections appear to be associated with an increased risk of leiomyomas, yet factors associated with cervical neoplasia are associated with a decreased risk. Additional study is needed regarding infectious agents and leiomyoma risk.
Pathophysiology
Leiomyomas have a median of 9% change, generally an increase in volume in a 6-month period. Growth appears to be race related. Black and white women had similar growth until 35 years of age, after which growth rates declined for white women. It has also been shown that the growth of a fibroid is not dependent on its position in the uterus. The size of fibroids may be important, as lesions greater than 5 cm in diameter have less short-term change.
Gonadal Steroids: Estrogen and Progesterone
There are substantial in vitro data supporting major roles for both estrogen and progesterone in the biology of uterine leiomyomas. The role of progesterone in myoma growth has moved beyond the simplistic concept of increasing mitosis to include inhibition of the apoptosis pathway via the induction of β-cell lymphoma 2 (Bcl 2). The apoptotic inhibitor Bcl2 is present in leiomyomas but is largely undetectable in myometrium. The role of Kruppel-like transcription factor 11 (KLF11) in integrating progesterone-mediated myoma cell signaling and proliferation has been shown. Likewise, regarding estrogen action, local action is likely key via an upregulation of the enzyme aromatase P450 and its gene CYP19 . Other elements of estrogenic response in myomas can also come into play, as myoma cells have a modest increase in type I isotype of 17β hydroxysteroid dehydrogenase.
Modulation of steroid receptors is also important. Leiomyomas have increased amounts of both estrogen and progesterone receptor (ER and PR) messenger ribonucleic acid (mRNA) compared with normal myometrial tissue. Both the A- and the N-terminally truncated B-isoforms of the PR appear to be present in both leiomyomas and myometrium; however, the A-isoform predominates. Similarly, ER-α rather than ER-β appears to be the predominant form in leiomyomas.
In addition to the direct action of ovarian steroids on the uterus, it is possible that the reproductive axis may also influence uterine metabolism through the direct action of pituitary gonadotropins. Gonadotropin-releasing hormone (GnRH), which is clinically used to reduce myoma size, abolishes differences in gene expression between normal myometrium and myomas. The placental glycoprotein chorionic gonadotropin (hCG) has been shown by several laboratories to have direct actions on myometrial metabolism. Additionally, work has shown that follicle-stimulating hormone (FSH), luteinizing hormone (LH), thyroid-stimulating hormone (TSH), and their common α-subunit can all have stimulatory effects on uterine prolactin production. There appears to be a variant LH/hCG receptor present in human uterine tissue that may modulate this action. LH has also been associated with myoma formation but not growth independent of the patient’s age. Genome-wide microarray studies have shown a strong role of glucocorticoids in the pathogenesis of fibroids. There are new data suggesting that the Nr4A nuclear receptor, which does not have a ligand, may be playing a role in fibroid pathogenesis. This receptor family is important because of its regulation of the profibrotic/extracellular matrix (ECM) pathways. Finally, there is increasing evidence that stem cells may be playing a key regulatory role in leiomyoma biology.
Fibrotic Factors
Leiomyomas can also be viewed as fibrotic tumors with a dynamic ECM playing an important role in their pathophysiology ( Fig. 26.2 ). This hypothesis dates back to the 1990s where experiments demonstrated that the ECM characterizing fibroids contains significant amounts of collagen types I and III protein, and that upregulation of mRNA levels occurs during the proliferative phase of the menstrual cycle in leiomyomas but not in myometrium. Other matrix components including matrix metalloprotease stromelysin 3 (MMP 11) and dermatopontin (a collagen-binding protein, also having decreased expression in keloid scars) have also been shown to be dysregulated in leiomyomas. Morphologic arrangement of extracellular proteins is also abnormal in myomas, and increased stiffness of these ECM alterations leads to altered gene expression through solid-state signaling. These modified mechanical stresses on cells lead to the activation of Rho-dependent signaling. Activation of this solid-state signaling and altered state of stress may also contribute to fibroid growth.
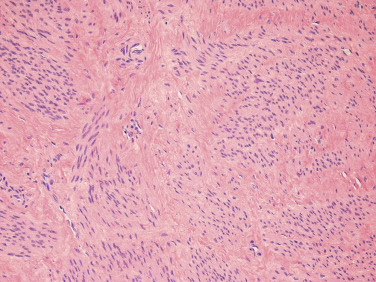
The TGF-β system also appears to be involved in the pathophysiology of leiomyomas as in other fibrotic processes. A complete review of this topic is beyond the scope of this chapter. Leiomyomas appear to have higher levels of TGF-β and particularly TGF-β3 mRNA and protein; this, in turn, affects cellular proliferation. Vitamin D supplementation has been shown to reverse TGF-β3–induced fibrosis in fibroids. Additionally, granulocyte-macrophage colony-stimulating factor (GM-CSF), connective tissue growth factor (CTGF), TGF-β4 (also known as lefty or ebaf, endometrial bleeding-associated factor), the sma- and mad-related (SMAD) family of transcriptions factors, and the mitogen-activated protein kinase (MAPK) signaling pathway appear to be part of the fibrotic pathway that is dysregulated in myomas, or in the myometrium or endometrium of the uterus in women with leiomyomas or abnormal uterine bleeding (AUB).
Angiogenesis
Angiogenesis, the formation of new blood vessels, is physiologic in the female reproductive tract as opposed to most other tissues, where it is pathologic. Abnormalities in uterine blood vessels and angiogenic growth factors also appear to play a role in the pathobiology of myomas. The myomatous uterus shows increased numbers of arterioles and venules as well as venule ectasia. These changes are not confined to the leiomyoma itself but also involve the myometrium and the endometrium. Although such venous abnormalities were originally postulated to be the result of physical compression of the vascular structures by bulky myomas, it is likely that molecular alterations are actually responsible for increased vessel numbers or abnormal function.
The process of angiogenesis involves interactions with specific components of the ECM, such as collagens type I and III, that are dysregulated in fibroids. There are also conflicting data regarding whether the resident immune cells (especially mast cells) contribute to the physiology of myomas by modulating angiogenesis.
The basic fibroblast growth factor (bFGF) receptor/ligand system appears to be a significant factor in the pathophysiology of leiomyomas. In addition to promoting angiogenesis, bFGF is a smooth muscle cell mitogen and acts similarly to estradiol on leiomyoma smooth muscle cells. Leiomyomas have increased levels of bFGF mRNA compared with matched myometrium, a reservoir of bFGF protein in the ECM, and dysregulation of the endometrial type I bFGF receptor.
Cytogenetic and Molecular Genetics
There appear to be multiple genetic pathways to the phenotypic leiomyomas. Leiomyomas are monoclonal, and each tumor is an independent clonal event. Although this fact was originally investigated using G6PD polymorphisms, androgen receptor polymorphism studies concur.
Second, certain cytogenetic rearrangements characterize leiomyomas. Although 40% of fibroids have 46,XX karyotypically normal cells, there are specific karyotypic abnormalities that have been consistent in a number of studies: t(12;14) translocations between chromosomes 12 and 14; trisomy 12; rearrangements of 6p, 10q, and 13q; and deletion of 3q, 7q, and 1p. There is evidence that karyotypic evolution is a late event in the pathogenesis of leiomyomas. There is also some evidence that genotype is related to both fibroid size and location and that specific karyotypic groups have specific gene expression profiles. Thus many of the characteristics we attribute to submucous fibroids, for example, may be related to genotype; therefore the clinical heterogeneity we see may be more intelligible when genotypic information is available. A genomewide association study (GWAS) from Japan determined significant associations with uterine fibroids in three chromosomes 10q24.33, 22q13.1, and 11p15.5. Subgroup analyses revealed a strong association of marker single nucleotide polymorphisms (SNPs) with uterine fibroids regardless of the presence or absence of heavy or painful menstrual bleeding, suggesting that these SNPs are associated with predisposition genes.
Hierarchical gene clustering has revealed at least four key pathogenic subgroups of fibroids, depending on somatic mutations or chromosomal alterations in key genes: the mediator complex subunit 12 (MED12) group, the high-mobility group AT-hook2 (HMG2), the fumarate hydratase (FH) group, and a rare group associated with deletion of collagen IV α5 (COL4A5) and COL4A6. It now appears that chromothripsis, a global event causing multiple chromosome rearrangements at once, may also play a role in leiomyomas. MED12 is a transcriptional factor, a mediator of both global and specific gene transcription located on chromosome Xq13.1 that has been described in the pathogenesis of uterine fibroids. MED12 was altered in 70% of tumors from 80 patients studied in this report from Scandinavia, and pathway analysis suggested that ECM-receptor interaction, Wingless family (Wnt) signaling, and focal adhesion pathways were altered by this change. Whole-genome sequencing has shown MED12 mutations to be present frequently in fibroids in racially and ethnically diverse American women, confirming its importance as a key molecule in fibroid pathobiology. There is increasing evidence that the downstream Wnt/β-catenin signaling pathway may also play a key role in fibroid pathogenesis.
High-mobility-group protein A2 (HMGA2, formerly called HMGI-C) is an architectural transcription factor located on chromosome 12 that is involved in the pathogenesis of fibroids with t(12;14). The dysregulation of HMGA2 might be associated with fibroid growth by the increased expression of CDKN2A (which encodes ARK[p14]). Intact ARF (p14) maintains senescence in fibroids.
The mutation of tumor repressor REST and activation of the mammalian target of rapamycin (mTOR) signaling pathway may also play a key role. The Eker rat model for leiomyomas has a germline defect in the tuberous sclerosis complex 2 (Tsc-2) tumor suppressor gene. Tsc-2 also activates mTOR , which makes the latter a particularly interesting gene in the pathogenesis of leiomyomas.
A significant limitation of genetic studies is that they have largely been conducted in areas where white people predominate and may not accurately reflect the karyotypes seen in black women. Given the different clinical behavior of myomas in black women, it is reasonable to believe that unique genes may be contributing to this risk. Association of a polymorphism in the COMT gene, seen more frequently in African-American women, was linked to leiomyoma risk. In vitro work also suggests that there may be differences in growth-factor regulation in leiomyomas from African-American women. Linkage analysis for FH in nonsyndromic leiomyomas has demonstrated a significant effect of race, with linkage only seen in white women and a negative relation in black patients.
Genetic Influences: Clinical
There are several lines of evidence that suggest that fibroids have a genetic component. First, monozygotic twins have twice the rate of concordance for hysterectomy as compared with dizygotic twins. There is also familial clustering, with a two- to sixfold increased risk of a woman having fibroids if she has an affected first-degree relative. Finally, there are also specific syndromes that have a demonstrated genetic component and whose phenotype includes uterine leiomyomas in association with other specific lesions:
Hereditary Leiomyomatosis and Renal Cell Cancer (Mendelian Inheritance in Man 605839) *
* The following website contains the full online catalog of these genetic disorders: http://www-ncbi-nlm-nih-gov.easyaccess1.lib.cuhk.edu.hk/omim/
This syndrome is autosomal dominant, and affected families manifest cutaneous leiomyomas and papillary renal cell carcinoma (RCC). Affected women can have uterine leiomyomas as well as uterine leiomyosarcomas. Both malignancies (sarcomas and RCCs) are atypical in their presentation compared with their sporadic counterparts; uterine sarcomas can appear in young premenopausal women, and the papillary RCC is often metastatic at presentation and more likely to be seen in women. Two other syndromes with cutaneous and uterine leiomyomas have been described, but lessons from molecular genetics suggest that these are incomplete forms of the hereditary leiomyomatosis and renal cell cancer (HLRCC) syndrome and should be of historical interest only (Reed syndrome or multiple cutaneous and uterine leiomyomas [MCUL], Mendelian Inheritance in Man [MIM] 150800).
FH, an enzyme that is part of the Krebs tricarboxylic acid cycle, is the gene mutation at 1q 42-43 responsible for HLRCC syndrome. Germline mutations appear to result in absent or nonfunctional proteins; thus FH appears to act as a tumor suppressor. FH appears to play a role in a small percentage of nonsyndromic leiomyomas seen in white women.
Although work on elucidating the pathogenesis of the HLRCC syndrome continues, FH mutations appear to induce a change toward a hypoxic phenotype. Thus the hypothesized relationship between hypoxia and the pathogenesis of myomas appears linked to this subset of leiomyomas.
Currently the identification of women at higher risk for malignancy due to the HLRCC syndrome is an important clinical task, as is the identification of women whose families carry the breast cancer (BRCA) gene mutations. However, in the future, individualized therapy will likely be possible based on genotype and underlying predispositional genes.
Cowden Disease (MIM 158350 )
This disease is a type of hamartomatous polyposis syndrome characterized by leiomyomas as well as other benign tumors, including lipomas and hamartomas. It is autosomal dominant in inheritance and involves the candidate gene phosphatase and tensin homologue (PTEN) . Patients with Cowden disease are at increased risk for endometrial, thyroid, kidney, and colorectal cancers. Around 40% of women with Cowden disease are reported to have fibroids.
Finally, deletions of collagen genes COL4A5 and COL4A6 have also been shown to be associated with a familial syndrome known as diffuse leiomyomatosis with Alport syndrome and rarely with nonsyndromic fibroids. There may be a synergistic effect between the inactivation of COL4A5, COL4A6 , and insulin receptor substrate 4 (IRS4) underlying the pathogenesis of Alport syndrome.
Other Influences
Epidermal growth factor (EFG) is a growth factor mitogenic for smooth muscle cells. EGF mRNA is upregulated in leiomyomas only in the secretory phase of the cycle. Receptor levels appear to be similar in leiomyomas and myometrium. The latest work concentrates on the role of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase–derived reactive oxygen species (ROS) for signaling EGF and the platelet-derived growth factor (PDGF) signaling pathway, leading to myomatous cell proliferation.
Heparin-binding growth factors are important biologic regulators in leiomyomas since they can be secreted and bound to the reservoir of heparin sulfate proteoglycans filling the leiomyomatous ECM. Heparin-binding epidermal growth factor (HBEGF), vascular endothelial growth factor (VEGF), PDGF, hepatoma-derived growth factor (HDGF), and the previously described basic FGF are all found in myomas. Many have also been documented to be stored in the ECM.
Insulin-like growth factors (IGFs) can act as smooth muscle cell mitogens and were originally shown to have greater binding to leiomyomas as compared with myometrium. However, assessment of mRNA levels suggests that gene expression has differed among studies. Later studies suggested specific modulation of the IGF-binding proteins. Recent work has demonstrated the role of activation of tyrosine kinases and especially the IGF-1 signaling pathway in fibroids. Regulation of these factors following GnRH-agonist treatment has also been reported. There may also be increased prevalence of leiomyomas in women with acromegaly.
Prolactin also appears to play an important role in myoma pathogenesis. In vitro studies suggest that it is mitogenic for leiomyoma and myometrial smooth muscle cells and that the prolactin receptor is present in these tissues, setting up an autocrine or local endocrine system. Additionally, agents that appear to cause clinical regression of uterine leiomyomas also appear to decrease prolactin production in vitro.
The resident immune cells also appear to influence leiomyoma biology. Mast cells have been implicated in leiomyoma pathobiology, given that they are generally uniformly distributed in myometrium but highly variable in leiomyomas. Recent work has suggested a correlation of mast cell number with vasculature. A number of cytokines have also been shown to be differentially regulated in leiomyomas and myometrium. Interleukin 8 (IL8) has decreased expression of both the ligand and its receptor in myometrium compared to leiomyomas. The functional significance of this is shown by the fact that neutralizing antibody to IL8 decreases cellular proliferation in vitro. Monocyte chemotactic protein-1 (mcp1) is largely undetectable in normal samples of leiomyoma and myometrium but is increased significantly following GnRH-agonist therapy.
Wnt 7a , the human homologue of the wingless Drosophila genes involved in anteroposterior (AP) axis formation and smooth muscle cell patterning, appears to be suppressed in leiomyomas as compared with normal myometrium and to be inversely related to ER-α expression. In contrast, secreted frizzled related protein 1 (sFRP1), a modulator of Wnt signaling, is increased in leiomyomas (particularly in the late proliferative phase) and increased by estradiol treatment and hypoxia. HOX gene expression does not appear to differ between leiomyomas and myometrium. The mRNA for protooncogenes cfos and cjun is also overexpressed in leiomyomas compared with normal myometrium.
Parathyroid hormone–related peptide (PTHrP) mRNA is also overexpressed in leiomyomas compared with normal myometrium. Serum overexpression of this protein originating from a fibroid simulating the hypercalcemia of malignancy has been reported in the literature.
Micro-RNAs
Micro-RNAs (mi-RNAs) are small noncoding RNAs that generally inhibit gene expression and appear to have a key role in the pathogenesis of leiomyomas. Although early studies showed differential expression of specific miRNAs between leiomyomas and normal myometrium and an association of key miRNAs with leiomyoma size and patient race, more recent studies have started to define the key regulatory pathways that are influenced by mi-RNAs.
Principles of Treatment
- ◆
An initial assessment of whether bleeding, bulk-related symptoms, or both are prompting therapy helps to guide appropriate therapeutic options. Additionally, desire for future fertility can further refine the available options.
- ◆
Surgical therapies primarily include hysterectomy and myomectomy. Both options can be offered using various surgical approaches (open vs. laparoscopic vs. robot assisted).
- ◆
Uterine artery embolization is being used increasingly as the first-line alternative to hysterectomy for women with bulk-related symptoms and no desire for future pregnancy.
- ◆
MRI guided focused ultrasound surgery provides a noninvasive FDA approved ablation method for uterine leiomyomas.
- ◆
Medical therapies that include oral contraceptives, progestins, nonsteroidal antiinflammatory drugs, antifibrinolytic agents, and progestin-loaded IUDs are all used for heavy menstrual bleeding associated with leiomyomas. There is, however, a lack of randomized trials that demonstrate their effectiveness in the medical management of fibroids.
- ◆
Progesterone receptor modulators like Ulipristal acetate have shown promising results from European reports.
- ◆
Other therapeutic agents like gonadotropin-releasing hormone agonists and antagonists and androgenic compounds also have a role in medical management of uterine fibroids.
Uterine leiomyomas do not always necessitate treatment. In general, expectant management is appropriate until the woman develops enough symptoms that she requests treatment. The US Agency for Healthcare Research and Quality on comparative management of fibroids has noted the lack of published data that examines the effectiveness of treatment strategies. There are two important caveats to this generalization. First, although bleeding symptoms are usually evident, bulk symptoms can be insidious in their onset and are often attributed to other processes such as aging. An initial assessment of whether bleeding, bulk-related symptoms, or both are prompting therapy helps to guide appropriate therapeutic options. Women not electing therapy cannot reflexively be termed asymptomatic; they may have substantial symptoms but view the therapies they are offered as worse than the disease. As a second step, assessing the patient’s desire regarding reproduction helps refine the available options. In general women with complaints of heavy menstrual bleeding (HMB) alone tend to have more options for therapy (e.g., endometrial ablation, hysteroscopic myomectomy, and hormonal therapy including a progestin-containing intrauterine device [IUD]) than women with concurrent bulk-related symptomatology.
Finally, menopause can be a cure for women with myomas. Clearly HMB ceases with the onset of menopause. However, not all women have enough volume reduction to alleviate their symptoms. In addition, bleeding symptoms may continue for women who elect postmenopausal HRT, and studies have suggested that myomas continue to grow in women who take HRT.
Surgical Therapies
Hysterectomy provides the only cure for fibroids and will remain a viable treatment option for the near term. In addition, short-term outcome studies suggest that women with myomas experience an improved quality of life following hysterectomy. Hysterectomy also eliminates concomitant conditions including adenomyosis, endometrial polyps, and abnormal pap smears. Observational data suggest that women who have undergone hysterectomy have an improved quality of life over the next 1 to 10 years. Unlike the case in hysterectomy for endometriosis, the ovaries can be retained without losing therapeutic efficacy. Generally women weigh the risk of menopausal symptoms against the risk of ovarian tumors in making this decision. The attention paid to the ovary’s production of androgens in the postmenopausal period and their possible importance in mood and libido appears to be leading to an increase in the number of women who retain their ovaries even if they are perimenopausal. The fact that hysterectomy, even without oophorectomy, decreases the risk of ovarian cancer may also affect decision making on this issue. Laparoscopic rather than open hysterectomy, if possible, should be a goal if this treatment modality is chosen. As robot assistance has shown a reduction in the likelihood of conversion to laparotomy at the time of hysterectomy, there may be hidden potential in this modality compared with traditional aggressive surgery. However, this benefit must be weighed against the risk of dissemination of undiagnosed cancer when power morcellation is used for specimen removal, given concern regarding peritoneal dissemination and its effect on survival. Although the magnitude of this risk is debated, recent guidance from the US Food and Drug Administration (FDA) recommends limiting the use of power morcellation to hysterectomy in premenopausal women who are not candidates for en bloc resection and counseling all women about the risks of power morcellation. Despite definitive treatment, hysterectomy alone (without oophorectomy) has been linked to an increased incidence of cardiovascular morbidity, prolapse, and worsening cognitive function, including Alzheimer and Parkinson diseases. These long-term adverse outcomes should be kept in mind in recommending hysterectomy as a surgical therapy.
Finally, the use of supracervical or subtotal hysterectomy in women with leiomyomas is debated. The fact that 7% of women in an unselected population have cyclic bleeding following this type of hysterectomy deserves further study to see whether these women had bleeding complaints and/or fibroids prior to hysterectomy. Women may also run the theoretical risk of forming cervical fibroids following supracervical hysterectomy. Finally, accumulating data suggest that, at least in the short term, sexual functioning is not improved with supracervical hysterectomy.
Nonetheless, as women seek less invasive options and the health care system seeks less costly ones, alternatives to hysterectomy will become more widely used. All surgical alternatives to hysterectomy, however, share the risk of new myoma formation (what is commonly but incorrectly termed fibroid recurrence ). Unlike the similar phenomenon after surgery in malignant disease, this is unlikely to be persistence of the same tumor but instead growth of additional tumors that may have been missed, not treated, resistant to treatment, or not yet present at the time of initial therapy. Thus, following a variety of techniques including abdominal myomectomy and UAE, the risk of subsequent procedures is significant.
Since the 1930s, abdominal myomectomy ( ) has been the traditional alternative to hysterectomy because it preserves the uterus and allows childbearing. However, open myomectomy does have morbidity similar to that of hysterectomy and poses a significant risk of subsequent surgery. Myomectomies are now increasingly being performed laparoscopically, with or without robotic assistance. Rates of conversion to laparotomy have been reported to be as low as 2% after laparoscopic myomectomy, and the procedure has shown fewer complications as compared with open myomectomy. Patients undergoing traditional myomectomy via laparotomy, compared with robot-assisted myomectomy, had more estimated blood loss and a longer length of stay in the hospital. When outcomes from robot-assisted laparoscopic myomectomy, standard laparoscopic myomectomy, and open myomectomy were compared, patients with laparoscopic and robot-assisted procedures had similar blood loss and length of stay, both of which were reduced compared with the open procedure. Short-term surgical outcomes are similar after robot-assisted myomectomy and standard laparoscopic myomectomy. Reports have shown increased operating time with robotic procedures, but the rate of complications is low compared with open surgery. However, recent safety concerns regarding the use of power morcellation and the need for having a larger abdominal wall incision for the delivery of surgical specimens intact has made conventional abdominal myomectomy relevant again. Using single-port laparoscopy or laparoendoscopic single-site surgery (LESS) has led to success for myomas; however, since this is the newest form of laparoscopic innovation, data are limited.
Abdominal myomectomy permits healthy pregnancies after surgery and pregnancy rates have been reported to be in the range of 50% to 60%. Uterine rupture following myomectomy is very rare, at 0.5% to 1%; it is suggested that this may be related to surgical technique. The common clinical practice of counseling women who have had a myomectomy with a transmural uterine incision to undergo an elective cesarean section is based on this risk of uterine rupture following classical cesarean delivery. However, there is no evidence they are analogous situations. There is enough evidence to question the rationale for the conventional practice and recommendation of cesarean delivery after myomectomy, even if the endometrial cavity has been breached.
Although laparoscopic myomectomy involves much smaller incisions and a quicker recovery, it does require a surgeon skilled in laparoscopic suturing, and not all women have the number and size of fibroids amenable to this technique. Whereas updated series suggest that the risk of uterine rupture is low following laparoscopic myomectomy, rare cases continue to be reported. Because these uterine ruptures typically occur remote from term, appropriate counseling for patients contemplating pregnancy is important, especially if devascularization with cautery occurs intraoperatively. Data are very limited on obstetric outcomes following robot-assisted myomectomy, but the few reports that are available have been reassuring.
Myolysis is a variation on the technique of laparoscopic myomectomy in which the leiomyoma tissue is coagulated rather than removed. Although this technique is easier to master than laparoscopic morcellation or suturing, localized destruction without repair may also increase the chance of uterine rupture and adhesion formation. A laparoscopically deployed radiofrequency ablation device for fibroids is now approved by the FDA, and concomitant use of intraperitoneal ultrasound diagnosis with this technique can optimize the detection of fibroids. In a single randomized trial, radiofrequency ablation resulted in a shorter length of stay and less blood loss compared with laparoscopic myomectomy.
For women with submucous myomas, the use of hysteroscopic myomectomy ( ) has distinct advantages. With their accessible location, type 0 and 1 (European Society of Hysteroscopy classification) myomas can be resected with an intrauterine operative endoscope with good long-term results. Although this procedure requires a highly skilled practitioner, it can be done as outpatient surgery, often with a regional or local anesthetic and sedation that eases recuperation. Symptomatic relief is good, with fewer than 16% of women in one large series who were treated for menorrhagia reporting second surgeries after 9 years. Fertility rates after hysteroscopic myomectomy appear excellent, and there have been no case reports of uterine rupture after uncomplicated hysteroscopic myomectomy.
For women who have completed childbearing and for whom bleeding is the primary problem, endometrial ablation, either alone or in combination with hysteroscopic myomectomy, may give relief with minimal invasiveness. Increasingly, a levonorgestrel IUD can be used to implement a “reversible endometrial ablation.” In addition to providing effective control of bleeding, it provides contraception for women in this premenopausal age group, in contrast to surgical endometrial ablation, which leaves women at risk for tubal and cervical and cervical ectopic pregnancies.
Uterine Artery Embolization
Transcatheter arterial embolization has long been an effective percutaneous technique for controlling bleeding in a wide variety of disorders. Its use for the treatment of leiomyomas was first reported in 1995. Although initially used as an alternative for patients who were felt to be poor surgical candidates, the resolution of symptoms in the initial cohort encouraged the use of this technique as a primary therapy.
UAE is increasingly the first-line alternative to hysterectomy for women with bulk-related symptoms and no desire for future pregnancy ; it has been recommended by the American College of Obstetrics and Gynecology (ACOG) as a safe and effective form of uterus-preserving treatment for fibroids. The procedure can be completed in 98% to 100% of patients. It provides a decrease in HMB in 85% to 94%, improves dysmenorrhea in 77% to 79%, and improves bulk-related symptoms in 60% to 96% of women, whereas mean uterine volume reduction is seen in 30% to 60% of women. The largest reported series of 1278 patients 3 years after UAE reported improvement of symptoms and quality of life in 95% of women. A rate of amenorrhea of 29% after procedures for fibroids was seen in 14.4% of women (hysterectomy 9.8%, myomectomy 2.8%, repeat UAE 1.8%). In other reports, the need for a second form of intervention after UAE was noted to be somewhere between 9% and 32%. Series with follow-up over 5 years report higher rates of secondary intervention. Although 75% of women continue to report normal or improved bleeding, approximately 20% report having undergone a second procedure in the form of hysterectomy, myomectomy, or a repeat UAE procedure. Previously thought of as a contraindication, fibroids greater than 10 cm in diameter have been shown to be treated successfully with UAE.
A series of randomized clinical trials and systematic reviews have compared UAE to surgery (hysterectomy and myomectomy). These reports have noted a shorter duration of hospitalization at initial treatment for patients undergoing UAE compared with surgery. Additionally, patients after UAE had similar improvement of symptoms at 5 years as compared with surgery.
It is relatively common for submucous myomas to be expelled vaginally after treatment ; thus most hysteroscopically resectable fibroids are still approached surgically. Similarly, the presence of pedunculated subserosal fibroids has historically been considered a relative contraindication to UAE therapy, although no cases of intraperitoneal expulsion have been reported. There are some data suggesting that UAE can still be performed in cases of pedunculated fibroids. A laparoscopic approach is generally preferred in such patients. Studies also suggest that high T2 signals predict greater volume reduction and complete devascularization predicts outcome at 5 years.
Most patients develop significant pain and some vaginal discharge following the procedure and usually require intravenous narcotics for pain control. Postembolization syndrome, defined as the combination of diffuse abdominal pain, mild fever, and mild leukocytosis, is common and can occur in 30% to 40% of patients; it gradually improves over a week. Serious complications after UAE are rare but are more likely when there is a single large leiomyoma. Since preoperative imaging and endometrial biopsies cannot distinguish between benign leiomyomas and myometrial neoplasms of low- or high-grade malignancy (e.g., leiomyosarcoma), a misdiagnosed sarcoma may be treated with UAE rather than surgical resection. Fortunately, given the rarity of these tumors, a review of the literature has noted only six cases (out of 8084 procedures) where women with uterine sarcomas underwent UAE. In such cases UAE did not seem to spread the disease, but it impaired prognosis by delaying diagnosis. Because the risk of leiomyosarcoma increases with age, UAE is not recommended for menopausal women with new onset or worsening symptoms related to presumed leiomyomas.
It is important to assess the effects of UAE on the ability of women to become pregnant subsequently or to carry a pregnancy to term. Thus far, there are a number of reported patients who became pregnant following UAE. Although no difference in intrapartum adverse outcomes was noted when patients after UAE were compared with patients after myomectomy, cesarean delivery rate was increased in both groups. Pregnancy clearly can and does occur following UAE with success rates reported from 20% to 60% ; however, the risk of spontaneous miscarriage in these women has been noted to be higher compared with women with fibroids who had not undergone UAE treatment. There are two major areas of caution for women wishing to optimize their fertility potential: effects on ovarian function and myometrial wall integrity. Because of increased placental implantation issues after UAE, close monitoring of placental status has been recommended. In one series, approximately 13% of women who were nulliparous and had no other risk factors had some form of placenta previa or accreta.
The early data on ovarian damage used amenorrhea as the indicator of perturbed ovarian function. It is clear that amenorrhea risk is age-related, with women under age 40 years having a 3% risk and women over age 50 years a 41% risk. Newer studies evaluate FSH and anti-müllerian hormone (AMH) levels to detect more subtle damage. Although studies with short-term outcomes may not show impact, most have shown an age-dependent risk, with risk increasing more after age 45 years. Compared with hysterectomy, UAE causes more of a decrement in AMH levels after a 2-year follow-up. However, it is often overlooked that surgery also has an adverse impact on ovarian reserve. A putative mechanism is suggested in another report indicating significantly increased FSH levels following UAE in patients with uteroovarian vascular anastamoses.
Focused Ultrasound—Noninvasive Treatment
MRgFUS provides a noninvasive ablation method that has been FDA-approved for the treatment of uterine fibroids since 2004. Although it was pioneered for the treatment of uterine fibroids, this modality can be used to treat multiple diseases and may prove to be the next step in surgical innovation from open to minimally invasive to noninvasive approaches.
Just as a laser amplifies and collates light into a therapeutic modality, focused ultrasound (FUS) can deliver a large amount of energy to target tissues in a noninvasive way. Treatment is accomplished by placing a transducer against the abdomen and targeting an intraabdominal myoma without breaching the skin. The intensity of FUS used for treatment is significantly higher than that used in diagnostic ultrasound and can rapidly increase temperature at the focal point in excess of 70°C. At this temperature, coagulative necrosis will occur. The procedure is performed under conscious sedation. After T2-weighted images are obtained (to develop a treatment plan), FUS sonications are targeted at the fibroid while MRI provides continuous thermal feedback.
It is not typically size alone that limits treatment, but size, vascularity, access, and other factors also play major roles. The strongest predictor of MRgFUS success is the nonperfused volume (NPV) or the area devascularized by treatment that is nonperfusing in posttreatment gadolinium imaging. In one of the pivotal trials, 71% of women reached a target symptom reduction score on the uterine fibroid symptom and quality-of-life (UFS-QOL) questionnaire at 6 months, and 51% maintained this at 12 months. In newer studies, as higher NPV rates were achieved, quality of life improved. Volume reduction is again proportional to NPV achieved after treatment. Up to 40% reduction in volume has been noted in fibroids after MRgFUS treatment. When low NPV (of around 26%) was achieved, 28% of patients ended up with an alternative form of treatment at 12 months after MRgFUS. Newer reports with higher NPV achieved (around 60% to 80%) led to failure of treatment in only 8% to 12% of women.
Complications from MRgFUS are very rare. The most common complication is skin burns. These can occur because of poor coupling or abdominal scars on the patient that are encountered within the FUS beam pathway. An early trial reported around 5% risk of burns. Only one case of extensive skin burns has been reported. Use of acoustic reflectors (like cork or foam) and an energy-blocking patch on scars has shown success in preventing burns.
A parallel enrollment trial compared MRgFUS with abdominal hysterectomy and recorded significant clinical complications on the short-form health survey (SF-36) at 1, 3, and 6 months. Clinically significant complications were lower in the MRgFUS group, with SF-36 scores improved in both arms at 6 months. However, scores were significantly better in the hysterectomy group. There is currently a randomized clinical trial funded by the National Institutes of Health (NIH) comparing MRgFUS with UAE (NCT00995878, clinicaltials.gov ), which should provide important information. Studies have also shown MRgFUS to be in the range of currently accepted criteria for cost-effectiveness.
Subsequent pregnancy-related complications after MRgFUS treatment are minimal; it is possible that MRgFUS could be the treatment of choice for patients desiring future fertility. Around 100 cases of pregnancies after MRgFUS have shown no impact on the rate of miscarriage or other obstetric parameters. In the only published series, 45 pregnancies in 51 women were reported. A live birth rate of 41%, mean birth weight of 3.3 kg, spontaneous abortion rate of 28%, and term delivery rate of 93% were noted. At least one patient with a fibroid impinging on the cavity and unexplained infertility conceived spontaneously after MRgFUS treatment.
Ultrasound-guided FUS (typically referred to as high-intensity focused ultrasound [HIFU]) has been used to treat several solid tumors outside the United States. Since feasibility studies showed promising results for the use of ultrasound-guided HIFU for uterine fibroids, larger studies have been reported. These reports have not only demonstrated safety and efficacy of ultrasound guided-HIFU for treatment of uterine fibroids, but one report also showed safety of pregnancy within 1 year of treatment.
Medical Therapies
There is a lack of randomized trials to demonstrate effectiveness of medical management of fibroids. Oral contraceptive pills (OCPs), progestins, nonsteroidal antiinflammatory drugs (NSAIDs), antifibrinolytic agents, androgenic compounds, and progestin-loaded IUDs, all of which are useful in the treatment of idiopathic HMB, have not been studied with leiomyoma-related bleeding. Nonetheless, they are widely used and are likely effective in at least a subset of women with fibroids. A systematic review reports that in trials where women were assigned to medical therapy, at least 60% underwent surgery by 2 years.
Progesterone Modulators
Clinical data regarding the efficacy of progesterone modulators have confirmed the importance of progesterone in myoma biology. A concern of paramount importance in using progesterone receptor modulators (PRMs) is the potentially increased risk of endometrial hyperplasia or cancer. Pathologists have, however, shown a unique histologic pattern in patients on these medications : simple endometrial cystic dilation. These PRM-associated endometrial changes do not have the typical molecular signatures of malignant progression ; however, long-term data are still lacking.
Ulipristal acetate is a PRM that is approved for both preoperative and intermittent repeated courses of therapy in the European Union under Conformité Européene marking; it is also undergoing further evaluation in other countries. Ulipristal has been compared with placebo at 5 or 10 mg once daily for 13 weeks in a randomized controlled trial. Treatment with this medication has resulted in the resolution of HMB and a significant reduction in fibroid volume in women with uteri of less than 16 weeks’ gestational size. No findings of endometrial hyperplasia were associated with its use. In another noninferiority trial, ulipristal was compared with GnRH agonists, at 5 or 10 mg a day for 13 weeks. Both arms had comparable rates of resolution of HMB. The ulipristal arm had more rapid induction of ammenorrhea; however, smaller reduction in myoma size was noted in this group compared to GnRH agonist arm. Data from the supplementary appendix of these studies suggest that PRMs provide more prolonged volume reduction after treatment is discontinued compared with GnRH agonists. Endometrial biopsies from women on ulipristal revealed a carryover effect of this medication after 3 months of therapy, which lasted up to 6 months. The use of ulipristal use has been reported for up to four 3-month courses separated by a spontaneous menstrual flow upon withdrawal or one brought on by norethindrone acetate. No cases of endometrial hyperplasia or cancer were noted in either group. A sustained long-term effect of 3 to 12 months of treatment with ulipristal on fibroid volume reduction and menstrual bleeding has been suggested from a series of 18 successful pregnancies occurring for up to 6 years following completion of therapy. A longer duration of treatment has not yet been evaluated. However, given these reassuring data, women with symptomatic fibroids may have the option of a unique intermittent therapy with this medication. Unfortunately the available FDA-approved formulation (30-mg tablet) makes off-label use of ulipristol for fibroids problematic in the United States.
Mifepristone (RU486) is a steroidal derivative of norethindrone; it acts primarily as an antiprogestin. It has, at high doses (50 mg/day), shown a reduction in myoma size comparable to that produced by GnRH agonists. Thus the clinical benefit was equivalent to that seen with GnRH agonists, yet follicular levels of estradiol were maintained to support bone mass and provide symptomatic relief. Identical results were found with a reduction in dose to 25 mg/day ; however, with a dose of 5 mg/day, although acyclicity was maintained, volume reduction was reduced to 30%. More recent studies suggest that daily doses of 5 and 10 mg produce volume reduction equivalent to that elicited by the higher doses, but they produced amenorrhea in only 60% to 65% of women while also resulting in less menstrual blood loss. Nonetheless, this regimen provides significant symptomatic improvement. Mifepristone has mild side effects compared with GnRH agonists. Adverse effects included mild and infrequent hot flashes in approximately 20% of patients during the first month of treatment only with higher doses; however, more persistent symptoms appeared in another study. Mifepristone is not approved for use by the FDA. A major impediment to off-label use is the fact that the currently available dose of RU486 is not appropriate (200 mg once for pregnancy termination vs. 5 to 10 mg/day for fibroid treatment for 6 months).
A unique pattern of endometrial changes has been observed following treatment with PRMs termed progesterone receptor modulator–associated endometrial changes (PACESs). The clinician should be aware of these cystic glandular changes causing thickening of the endometrium. True endometrial hyperplasia and atypical hyperplasia have not been observed following PRM therapy, and no woman has developed endometrial carcinoma.
Gonadotropin-Releasing Hormone Agonists
Since the action of native GnRH depends on its pulsatile release, the effects of GnRH agonists depend upon their continuous presence. They first cause a time-limited increase in gonadotropin release, termed the flare . This subsequently leads to receptor downregulation, followed 1 to 3 weeks later by a hypogonadotropic hypogonadal state. It is this downregulated phase that is useful clinically in the treatment of myomas. Alterations of the GnRH molecule, typically at positions 6 and 10 of the two glycine (G) residues, produce a longer half-life and are more useful for clinical purposes.
Many studies have focused on the efficacy and benefits of treatment with a GnRH agonist in women with fibroids. Most women experience a substantial reduction in mean uterine volume of 30% to 60% over 3 to 6 months of therapy. However, there is a wide range of responsiveness, with rare individuals achieving no volume reduction. Both the estradiol levels at week 12 and the woman’s weight are correlated with the degree of uterine shrinkage.
The other primary benefit of treatment with a GnRH agonist is the induction of amenorrhea. Menses typically return 4 to 10 weeks following the end of treatment. Fibroid and uterine volume usually returns to pretreatment size within 3 to 4 months. The rapid return of ovarian steroidogenesis, coupled with an increase in the concentration of ERs in fibroids recently treated with GnRH agonists, may contribute to the rapid regrowth of these tumors.
GnRH agonists can have significant adverse effects, the most important of which is bone loss. Six months of GnRH-agonist treatment can cause a 6% loss in trabecular bone, not all of which is reversible on discontinuation of therapy. Symptomatic side effects of GnRH-agonist therapy are common. Hot flashes are universal in women undergoing treatment. Other less common side effects include sleep disturbance, irregular vaginal bleeding, vaginal dryness, headache, depression, hair loss, and musculoskeletal symptoms.
Because of the concerns regarding bone loss with GnRH agonists, clinical use of these drugs is typically confined to use as preoperative therapy or in women for whom a short period of treatment will be effective. The GnRH agonist leuprolide is FDA-approved in conjunction with iron administration for the presurgical treatment of uterine fibroids and to correct anemia. This is the only medical treatment approved by the FDA for the treatment of this disease.
Administration prior to either hysterectomy or myomectomy is the most common current use of these agents. Length of therapy varies from 1 to 6 months, depending on the surgical and hematologic goals and the planned procedure. The amenorrhea induced by GnRH therapy leads to improved hemoglobin concentrations, which permits women who are anemic to correct this problem and potentially to donate their own blood for transfusion. Preoperative GnRH therapy has also been shown to reduce intraoperative blood loss significantly. Although current guidelines from ACOG suggest that the use of GnRH agonists is beneficial preoperatively, they also state that for each individual the benefit must be weighed against the cost and the side effects.
Gonadotropin-Releasing Hormone Agonist Therapy With an Estrogen/Progestin Add-Back Regimen
For many women, 3 to 6 months of symptomatic relief from leiomyomas will not allow them to avoid surgery but it does afford them the opportunity to prepare themselves optimally for an operation. Therefore the concept of adding additional therapy to minimize the side effects of prolonged therapy was developed, the so-called add-back regimen. The goal of this approach was to achieve a window of therapeutic efficacy during which side effects would be lessened or eliminated yet no regrowth of the myomas would occur.
Studies have used one of two treatment strategies: simultaneous and sequential administration. With simultaneous treatment regimens, both the GnRH agonist and the add-back regimen are started at the same time. In sequential treatment regimens, the GnRH-agonist is given alone for up to 6 months before steroid hormone treatment is added, reducing a period of hypoestrogenism before steroid add-back therapy is started. Studies have suggested that sequential treatment is superior for therapeutic efficacy in the treatment of fibroids.
Gonadotropin-Releasing Hormone Antagonists
GnRH antagonists have also been studied in the treatment of uterine leiomyomas. The lack of flare effect and rapid onset of action give them an advantage over GnRH agonists. Although they are not FDA-approved for this indication, they have several significant advantages over GnRH agonists. However, in the United States, these agents are marketed for use of ovulation induction, and long-term preparations are not available. This could make the treatment of fibroids with GnRH antagonists cumbersome.
Aromatase Inhibitors
Aromatase inhibitors have been shown to decrease symptoms from fibroids and to effect shrinkage in size when given to women that were pre- or perimenopausal. A randomized trial compared letrozole at 2.5 mg/day to triptorelin (3.75 mg/month) for 12 weeks in 70 women with a single fibroid 5 cm or greater size. A statistically significant volume reduction in myoma size was noted in the letrozole group versus the triptorelin arm (45% vs. 33%). Serum hormone levels were also significantly reduced in the triptorelin arm versus the letrozole group. Extensive data regarding the safety, efficacy, and cost-effectiveness of this class of medications as medical therapy for uterine fibroids are still lacking.
Selective Estrogen-Receptor Modulators
Selective estrogen-receptor modulators (SERMs), which exhibit tissue-specific agonist or antagonist activity, appear to work better in animal models of myomas than in clinical trials. Studies have examined both tamoxifen and raloxifene. Clomiphene has not been studied and has been reported to cause growth of a myoma in a single case report. Despite promising results in animal models, clinical studies have had less impressive results. However, in premenopausal women, raloxifene alone or when combined with GnRH agonists demonstrated little efficacy despite use at three times the conventional dose.
Androgens
Danazol, an androgenic steroid most commonly used for the medical treatment of endometriosis, can be used to induce amenorrhea to control anemia due to fibroid-related HMB. A second androgenic steroid, gestrinone, has been shown to cause volume reduction and amenorrhea in women with myomas. A great advantage of this drug is that, after it is discontinued, there is a carryover effect like that of PRMs; in one study, 89% of the women maintained a decreased uterine volume 18 months after cessation of therapy. Unfortunately gestrinone is not available in the United States. However, androgenic side effects including acne, hirsutism, and irreversible deepening of the voice have limited its clinical use.
Future Directions for Medical Therapy
Growth Factor–Directed Treatments
Particular factors that appear to be relevant to leiomyoma biology include basic fibroblast growth factor (bFGF), which is angiogenic; TGF-β, which is fibrotic; and insulin-like growth factors I and II (IGF-I and -II), which mediate the effects of growth hormone (GH). These molecules, as well as other growth factors, are likely to be targets for leiomyoma treatment in the future.
Growth Hormone- and Insulin-Like Growth Factor–Directed Therapy
Both GH and the IGFs appear to have metabolic effects on uterine leiomyomas and the surrounding myometrium. Because women with acromegaly (an excess of GH) have a high incidence of leiomyomas, researchers decided to test the hypothesis that interfering with the GH axis might work as a treatment for leiomyomas. Lanreotide (a long-acting somatostatin analogue) has been used in seven premenopausal women with uterine myomas in a pilot study in Italy. Over the 3 months of treatment, both uterine volume and the volume of the largest leiomyoma were significantly reduced by 24% and 42%, respectively. Three months following the discontinuation of therapy, there was some regrowth, but a significant reduction in uterine volume persisted at 17% and 29%, respectively. Levels of estradiol were not affected by this treatment, although both plasma GH and IGF-I levels were significantly reduced, suggesting that additional pathologic modulators may be effective.
Antiangiogenic Therapies
There is significant evidence that the angiogenic factor bFGF and its type I receptor are important in the pathogenesis of leiomyoma-related bleeding. In a variety of systems, interferons (IFNs)-α or -β antagonize the effects of bFGF and have proven clinically useful in the treatment of a variety of vascular tumors. In vitro studies of leiomyomas demonstrate that IFN-α is an effective inhibitor of serum-stimulated and bFGF-stimulated DNA synthesis in both leiomyoma and normal myometrial cells as well as in endometrial cells. A case report also raises the possibility that IFNs may provide effective treatment for fibroids. A premenopausal woman who was treated with IFN-α for hepatitis C was noted to have significant shrinkage of a leiomyoma after 7 months of interferon therapy. Tranilast (N-[3′4′-dimethoxycinnamonyl] anthranilic acid [N-5′]), a drug currently used in the treatment of a variety of allergic conditions, has been shown in vitro to decrease leiomyoma cellular proliferation by arresting cells at the transition from G0 to G1 phase. Although this drug acts as an angiogenesis inhibitor, it also works as a mast cell stabilizer and a fibrosis inhibitor, which may have relevance for leiomyomas.
Current research also involves work on the role of retinoic acid, vitamin D, and green tea components in preventing fibroid formation.
Adenomyosis
- ◆
Adenomyosis is a very common benign uterine disease associated with increased parity.
- ◆
Although the pathophysiology of the disease process is not well understood, gonadal steroids and the physiology of the uterine junctional zone may play a key role in the disease process.
- ◆
Typically diagnosed by histology on posthysterectomy specimens, adenomyosis can now be diagnosed by transvaginal ultrasonography (TVS) and MRI.
- ◆
Hysterectomy is considered the definitive therapy; the role of uterus-sparing surgery is still undetermined.
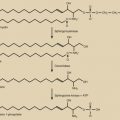
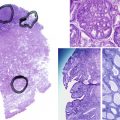
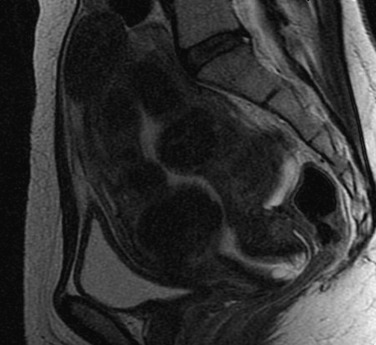
Adenomyosis, formerly termed endometriosis interna, is a benign uterine disease characterized by the presence of ectopic endometrial glands and stroma within the myometrium ( Fig. 26.3 ). Furthermore, the surrounding myometrium is usually altered to produce hypertrophy. Disease ranges from grossly visible nodules, termed adenomyomas , which can clinically resemble leiomyomas, to disease that is detectable only by microscopy. Definitions vary for the abnormal presence of glands within the stroma, with most settling on a definition of glands found one to three low-power fields from the endomyometrial junction. Clearly differences in definition will lead to differences in perceived rates of occurrence.
Classically, an adenomyotic uterus is termed boggy, globular, and symmetrically enlarged . However, this disease coexists with many other uterine conditions. One study has argued that adenomyosis is not indeed a true disease but a variant of the norm as women have had similar symptoms for hysterectomy with and without adenomyosis. Most women in this study were perimenopausal, which could have been a major selection bias.
Adenomyosis can affect around 20% to 65% of women, although the accuracy of these numbers can be questioned, since diagnosis can be made with certainty only by microscopic examination of the uterus typically after a hysterectomy. In another series of hysterectomies, adenomyosis appeared in about one quarter of all uterine specimens, but it is no more likely to coexist with symptomatic leiomyomas (23.3%) than with endometrial cancer (28.2%) or ovarian cancer (28.1%).
Unlike leiomyomas, adenomyosis is associated with increasing parity. It is estimated that at least 80% of women with this disorder are parous. However, this may be a confounding variable, since women with a history of multiple pregnancies may simply have had more indications and/or inclination to proceed to hysterectomy, during which the diagnosis could be made. Studies suggesting the presence of adenomyosis with imaging modalities rather than histopathology have suggested the presence of this disease process in adolescents as well. The California Teachers Study noted clinical differences in women with endometriosis and adenomyosis. Women with adenomyosis were older, had higher parity, early menarche, and shorter menstrual cycles, and were more obese compared with women with endometriosis. Another study compared women with fibroids and adenomyosis with women who had fibroids only. Women with both fibroids and adenomyosis had more pelvic pain and dysmenorrhea, higher parity, a history of previous uterine surgery and more clinical depression compared with those who had fibroids only. Women with histopathology-proven adenomyosis were more likely to have a history of previous uterine surgery in several reports. Data regarding smoking as a risk factor for adenomyosis are controversial.
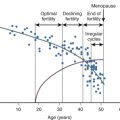
Clinically, adenomyosis has similarities to leiomyomas in that its peak incidence is in women from 40 to 50 years of age, with approximately 60% of women reporting AUB, chiefly HMB. The clinical presentation of adenomyosis is heterogeneous, with AUB (classified as AUB from adenomyosis, or AUB-A type of bleeding ; see section on AUB later in this chapter) and dysmenorrhea being the two most commonly reported symptoms. Abnormal distribution of thick and dilated vessels in the endometrium, particularly in the secretory phase of the menstrual cycle, is one explanation for heavy menstrual flow in such women. Dysmenorrhea, occurring in approximately one quarter of all cases, has been correlated with deep penetration and/or a high density of endometrial glands within the myometrium.
The most widely quoted hypothesis regarding the pathogenesis of adenomyosis is that invasion of the myometrium by the endometrium induces hypertrophy and hyperplasia of the myometrium. Proponents of this theory often quote the association of parity with adenomyosis to suggest that disruption of the layers of the uterus at the time of pregnancy and cesarean delivery may predispose to this condition. However, experimental evidence indicates instead that adenomyosis can be a metaplastic process or a developmental defect. First, adenomyosis has been diagnosed in a woman with Rokitansky–Kuster–Hauser syndrome who lacked eutopic endometrium. Additionally, studies comparing the molecular expression of growth factors show distinct differences between ectopic and eutopic endometrium. Factors that appear common to the pathogenesis of leiomyomas and adenomyosis include angiogenic factors such as bFGF, fibrotic factors including GM-CSF, the gonadotropin receptor LH, and resident immune cells. The efficacy of some conventional and investigational therapies may be mediated through these systems.
The junctional zone of the myometrium may also play a role in the disease process. This structurally distinct region appears as a dark band on T2-weighted MRI and separates the subendometrial myometrium from the outer myometrium. Ultrastructural changes and differential growth factor expression in this region may influence disease physiology.
Gonadal steroid hormones also play a role in the pathophysiology of adenomyosis. Adenomyotic implants express higher aromatase and estrone sulfatase activity, and also have polymorphisms in estrogen receptors (ERs). In vitro studies have shown normalization of aromatase activity by GnRH agonists and danazol, but there is a lack of data to show these effects in vivo. The role of estrogen and ERs in adenomyotic implants is further supported by the fact that endometrial hyperplasia was more prevalent in women with adenomyosis in one report. A murine model of adenomyosis also supports this, as early tamoxifen exposure in these mice led to the development of adenomyosis and abnormal myometrium.
Interestingly, another murine model of adenomyosis was developed by placing a graft of pituitary tissue in a uterine horn. Prolactin appears to be the key pathogenic agent in this model: the mice have elevated levels of plasma prolactin, and administration of bromocriptine prevents the development of adenomyosis. In this model, there does appear to be endometrial cell invasion due to degeneration of myometrial cells. Indirect exposure of the uterus due to hyperprolactinemia secondary to treatment with selective serotonin reuptake inhibitors (SSRIs) can also induce adenomyosis. This theory is further strengthened by work showing both clinical depression and antidepressant use to be increased in women with adenomyosis. A second model using the FORKO (follitropin receptor knockout) mouse suggests that the rising levels of FSH seen with aging may also play an important pathogenic role in this disease.
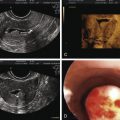
Although a definitive diagnosis of adenomyosis requires histology, imaging techniques are increasingly able to suggest the appropriate diagnosis. Both TVS and MRI, especially T2-weighted images, are used for this purpose. MRI is a better imaging modality for adenomyosis, but it is expensive. It can also differentiate very well between an adenomyoma and a fibroid. Visual evidence of adenomyosis with TVS includes (1) asymmetric thickening of the myometrium (with the posterior uterine wall typically thicker), (2) myometrial cysts, (3) linear striations radiating out from the endometrium, (4) loss of a clear endomyometrial border, and (5) increased myometrial heterogeneity. With MRI, adenomyosis can be diagnosed with accuracy when the maximal junctional zone thickness is 12 mm or greater; a maximal junctional zone thickness of 8 mm or less usually rules out adenomyosis. Although TVS is a less expensive imaging technique, it is known to be operator-dependent. Three-dimensional (3D) TVS can allow better visualization of the junctional zone compared with standard TVS and hence may be more advantageous. A review of 23 articles comparing the sensitivity and specificity MRI and TVS revealed both techniques to have similar sensitivity (0.72 for TVS and 0.77 for MRI) and specificity (0.81 for TVS and 0.89 for MRI). Computed tomography has no role in the diagnosis of adenomyosis, and needle biopsy should be reserved for cases where malignancy must be ruled out.
The only definitive treatment for adenomyosis is total hysterectomy. GnRH-agonist treatment has been shown to produce amenorrhea, a transient decrease in uterine size, and even in the ability to conceive. Other medical therapies include use of a levonorgestrel-releasing IUD ; there is also a single case report of a danazol containing IUD. Unfortunately resumption of pretreatment uterine size and recurrence of symptoms are usually documented within 6 months of cessation of therapy.
Data regarding conservative surgery (if an adenomyoma is present) are scarce. The absence of a surgical plane separating adenomyotic tissue from normal myometrium makes adenomyomectomy difficult. Furthermore, the consistency of the adenomyotic uterus is described as “woody,” and suturing is difficult in this environment. Adenomyomectomy has been reported to improve the symptoms of adenomyosis, and one study reports conservative surgery and GnRH medical therapy following treatment to be superior to surgery alone. Other reported techniques include endomyometrial ablation and laparoscopic myometrial electrocoagulation, which, on the basis of 3 years of follow-up data, appear to decrease symptoms in more than half of patients.
Both UAE and MRgFUS have been reported for the treatment of adenomyosis. Several studies show small case series with symptomatic adenomyosis diagnosed by MRI or TVS. A review article found that in 511 women from 15 studies, UAE led to improvement of symptoms in 75.5% of patients after a median follow-up of 26.9 months. In another report, after a median follow-up of 58 months, around 18% of women ended with a hysterectomy; however, 73% of women were completely asymptomatic. There has been limited experience with treatment outcomes following MRgFUS for adenomyosis. The largest study to date included 20 patients with a 6-month follow-up and indicated safe and effective MRgFUS therapy in all subjects enrolled. Another MRgFUS case reports a spontaneous pregnancy with full-term delivery after treatment. Ultrasound-guided high-intensity focused ultrasound ablation has also been studied in one report, wherein 78 patients with adenomyosis were enrolled. After a mean follow-up of 24 months, around 90% of the patients had complete relief of symptoms. Based on these findings, hysterectomy still appears to be the treatment of choice for women with significant symptoms who have completed childbearing. Exploration of uterine-sparing alternative treatments for symptomatic relief is warranted.
As imaging techniques get better, adenomyosis is being diagnosed with increasing frequency in women of reproductive age. Data on these women are limited to small case series. Evidence shows a link between adenomyosis and infertility ; however, there is no direct link. There is currently no consensus regarding the impact of adenomyosis on embryo implantation potential. Although some studies have identified alterations in the endometrial milieu in adenomyosis patients that may impact implantation, others have shown no such impairment. Increased risk of preterm birth and preterm premature rupture of membranes in women with adenomyosis (diagnosed with TVS or MRI) is noted in certain reports.
Endometrial Polyps
- ◆
Endometrial polyps arise from the endometrium, causing AUB.
- ◆
Most polyps are benign, although malignant transformation has been noted in certain high-risk women.
- ◆
Polyps can be diagnosed by imaging in the form of TVS, saline infusion sonograms, hysterosalpingograms, or hysteroscopy.
- ◆
Polypectomy under hysteroscopic guidance is the recommended treatment.
Endometrial polyps, as their name suggests, arise from the endometrial layer of the uterus. They are characterized by glandular proliferation surrounding a central core of prominent blood vessels in the stroma. Polyps are associated with AUB, particularly spotting and irregular bleeding, but the underlying mechanism has not been articulated. Several mechanisms are thought to play a role in development of endometrial polyps. These include overexpression of endometrial aromatase activity ; monoclonal endometrial hyperplasia ; genetic factors, particularly cytogenetic rearrangements of chromosomes 6p, 12q, and 7q ; and alterations in endometrial levels of matrix metalloproteinases and cytokines. Other work has also shown the role of increased TGF-β, VEGF, and bcl-2 in the pathogenesis of endometrial polyps. Historically polyps have been known to have ERs, though current literature suggests the presence of both ERs and PRs. It is suggested that both estrogen and progesterone contribute to the elongation of endometrial glands, stroma, and blood vessels to give them a characteristic appearance. Progestins also have an antiproliferative function on polyps, as do androgens; however, data suggest that testosterone does not substitute for progestational activity for endometrial polyps.
The estimated prevalence of polyps varies widely from 7.8% to 34.9% depending on the definition and diagnostic modality used. The reported prevalence of polyps is as low as 0.9% in women less than 30 years of age and increases with age. Risk factors for development of endometrial polyps include obesity, hypertension, diabetes, and advancing age, especially postmenopausal status. At least one paper has challenged these classical risk factors and has shown only age to be a statistically significant risk factor. Women with Lynch and Cowden syndromes may also have an increased incidence of endometrial polyps compared with the general population, possibly accompanied by an increased risk of associated endometrial cancer.
Tamoxifen use in postmenopausal women can lead to the development of polyps in around 2% to 36% of women. Tamoxifen-induced polyps may be large in number and size and also show unique molecular alterations. The levonorgestrel-loaded IUD has been shown to reduce tamoxifen-induced polyp development in patients being treated for breast cancer who were followed for a year. Data regarding a relationship between endometrial polyps and postmenopausal hormone therapy are contradictory.
The most frequent symptom in women with endometrial polyps is AUB, or AUB-P in the updated FIGO classification, and is reported in 50% of symptomatic cases. Conversely, of women who have abnormal bleeding, approximately 30% have evidence of endometrial polyps. Although abnormal bleeding is the most common clinical representation, most polyps tend to remain symptom free and are an incidental finding on imaging. The size, number, and location of polyps do not correlate with clinical symptomatology.
Most polyps are benign and malignant transformation can occur in 0% to 12.9%. Microsatellite instability has been noted in patients with multiple polyps or polyps with hyperplasia. A meta-analysis revealed that the prevalence of malignant polyps was higher in postmenopausal women compared with women in their reproductive years (5.42% vs. 1.7%) and that malignant polyps were more likely to bleed than nonmalignant ones. Risk factors associated with malignant endometrial polyps include age greater than 60 years, polyp size greater than 1.5 cm in length, menopausal status, having polycystic ovarian syndrome, and abnormal bleeding.
Endometrial polyps are still diagnosed following dilation and curettage (D&C) or hysterectomy, although new methods of diagnosis and treatment are being used more frequently. Increasingly, saline-infusion sonography (SIS), (also termed sonohysterography or, less commonly, office hysteroscopy ) is used to diagnose polyps ( Fig. 26.4A to C ). A pipelle biopsy has a positive predictive value of 56.3% for polyps and is more useful in postmenopausal women compared with premenopausal women. Polyps can also be diagnosed by hysterosalpingography if the uterine cavity is also visualized. Newer 3D ultrasound probes provide more accurate visualization between the endometrium and myometrium at the fundus and the cornual angles, hence improving the diagnostic accuracy compared with standard ultrasound. Another review reported a similar performance of TVS, saline infusion sonography, and hysteroscopy for the detection of endometrial polyps. Both hysteroscopy and SIS give a better sense of the endometrial cavity than TVS; however, SIS has the added advantage of providing the examiner with information regarding the adnexa and myometrium. Newer reports demonstrate the usefulness of 3D-SIS in the identification of endometrial polyps.
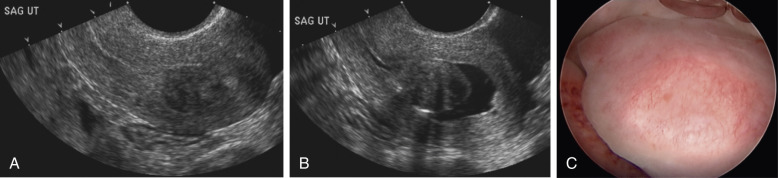
Medical management of polyps has a limited role. There are data regarding the use of a levonorgestrel-releasing intrauterine system (LNG-IUS) for the prevention of polyp formation in women being treated for breast cancer with tamoxifen. Observing asymptomatic polyps can be an option in low-risk individuals. When left untreated, asymptomatic polyps can regress spontaneously. In one trial, 27% of lesions resolved within 1 year of follow-up in premenopausal patients. Polyps with a mean length of 1.5 cm or more were less likely to resolve. Other studies have shown complete resolution of polyps less than 1 cm. The majority of cases of endometrial polyps are, however, treated with hysteroscopic resection or hysteroscopically guided D&C rather than the traditional D&C. Visualization and direct removal with this technique is reported to be more effective in reducing recurrence rates. Lower recurrence rates after hysteroscopic polypectomy are seen in women with fewer polyps at the time of initial surgery if the initial surgery is combined with an endometrial ablation procedure or LNG-IUS placement, if intrauterine morcellation rather than hysteroscopic resection is used, and if such women have a shorter duration of follow-up. It has been reported that the volume of menstrual loss in women who underwent polypectomy did not differ from that of women who did not undergo polypectomy, but intermenstrual bleeding was significantly improved by polypectomy.
There are few data regarding the effects of polyps on infertility. There are many theories of how polyps can affect implantation. These include mechanical obstruction hindering ostium function and affecting sperm migration, as well as decreased endometrial receptivity due to cytokines and cellular adhesion molecules. The only randomized trial showed an improvement in pregnancy rates after polypectomy in women undergoing intrauterine insemination (IUI). Other retrospective data have shown no difference in pregnancy rates, especially if the polyp was less than 1.5 cm in length. In light of conflicting data, expert opinion recommends removal of polyps when diagnosed prior to in vitro fertilization (IVF) treatment. When lesions are seen during stimulation, the treatment decision is best made on an individual basis.
Abnormal Uterine Bleeding
- ◆
AUB can affect up to one third of women of reproductive age and accounts for a third of all outpatient gynecologic visits.
- ◆
The Federation Internationale de Gynecologie et d’Obstetrique (FIGO) has created a universal classification of causes of AUB, which has helped in organizing the approach totreatment.
- ◆
The pathophysiology of abnormal uterine disease depends on the cause of the bleeding, and this also dictates treatment options.
AUB affects up to one third of reproductive-age women and can occur in conjunction with the pathologic processes we have already discussed or in their absence. AUB also accounts for around one third of all outpatient gynecologic visits. In the United States, AUB (previously called dysfunctional uterine bleeding ) is commonly equated with anovulatory bleeding, whereas in Europe it is a diagnosis of exclusion of excessive bleeding not due to demonstrable pelvic disease, complications of pregnancy, or systemic disease. HMB may occur in 10% to 30% of women and in up to 50% of perimenopausal women. However, the self-reporting of menstrual pattern and flow is highly variable. Change in pattern of flow is probably the most important sign of pathology. HMB is the type of abnormal bleeding frequently associated with benign uterine pathology, including leiomyomata and adenomyosis. Fragile, large, thin-walled vessels and an aglandular endometrium appear to underlie the menorrhagia associated with some submucous myomas. These vessels may arise from abnormalities in angiogenesis associated with growth factors released by myomata (e.g., bFGF, VEGF).
There has been a push toward changing and standardizing the terminology for AUB, as confused terminology has made research collaborations and the interpretation of clinical trials difficult. Terms like menorrhagia, metrorrhagia , and dysfunctional uterine bleeding should be abolished. To standardize communication, FIGO created a universal classification of causes of AUB for women in their reproductive years. The PALM-COEIN nomenclature has four categories that are defined by structural criteria. These are, first, PALM : bleeding due to polyps, adenomyosis, leiomyomas and malignancy or hyperplasia. The other four are unrelated to structural abnormalities and include COEIN : bleeding due to coagulopathy, ovulatory disorders, endometrial causes, iatrogenic and (pathologies) not classified. It is hoped that this classification system will facilitate collaborations to study AUB; however, their ease of use has to be demonstrated. The four structural causes of AUB, excluding bleeding due to malignancy, have already been discussed in this chapter. Previously classified true dysfunctional uterine bleeding equates to the four nonstructural causes of bleeding in the FIGO classification.
Around 13% of women with HMB have some form of coagulopathic disorder. Systemic disease as a cause of HMB should be suspected in adolescents where coagulopathies including thrombocytopenia, von Willebrand disease, or others may be the underlying cause.
Ovulatory disorders include both ovulatory and anovulatory dysfunction leading to AUB. Anovulatory dysfunctional uterine bleeding is characterized by irregular and prolonged bleeding secondary to disturbances in the hypothalamic-pituitary-ovarian axis. It is most common at the extremes of reproductive life and in association with polycystic ovary syndrome. The unopposed action of estrogen on the uterus, resulting in dilated veins and the lack of suppression of spiral arteriole development, may represent the underlying pathophysiology. Large, thin-walled, tortuous vessels can be demonstrated on the surface of the hyperplastic endometrium. Unopposed estrogen reduces vascular tone either through direct effects of estrogen on vascular smooth muscle cells or increased production of nitric oxide (NO), leading to vasodilatation. The endometrium often breaks down unevenly in these circumstances. Scattered patches of thrombotic foci and necrotic degeneration are found adjacent to abnormally proliferated endometrium.
Ovulatory dysfunctional uterine bleeding is characterized by regular episodes of heavy menstrual flow, usually with the heaviest loss during the first 3 days of menstruation. Although many ovulatory disorders can be traced back to endocrinopathies, the underlying abnormalities appear to be defects in processes that regulate the loss of blood during menstruation, primarily angiogenesis, vasoconstriction, and hemostasis. In contrast to anovulatory dysfunctional uterine bleeding, the surface vessels of the endometrium appear to be grossly normal, and only minor abnormalities, such as venular ectasia, have been described in endometrial and myometrial veins.
Endometrial causes of AUB are similar in pathogenesis to those of ovulatory disorders. Hypoxia, inflammation, hemostasis, and angiogenesis all play critical roles in the shedding and subsequent scarless repair of the functional upper layer of the endometrium. Perturbation of local glucocorticoid metabolism, aberrant prostaglandin synthesis, and excessive plasminogen have all been implicated in AUB-E. The disruption of local endometrial hemostatic processes can be due to deficient production of vasoconstrictors such as endothelin-1 and prostaglandin F2α, increased production of vasodilators such as prostaglandin E2 and prostacyclin, and excessive breakdown of clot in the endometrium by abnormal production of plasminogen activator. In addition, vascular smooth muscle cell proliferation is reduced in the spiral arterioles in the mid- and late secretory stages in women with menorrhagia, possibly contributing to vessel instability. Endometrial bleeding-associated factor (EBAF) (also known as TGF-β4), a member of the TGF-β family of growth factors, suppresses production of collagen and promotes expression of collagenolytic and elastinolytic enzymes by antagonizing the normal signaling pathway activated by TGF-β growth factors. Abnormal expression of EBAF, which in a normal cycle occurs only in the late secretory and menstrual phases, has been reported in the endometrium of women with HMB. Angiopoietin 1 and 2 (Ang-1 and Ang-2) may also be involved in the pathogenesis of HMB. Ang-1 promotes vascular maturation, while Ang-2 destabilizes vessels and initiates neovascularization. An altered ratio of Ang-1 to Ang-2 in the endometrium due to the down-regulation of Ang-1 expression is also associated with HMB.
AUB due to iatrogenic causes (AUB-I) usually includes the use of exogenous steroid therapy in the form of a combined estrogen and progestin pill, patch, or ring and levonorgestrel-releasing IUD. The “not classified” category (AUB-N) includes a number of entities that do not fall into any group—for example, AUB due to a uterine arteriovenous malformation. Furthermore, this category has been left open for new entities that may cause AUB and have not yet been discovered. It is important to note that a woman can have AUB due to more than one cause; for example, a woman with HMB due to a submucosal fibroid (AUB-L) can also have concomitant anovulation if she has polycystic ovarian syndrome (PCOS), and she would have AUB-O type of bleeding as well.
These pathophysiologic mechanisms proposed to underlie dysfunctional uterine bleeding are targets for novel therapeutic interventions ( Fig. 26.5 ). Use of the nonhormonal oral antifibrinolytic medication called tranexamic acid has shown a 66% response rate for HMB and appears to be extremely helpful in patients with AUB. Although tranexamic acid has been widely used internationally for many years, its recent FDA approval is leading to increasing use in the United States. Other options would include the use of antiprogestins or selective PR modulators to suppress endometrial growth and stabilize the endometrial vasculature; MMP inhibitors to prevent ECM catabolism, including the matrix of the vessel walls; and NSAIDs or selective cyclooxygenase 2 (COX-2) inhibitors to suppress prostanoid synthesis. Even though their use is not novel, combined estrogen and progestin formulation in the form of a patch, ring, or pill; cyclical oral progestins; and progestin-releasing IUDs are still unfortunately being used as the primary therapy for such patients. Surgical destruction or removal of endometrium via endometrial ablation with newer nonresectoscopic, operator-friendly devices has shown impressive success rates and has also shown to be safe. Hysterectomy is a definitive but less desirable option for AUB patients who are not concerned about fertility preservation.
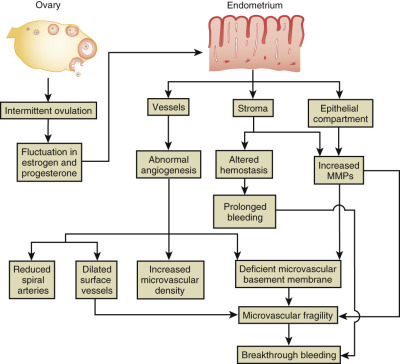
Intrauterine Adhesions
- ◆
The formation of intracavitary adhesions in an organ that routinely undergoes sloughing and regrowth every month is not well understood.
- ◆
In the developed world any form of uterine instrumentation, especially after childbirth or in the setting of an infection, is the most common cause for adhesion formation. In the developing world the most common cause is intrauterine infection, particularly Mycobacterium tuberculosis.
- ◆
Women with intrauterine adhesions may have no symptoms or may experience hypo- or amenorrhea.
- ◆
Treatment includes restoration of the normal cavity by a skilled hysteroscopic surgeon.
The formation of intracavitary synechiae in an organ that routinely undergoes sloughing and regrowth without scarring is not well understood. The true prevalence of intrauterine adhesions is difficult to establish, as it is a rare and often asymptomatic condition. Prevalence ranges from 1.5% as incidental finding on hysterosalpingography to 21.5% in women with a history of postpartum curettage. The clinical literature consistently reports that pregnancy frequently precedes the formation of intrauterine adhesions. The relationship between intrauterine adhesions and pregnancy is thought to be the result of defects in the regeneration of the endometrium after delivery, especially the area underlying the placenta. The placental site takes up to 6 weeks to repair, with the thrombosed vessels and superficial necrotic tissue exfoliated as growing endometrium undermines the area. Trauma to the regenerating endometrium involved in restoring stroma and epithelium at this site (e.g., as a consequence of curettage performed 1 to 4 weeks after delivery) may result in a permanent scar with adhesions. This is considered the primary reason for intrauterine adhesions in the industrialized world. In a meta-analysis that included over 900 women evaluated with hysteroscopy within 12 months following a spontaneous abortion (86% underwent curettage), the prevalence of intrauterine adhesions was 19.1%. In another study, women were either randomized to nonsurgical treatment or D&C after incomplete abortion. Of the women that underwent D&C, 7.7% had some form of intrauterine adhesions compared with no adhesions in the nonsurgical group. Curettage in the first 48 hours postpartum seems to cause fewer adhesions than when the procedure occurs later than that. Uterine compression sutures used to treat severe postpartum hemorrhage have been associated with the development of intrauterine adhesions. In four retrospective reviews, adhesions were diagnosed in 19% to 27% of women who received uterine compression sutures to treat postpartum hemorrhage. Not only D&C but any sort of intrauterine instrumentation can lead to the development of intrauterine synechiae. The average incidence of adhesion formation after hysteroscopic myomectomy, for example, has been reported around 10% at second-look hysteroscopy after surgery. In another study, the rate of adhesion formation was noted at 6.7% after uterine septum takedown, 31.3% after myoma resection, and as high as 45.5% after multiple myoma resection. The role of infection after abortion or delivery in the formation of uterine synechiae is controversial. A comparison of uterine cavities in women who delivered via cesarean section with and without endometritis at the time did not show any differences when it came to intrauterine adhesions.
In the developing world, infection of the endometrium, particularly with M. tuberculosis , is an important cause of uterine synechiae. With the human immunodeficiency virus–acquired immune deficiency syndrome (HIV-AIDS) epidemic and ease of air travel, genital tuberculosis should not be considered a threat to the developing world only. It is also important to note that most African studies have still attributed the major cause of intrauterine adhesions to be previous cavitary instrumentation. The estimated prevalence of genital tuberculosis varies widely between 2% and 25% in infertile women, depending on the diagnostic criteria used and the geographic location. Tuberculous adhesions can be present in as many as 35% of women with the infection and have a poor prognosis for future fertility. A characteristic “pipe stem” or “beaded” appearance on hysterosalpingography and culture with acid-fast stains on endometrial biopsy is helpful, but both tests have poor sensitivity. Newer tests such as interferon gamma release assays are more useful currently.
Women with intrauterine adhesions may have no symptoms or a variety of menstrual disorders including hypomenorrhea, oligomenorrhea, amenorrhea, dysmenorrhea, and very rarely HMB. Infertility, amenorrhea, and hypomenorrhea are the most common presenting complaints, with infertility rates reported as high as 43%. A number of classification systems have been proposed for women with intrauterine adhesions. All systems require a thorough evaluation of the endometrial cavity to note the consistency, extent, and location of the adhesions. There is paucity of data from comparison of these classification systems.
Hysterosalpingography performed to view the cavity, along with hysteroscopy, reveals the intrauterine lesions. Ultrasonography, sonohysterography, and MRI can also be valuable in certain cases ( Fig. 26.6 ). One group compared hysterosalpingography, sonohysterography, and TVS in patients with infertility and found both hysterosalpingography and sonohysterography imaging to have similar sensitivities of around 75% in detecting intrauterine adhesions. There was very limited use of TVS in detecting synechiae in this study. The “gold standard” for the diagnosis of intrauterine adhesions is the use of diagnostic hysteroscopy.
Since asymptomatic adhesions do not interfere with a woman’s general well-being, expectant management in such women has led to a resumption of regular menses in 1 to 7 years in up to 78% of women in one report. There is no role for medical therapy in the treatment of intrauterine adhesions. Before widespread use of the hysteroscope, blind D&C was used. Schenker and colleagues have reported an 84% rate of resumption of normal menses in women after D&C. Of these women, 51% conceived and 55% had term deliveries. Surgical removal of adhesions hysteroscopically (see ) with blunt dissection, scissors, electrocautery, or laser ablation is now recommended. Return of normal menses after hysteroscopic resection varies between 92% and 96%. The primary goal of surgery is to restore the normal volume and shape of the uterine cavity. In the nonindustrialized world, where hysteroscopic resection may not be feasible, there have been reports showing D&C to be equivalent to hysteroscopic resection, which is reassuring.
Postoperative management is focused on reducing the risk of the reformation of adhesions, the rate of which can be as high as one in three women with mild to moderate and two in three women with severe adhesions. Even though there is a need for larger randomized trials, there is some evidence that highlights the use of adhesion barriers after hysteroscopic procedures. There is, however, no consensus regarding the optimal methods for treating and preventing intrauterine adhesions. Insertion of an IUD or balloon catheter after lysis of adhesions is commonly employed to prevent recurrence. Stimulation of endometrial proliferation with exogenous estrogens alone or in combination with a progestin has been advocated by some. There is, however, a lack of high-quality evidence to assess the effectiveness of all the various antiadhesion therapies. Most recently the use of autologous peripheral blood bone marrow–derived stem cells as a treatment for patients with intrauterine adhesions has shown promising results.
Successful pregnancy rates after hysteroscopic resection of adhesions can be up to 40% to 80% and a live birth rate of 30% to 70%, with issues of placentation being most the serious complication of pregnancy. Of 696 births reported in a review, 17 pregnancies had placenta accreta. The preterm delivery rate was 40% to 50% in this group of patients.
Dysmenorrhea
- ◆
Primary dysmenorrhea is menstrual pain not associated with recognizable pelvic disease and is thought to be due to an intrinsic uterine dysfunction.
- ◆
Uterotonic agents like prostaglandins are thought to be key players in the pathophysiology of the disease.
- ◆
Treatment for dysmenorrhea is individualized, with the goal of providing pain relief.
Primary dysmenorrhea, or menstrual pain not associated with recognizable pelvic disease, is due to intrinsic uterine dysfunction. Occurring only in ovulatory cycles, the symptoms of dysmenorrhea usually commence a few hours before the onset of the menstrual flow. Pain is greatest when the endometrium is shedding rapidly, approximately 12 hours after flow begins. The diagnosis of primary dysmenorrhea is established when secondary causes of dysmenorrhea are ruled out and based on medical history and normal findings on pelvic and rectovaginal examination and imaging.
The prevalence of dysmenorrhea in women varies between 50% and 90% in most studies. Under 30 years old, smoking, irregular or heavy menstrual flow, BMI less than 20 kg/m 2 , menarche before age 12 years, history of sexual assault, and a strong family history of dysmenorrhea are a few of the many risk factors known for primary dysmenorrhea. There also appears to be a familial predisposition to primary dysmenorrhea. Women with dysmenorrhea, when compared with women without dysmenorrhea, have a greater sensitivity to experimental pain. This may increase their susceptibility to other chronic pain conditions and fibromyalgia later in life.
The painful cramps of dysmenorrhea are associated with uterine contractions; in women with dysmenorrhea, uterine contractile activity is heightened during menses, and the basal myometrial tone and amplitude of contractions are increased. These contractions are nonrhythmic, occur at high frequency (more than 4 to 5 times per minute), begin at an elevated basal tone (more than 10 mm Hg), and result in high intrauterine pressures, frequently more than 150 to 180 mm Hg. During intense contractions, there is a reduction in blood flow to the endometrium, suggesting that ischemia and the buildup of anaerobic metabolites stimulates type C neurons, which causes the pain of dysmenorrhea. There is also evidence from Doppler studies to show higher resistance in uterine vessels in women with primary dysmenorrhea, which can reduce blood flow to the endometrium. The uterine contractions are prompted by prostaglandins, which are potent uterotonic agents both in vitro and in vivo, acting through cell-surface prostaglandin receptors. Prostanoids may also directly sensitize uterine pain fibers. The notion that prostanoids are central to the pathogenesis of dysmenorrhea is supported by the observation that eicosanoids, most prominently prostaglandin F2-α (PGF 2α ), are found in high concentrations in menstrual fluid and that PGF 2α levels are higher in the endometrium and menstrual fluid of women complaining of dysmenorrhea than in those with pain-free menses. This high concentration of prostaglandins has been shown not only in the endometrium but also in the saliva of women during attacks of menstrual migraine associated with dysmenorrhea. More recently, patients with primary dysmenorrhea have also been hypothesized to have endothelial dysfunction.
Treatment of primary dysmenorrhea is individualized to each patient’s severity of symptoms. The goal of the treatment is to provide adequate relief of pain. At a minimum, pain relief should be sufficient to allow the woman to perform most or all of her usual activities. All drugs effective in inhibiting prostaglandin synthesis, including the potent NSAIDs (ibuprofen, naproxen, and mefenamic acid), alleviate symptoms of dysmenorrhea. A Cochrane database review included 80 randomized trials comparing NSAID therapy with placebo and paracetamol. NSAIDs were significantly more effective in reducing pain symptoms compared with placebo or acetaminophen. A combination of NSAID and paracetamol may also be helpful in relieving the pain of primary dysmenorrhea. Choosing an appropriate NSAID may be difficult, as it is not clear whether some NSAIDs work better than others. Some studies suggest that fenamates (a class that includes mefenamic acid, flufenamic acid, tolfenamic acid, and others) may have better pain-relieving propensities than phenylpropionic acid derivatives (a class that includes ibuprofen and naproxen). Although there may not be sufficient data to prove the superiority of medications from the fenamates class, a reasonable option would be to start with NSAIDs from the group of phenylpropionic acid derivatives and move onto the fenamate class of medications if insufficient relief is noted.
Combined OCPs are second-line medications after NSAIDs for the relief of pain from primary dysmenorrhea. No randomized trials have compared the efficacy of NSAIDs with OCPs. A systematic review compared OCPs with placebo and evaluated 10 randomized trials. A treatment benefit with OCPs was noted (pooled odds ratio [OR], 2.99, 95% confidence interval [CI], 1.76 to 5.07). Continuous rather than cyclic administration of OCPs has shown more success in treating patients with primary dysmenorrhea, although this difference is lost after 6 months of therapy. Contraceptive ring users have similar results to OCP users for dysmenorrhea, whereas contraceptive patches have not shown comparable results to OCPs. The use of a levonorgestrel-releasing IUD has been tried for primary dysmenorrhea with success in limited studies. Commonly, if treatment with NSAIDs is not successful after 3 months, OCPs are tried for another 3 months. If no relief is then noted, the reasons for secondary dysmenorrhea should be explored.
A long list of complementary and alternative medicines has been used in the treatment of primary dysmenorrhea. Most of them have shown similar efficacy to the conventional NSAIDs. Heat applied to the lower abdomen, aerobic exercise, yoga, acupuncture, massage with aromatic essential oils, and the use of far infrared emitting belts are among the few nonpharmacologic agents that have been tested for the treatment of dysmenorrhea in randomized clinical trials with promising results. Data on dietary supplements lack high-quality evidence to support the effectiveness of any particular supplement, and evidence of safety is lacking. A low-fat vegetarian diet, a diet rich in dairy foods, a diet rich in omega-3 fatty acids, and vitamin E supplementation have been effective in pain management from primary dysmenorrhea according to some reports.
Newer medications that include calcium antagonists like nifedipine are also effective because they prevent uterine contraction. Magnesium and glyceryl trinitrate have also been used to treat primary dysmenorrhea. Reductions in NO, which relaxes uterine smooth muscle, may also contribute to the intensified contractions associated with dysmenorrhea. Sildenafil citrate, a type 5 specific phosphodiesterase inhibitor, prevents the degradation of cyclic guanosine monophosphate (cGMP); thus it augments the local action of NO and has been shown to be an effective agent for the treatment of primary dysmenorrhea.
In addition to prostanoids, other uterotonic substances including lipoxygenase products, vasopressin, and oxytocin may have a role in dysmenorrhea. Thus, antagonists of the V 1 receptor and oxytocin receptor have a therapeutic effect in dysmenorrheic subjects.
References
- 1. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al: Uterine fibroids. Nat Rev Dis Primers 2016; 2: pp. 16043
- 2. Marshall LM, Spiegelman D, Barbieri RL, et al: Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol 1997; 90: pp. 967-973
- 3. Cramer SF, and Patel A: The frequency of uterine leiomyomas. Am J Clin Pathol 1990; 94: pp. 435-438
- 4. Day Baird D, Dunson DB, Hill MC, et al: High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003; 188: pp. 100-107
- 5. Rein MS, Barbieri RL, and Friedman AJ: Progesterone: a critical role in the pathogenesis of uterine myomas. Am J Obstet Gynecol 1995; 172: pp. 14-18
- 6. Kjerulff KH, Langenberg P, Seidman JD, et al: Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J Reprod Med 1996; 41: pp. 483-490
- 7. Faerstein E, Szklo M, and Rosenshein N: Risk factors for uterine leiomyoma: a practice-based case-control study. I. African-American heritage, reproductive history, body size, and smoking. Am J Epidemiol 2001; 153: pp. 1-10
- 8. Huyck KL, Panhuysen CI, Cuenco KT, et al: The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol 2008; 198: pp. 168.e1-168.e9
- 9. Wise LA, Palmer JR, Stewart EA, et al: Age-specific incidence rates for self-reported uterine leiomyomata in the Black Women’s Health Study. Obstet Gynecol 2005; 105: pp. 563-568
- 10. Wechter ME, Stewart EA, Myers ER, et al: Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol 2011; 205: pp. 492.e1-492.e5
- 11. Wilcox LS, Koonin LM, Pokras R, et al: Hysterectomy in the United States, 1988-1990. Obstet Gynecol 1994; 83: pp. 549-555
- 12. D’Aloisio AA, Baird DD, DeRoo LA, et al: Early-life exposures and early-onset uterine leiomyomata in black women in the Sister Study. Environ Health Perspect 2012; 120: pp. 406-412
- 13. Marshall LM, Spiegelman D, Goldman MB, et al: A prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomata. Fertil Steril 1998; 70: pp. 432-439
- 14. Ishikawa H, Reierstad S, Demura M, et al: High aromatase expression in uterine leiomyoma tissues of African-American women. J Clin Endocrinol Metab 2009; 94: pp. 1752-1756
- 15. Pan Q, Luo X, and Chegini N: Genomic and proteomic profiling I: leiomyomas in African Americans and Caucasians. Reprod Biol Endocrinol 2007; 5: pp. 34
- 16. Al-Hendy A, and Salama SA: Catechol-O-methyltransferase polymorphism is associated with increased uterine leiomyoma risk in different ethnic groups. J Soc Gynecol Investig 2006; 13: pp. 136-144
- 17. Sharan C, Halder SK, Thota C, et al: Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-o-methyltransferase. Fertil Steril 2011; 95: pp. 247-253
- 18. Halder SK, Goodwin JS, and Al-Hendy A: 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J Clin Endocrinol Metab 2011; 96: pp. E754-E762
- 19. Halder SK, Sharan C, and Al-Hendy A: 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol Reprod 2012; 86: pp. 116
- 20. Wise LA, Palmer JR, Stewart EA, et al: Polycystic ovary syndrome and risk of uterine leiomyomata. Fertil Steril 2007; 87: pp. 1108-1115
- 21. Boynton-Jarrett R, Rich-Edwards JW, Jun HJ, et al: Abuse in childhood and risk of uterine leiomyoma: the role of emotional support in biologic resilience. Epidemiology 2011; 22: pp. 6-14
- 22. Baird D, and Wise LA: Childhood abuse and fibroids. Epidemiology 2011; 22: pp. 15-17
- 23. Wise LA, Palmer JR, and Rosenberg L: Lifetime abuse victimization and risk of uterine leiomyomata in black women. Am J Obstet Gynecol 2013; 208: pp. 272.e1-272.e13
- 24. Wise LA, Palmer JR, Cozier YC, et al: Perceived racial discrimination and risk of uterine leiomyomata. Epidemiology 2007; 18: pp. 747-757
- 25. Templeman C, Marshall SF, Clarke CA, et al: Risk factors for surgically removed fibroids in a large cohort of teachers. Fertil Steril 2009; 92: pp. 1436-1446
- 26. Ross RK, Pike MC, Vessey MP, et al: Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J 1986; 293: pp. 359-362
- 27. Parazzini F, La Vecchia C, Negri E, et al: Epidemiologic characteristics of women with uterine fibroids: a case-control study. Obstet Gynecol 1988; 72: pp. 853-857
- 28. Baird DD, and Dunson DB: Why is parity protective for uterine fibroids? Epidemiology 2003; 14: pp. 247-250
- 29. Morgan Ortiz F, Pina Romero B, Elorriaga Garcia E, et al: Uterine leiomyomas during pregnancy and its impact on obstetric outcome. Ginecol Obstet Mex 2011; 79: pp. 467-473
- 30. Laughlin SK, Hartmann KE, and Baird DD: Postpartum factors and natural fibroid regression. Am J Obstet Gynecol 2011; 204: pp. 496.e1-496.e6
- 31. Laughlin SK, Herring AH, Savitz DA, et al: Pregnancy-related fibroid reduction. Fertil Steril 2010; 94: pp. 2421-2423
- 32. Amanti L, Sadeghi-Bazargani H, Abdollahi H, et al: Uterine leiomyoma and its association with menstrual pattern and history of depo-medroxyprogesterone acetate injections. Int J Gen Med 2011; 4: pp. 535-538
- 33. Wise LA, Palmer JR, Harlow BL, et al: Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. Am J Epidemiol 2004; 159: pp. 113-123
- 34. Harmon QE, Umbach DM, and Baird DD: Use of estrogen-containing contraception is associated with increased concentrations of 25-hydroxy vitamin D. J Clin Endocrinol Metab 2016; 101: pp. 3370-3377
- 35. Adams Hillard PJ: Menstruation in adolescents: what’s normal, what’s not. Ann N Y Acad Sci 2008; 1135: pp. 29-35
- 36. Wise LA, Radin RG, Palmer JR, et al: Intake of fruit, vegetables, and carotenoids in relation to risk of uterine leiomyomata. Am J Clin Nutr 2011; 94: pp. 1620-1631
- 37. Wise LA, Radin RG, Kumanyika SK, et al: Prospective study of dietary fat and risk of uterine leiomyomata. Am J Clin Nutr 2014; 99: pp. 1105-1116
- 38. Chiaffarino F, Parazzini F, La Vecchia C, et al: Diet and uterine myomas. Obstet Gynecol 1999; 94: pp. 395-398
- 39. Wise LA, Radin RG, Palmer JR, et al: A prospective study of dairy intake and risk of uterine leiomyomata. Am J Epidemiol 2010; 171: pp. 221-232
- 40. Wise LA, Palmer JR, Reich D, et al: Hair relaxer use and risk of uterine leiomyomata in African-American women. Am J Epidemiol 2012; 175: pp. 432-440
- 41. Wise LA, Palmer JR, Harlow BL, et al: Risk of uterine leiomyomata in relation to tobacco, alcohol and caffeine consumption in the Black Women’s Health Study. Hum Reprod 2004; 19: pp. 1746-1754
- 42. Nagata C, Nakamura K, Oba S, et al: Association of intakes of fat, dietary fibre, soya isoflavones and alcohol with uterine fibroids in Japanese women. Br J Nutr 2009; 101: pp. 1427-1431
- 43. Vines AI, Ta M, and Esserman DA: The association between self-reported major life events and the presence of uterine fibroids. Womens Health Issues 2010; 20: pp. 294-298
- 44. Baird DD, and Newbold R: Prenatal diethylstilbestrol (DES) exposure is associated with uterine leiomyoma development. Reprod Toxicol 2005; 20: pp. 81-84
- 45. D’Aloisio AA, Baird DD, DeRoo LA, et al: Association of intrauterine and early-life exposures with diagnosis of uterine leiomyomata by 35 years of age in the Sister Study. Environ Health Perspect 2010; 118: pp. 375-381
- 46. Parazzini F, Negri E, La Vecchia C, et al: Uterine myomas and smoking. Results from an Italian study. J Reprod Med 1996; 41: pp. 316-320
- 47. Wong JY, Chang PY, Gold EB, et al: Environmental tobacco smoke and risk of late-diagnosis incident fibroids in the Study of Women’s Health across the Nation (SWAN). Fertil Steril 2016; 106: pp. 1157-1164
- 48. Marshall LM, Spiegelman D, Manson JE, et al: Risk of uterine leiomyomata among premenopausal women in relation to body size and cigarette smoking. Epidemiology 1998; 9: pp. 511-517
- 49. Wise LA, Palmer JR, Spiegelman D, et al: Influence of body size and body fat distribution on risk of uterine leiomyomata in U.S. black women. Epidemiology 2005; 16: pp. 346-354
- 50. Terry KL, De Vivo I, Hankinson SE, et al: Anthropometric characteristics and risk of uterine leiomyoma. Epidemiology 2007; 18: pp. 758-763
- 51. Radin RG, Palmer JR, Rosenberg L, et al: Dietary glycemic index and load in relation to risk of uterine leiomyomata in the Black Women’s Health Study. Am J Clin Nutr 2010; 91: pp. 1281-1288
- 52. Baird DD, Dunson DB, Hill MC, et al: Association of physical activity with development of uterine leiomyoma. Am J Epidemiol 2007; 165: pp. 157-163
- 53. Radin RG, Rosenberg L, Palmer JR, et al: Hypertension and risk of uterine leiomyomata in US black women. Hum Reprod 2012; 27: pp. 1504-1509
- 54. Takeda T, Sakata M, Isobe A, et al: Relationship between metabolic syndrome and uterine leiomyomas: a case-control study. Gynecol Obstet Invest 2008; 66: pp. 14-17
- 55. Faerstein E, Szklo M, and Rosenshein NB: Risk factors for uterine leiomyoma: a practice-based case-control study. II. Atherogenic risk factors and potential sources of uterine irritation. Am J Epidemiol 2001; 153: pp. 11-19
- 56. Moore KR, Smith JS, Laughlin-Tommaso SK, et al: Cervical neoplasia-related factors and decreased prevalence of uterine fibroids among a cohort of African American women. Fertil Steril 2014; 101: pp. 208-214
- 57. Peddada SD, Laughlin SK, Miner K, et al: Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA 2008; 105: pp. 19887-19892
- 58. Baird DD, Garrett TA, Laughlin SK, et al: Short-term change in growth of uterine leiomyoma: tumor growth spurts. Fertil Steril 2011; 95: pp. 242-246
- 59. Yin P, Lin Z, Cheng YH, et al: Progesterone receptor regulates Bcl-2 gene expression through direct binding to its promoter region in uterine leiomyoma cells. J Clin Endocrinol Metab 2007; 92: pp. 4459-4466
- 60. Matsuo H, Maruo T, and Samoto T: Increased expression of Bcl-2 protein in human uterine leiomyoma and its up-regulation by progesterone. J Clin Endocrinol Metab 1997; 82: pp. 293-299
- 61. Xu Q, Takekida S, Ohara N, et al: Progesterone receptor modulator CDB-2914 down-regulates proliferative cell nuclear antigen and Bcl-2 protein expression and up-regulates caspase-3 and poly(adenosine 5′-diphosphate-ribose) polymerase expression in cultured human uterine leiomyoma cells. J Clin Endocrinol Metab 2005; 90: pp. 953-961
- 62. Yin P, Lin Z, Reierstad S, et al: Transcription factor KLF11 integrates progesterone receptor signaling and proliferation in uterine leiomyoma cells. Cancer Res 2010; 70: pp. 1722-1730
- 63. Velarde MC, Iruthayanathan M, Eason RR, et al: Progesterone receptor transactivation of the secretory leukocyte protease inhibitor gene in Ishikawa endometrial epithelial cells involves recruitment of Kruppel-like factor 9/basic transcription element binding protein-1. Endocrinology 2006; 147: pp. 1969-1978
- 64. Sumitani H, Shozu M, Segawa T, et al: In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology 2000; 141: pp. 3852-3861
- 65. Ye Y, Cheng X, Luo HB, et al: CYP1A1 and CYP1B1 genetic polymorphisms and uterine leiomyoma risk in Chinese women. J Assist Reprod Genet 2008; 25: pp. 389-394
- 66. El-Shennawy GA, Elbialy AA, Isamil AE, et al: Is genetic polymorphism of ER-alpha, CYP1A1, and CYP1B1 a risk factor for uterine leiomyoma? Arch Gynecol Obstet 2011; 283: pp. 1313-1318
- 67. Kasai T, Shozu M, Murakami K, et al: Increased expression of type I 17beta-hydroxysteroid dehydrogenase enhances in situ production of estradiol in uterine leiomyoma. J Clin Endocrinol Metab 2004; 89: pp. 5661-5668
- 68. Brandon DD, Erickson TE, Keenan EJ, et al: Estrogen receptor gene expression in human uterine leiomyomata. J Clin Endocrinol Metab 1995; 80: pp. 1876-1881
- 69. Brandon DD, Bethea CL, Strawn EY, et al: Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am J Obstet Gynecol 1993; 169: pp. 78-85
- 70. Viville B, Charnock-Jones DS, Sharkey AM, et al: Distribution of the A and B forms of the progesterone receptor messenger ribonucleic acid and protein in uterine leiomyomata and adjacent myometrium. Hum Reprod 1997; 12: pp. 815-822
- 71. Ishikawa H, Ishi K, Serna VA, et al: Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010; 151: pp. 2433-2442
- 72. Stewart EA, Austin DJ, Jain P, et al: RU486 suppresses prolactin production in explant cultures of leiomyoma and myometrium. Fertil Steril 1996; 65: pp. 1119-1124
- 73. Pedeutour F, Quade BJ, Weremowicz S, et al: Localization and expression of the human estrogen receptor beta gene in uterine leiomyomata. Genes Chromosomes Cancer 1998; 23: pp. 361-366
- 74. Chegini N, Verala J, Luo X, et al: Gene expression profile of leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. J Soc Gynecol Investig 2003; 10: pp. 161-171
- 75. Rosenberg SM, and Bhatnagar AS: Sex steroid and human chorionic gonadotropin modulation of in vitro prolactin production by human term decidua. Am J Obstet Gynecol 1984; 148: pp. 461-465
- 76. Stewart EA, Rein MS, Friedman AJ, et al: Glycoprotein hormones and their common alpha-subunit stimulate prolactin production by explant cultures of human leiomyoma and myometrium. Am J Obstet Gynecol 1994; 170: pp. 677-683
- 77. Kornyei JL, Lei ZM, and Rao CV: Human myometrial smooth muscle cells are novel targets of direct regulation by human chorionic gonadotropin. Biol Reprod 1993; 49: pp. 1149-1157
- 78. Stewart EA, Jain P, Penglase MD, et al: The myometrium of postmenopausal women produces prolactin in response to human chorionic gonadotropin and alpha-subunit in vitro. Fertil Steril 1995; 64: pp. 972-976
- 79. Reshef E, Lei ZM, Rao CV, et al: The presence of gonadotropin receptors in nonpregnant human uterus, human placenta, fetal membranes, and decidua. J Clin Endocrinol Metab 1990; 70: pp. 421-430
- 80. Stewart EA, Sahakian M, Rhoades A, et al: Messenger ribonucleic acid for the gonadal luteinizing hormone/human chorionic gonadotropin receptor is not present in human endometrium. Fertil Steril 1999; 71: pp. 368-372
- 81. Baird DD, Kesner JS, and Dunson DB: Luteinizing hormone in premenopausal women may stimulate uterine leiomyomata development. J Soc Gynecol Investig 2006; 13: pp. 130-135
- 82. Whirledge S, Dixon D, and Cidlowski JA: Glucocorticoids regulate gene expression and repress cellular proliferation in human uterine leiomyoma cells. Horm Cancer 2012; 3: pp. 79-92
- 83. Yin H, Lo JH, Kim JY, et al: Expression profiling of nuclear receptors identifies key roles of NR4A subfamily in uterine fibroids. Mol Endocrinol 2013; 27: pp. 726-740
- 84. Bulun SE, Moravek MB, Yin P, et al: Uterine leiomyoma stem cells: linking progesterone to growth. Semin Reprod Med 2015; 33: pp. 357-365
- 85. Ono M, Qiang W, Serna VA, et al: Role of stem cells in human uterine leiomyoma growth. PLoS ONE 2012; 7: pp. e36935
- 86. Moravek MB, Yin P, Ono M, et al: Ovarian steroids, stem cells and uterine leiomyoma: therapeutic implications. Hum Reprod Update 2015; 21: pp. 1-12
- 87. Nowak RA: Novel therapeutic strategies for leiomyomas: targeting growth factors and their receptors. Environ Health Perspect 2000; 108: pp. 849-853
- 88. Stewart EA, Floor AE, Jain P, et al: Increased expression of messenger RNA for collagen type I, collagen type III, and fibronectin in myometrium of pregnancy. Obstet Gynecol 1995; 86: pp. 417-422
- 89. Palmer SS, Haynes-Johnson D, Diehl T, et al: Increased expression of stromelysin 3 mRNA in leiomyomas (uterine fibroids) compared with myometrium. J Soc Gynecol Investig 1998; 5: pp. 203-209
- 90. Catherino WH, Leppert PC, Stenmark MH, et al: Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer 2004; 40: pp. 204-217
- 91. Leppert PC, Baginski T, Prupas C, et al: Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril 2004; 82: pp. 1182-1187
- 92. Rogers R, Norian J, Malik M, et al: Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol 2008; 198: pp. 474.e1-474.e11
- 93. Norian JM, Owen CM, Taboas J, et al: Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol 2012; 31: pp. 57-65
- 94. Leppert PC, Jayes FL, and Segars JH: The extracellular matrix contributes to mechanotransduction in uterine fibroids. Obstet Gynecol Int 2014; 2014: pp. 783289
- 95. Arici A, and Sozen I: Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril 2000; 73: pp. 1006-1011
- 96. Lee BS, and Nowak RA: Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-beta 3 (TGF beta 3) and altered responses to the antiproliferative effects of TGF beta. J Clin Endocrinol Metab 2001; 86: pp. 913-920
- 97. Stewart EA, and Nowak RA: Leiomyoma-related bleeding: a classic hypothesis updated for the molecular era. Hum Reprod Update 1996; 2: pp. 295-306
- 98. Deleted in review.
- 99. Kothapalli R, Buyuksal I, Wu SQ, et al: Detection of ebaf, a novel human gene of the transforming growth factor beta superfamily association of gene expression with endometrial bleeding. J Clin Invest 1997; 99: pp. 2342-2350
- 100. Chegini N, Tang XM, and Ma C: Regulation of transforming growth factor-beta1 expression by granulocyte macrophage-colony-stimulating factor in leiomyoma and myometrial smooth muscle cells. J Clin Endocrinol Metab 1999; 84: pp. 4138-4143
- 101. Xu J, Luo X, and Chegini N: Differential expression, regulation, and induction of Smads, transforming growth factor-beta signal transduction pathway in leiomyoma, and myometrial smooth muscle cells and alteration by gonadotropin-releasing hormone analog. J Clin Endocrinol Metab 2003; 88: pp. 1350-1361
- 102. Propst AM, Quade BJ, Nowak RA, et al: Granulocyte macrophage colony-stimulating factor in adenomyosis and autologous endometrium. J Soc Gynecol Investig 2002; 9: pp. 93-97
- 103. Chegini N, Luo X, Ding L, et al: The expression of Smads and transforming growth factor beta receptors in leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. Mol Cell Endocrinol 2003; 209: pp. 9-16
- 104. Luo X, Ding L, and Chegini N: Gonadotropin-releasing hormone and TGF-beta activate MAP kinase and differentially regulate fibronectin expression in endometrial epithelial and stromal cells. Am J Physiol Endocrinol Metab 2004; 287: pp. E991-E1001
- 105. Luo X, Ding L, and Chegini N: CCNs, fibulin-1C and S100A4 expression in leiomyoma and myometrium: inverse association with TGF-beta and regulation by TGF-beta in leiomyoma and myometrial smooth muscle cells. Mol Hum Reprod 2006; 12: pp. 245-256
- 106. Weston G, Trajstman AC, Gargett CE, et al: Fibroids display an anti-angiogenic gene expression profile when compared with adjacent myometrium. Mol Hum Reprod 2003; 9: pp. 541-549
- 107. Buttram VC, and Reiter RC: Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril 1981; 36: pp. 433-445
- 108. Torry RJ, and Rongish BJ: Angiogenesis in the uterus: potential regulation and relation to tumor angiogenesis. Am J Reprod Immunol 1992; 27: pp. 171-179
- 109. Ribatti D, Belloni AS, Nico B, et al: Tryptase- and leptin-positive mast cells correlate with vascular density in uterine leiomyomas. Am J Obstet Gynecol 2007; 196: pp. 470.e1-470.e7
- 110. Lindner V, and Reidy MA: Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci USA 1991; 88: pp. 3739-3743
- 111. Rauk PN, Surti U, Roberts JM, et al: Mitogenic effect of basic fibroblast growth factor and estradiol on cultured human myometrial and leiomyoma cells. Am J Obstet Gynecol 1995; 173: pp. 571-577
- 112. Lee BS, Stewart EA, Sahakian M, et al: Interferon-alpha is a potent inhibitor of basic fibroblast growth factor-stimulated cell proliferation in human uterine cells. Am J Reprod Immunol 1998; 40: pp. 19-25
- 113. Mangrulkar RS, Ono M, Ishikawa M, et al: Isolation and characterization of heparin-binding growth factors in human leiomyomas and normal myometrium. Biol Reprod 1995; 53: pp. 636-646
- 114. Anania CA, Stewart EA, Quade BJ, et al: Expression of the fibroblast growth factor receptor in women with leiomyomas and abnormal uterine bleeding. Mol Hum Reprod 1997; 3: pp. 685-691
- 115. Mehine M, Kaasinen E, Makinen N, et al: Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med 2013; 369: pp. 43-53
- 116. Bulun SE: Uterine fibroids. N Engl J Med 2013; 369: pp. 1344-1355
- 117. Linder D, and Gartler SM: Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science 1965; 150: pp. 67-69
- 118. Mashal RD, Fejzo ML, Friedman AJ, et al: Analysis of androgen receptor DNA reveals the independent clonal origins of uterine leiomyomata and the secondary nature of cytogenetic aberrations in the development of leiomyomata. Genes Chromosomes Cancer 1994; 11: pp. 1-6
- 119. Rein MS, Friedman AJ, Barbieri RL, et al: Cytogenetic abnormalities in uterine leiomyomata. Obstet Gynecol 1991; 77: pp. 923-926
- 120. Hodge JC, Kim TM, Dreyfuss JM, et al: Expression profiling of uterine leiomyomata cytogenetic subgroups reveals distinct signatures in matched myometrium: transcriptional profilingof the t(12;14) and evidence in support of predisposing genetic heterogeneity. Hum Mol Genet 2012; 21: pp. 2312-2329
- 121. Stewart EA, and Morton CC: The genetics of uterine leiomyomata: what clinicians need to know. Obstet Gynecol 2006; 107: pp. 917-921
- 122. Nibert M, and Heim S: Uterine leiomyoma cytogenetics. Genes Chromosomes Cancer 1990; 2: pp. 3-13
- 123. Hodge JC, Pearce KE, Clayton AC, et al: Uterine cellular leiomyomata with chromosome 1p deletions represent a distinct entity. Am J Obstet Gynecol 2014; 210: pp. 572.e1-572.e7
- 124. Brosens I, Deprest J, Dal Cin P, et al: Clinical significance of cytogenetic abnormalities in uterine myomas. Fertil Steril 1998; 69: pp. 232-235
- 125. Hodge JC, and Morton CC: Genetic heterogeneity among uterine leiomyomata: insights into malignant progression. Hum Mol Genet 2007; 16: pp. R7-R13
- 126. Brosens J, Campo R, Gordts S, et al: Submucous and outer myometrium leiomyomas are two distinct clinical entities. Fertil Steril 2003; 79: pp. 1452-1454
- 127. Cha PC, Takahashi A, Hosono N, et al: A genome-wide association study identifies three loci associated with susceptibility to uterine fibroids. Nat Genet 2011; 43: pp. 447-450
- 128. Makinen N, Mehine M, Tolvanen J, et al: MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011; 334: pp. 252-255
- 129. Osinovskaya NS, Ivashchenko TE, Dolinskii AK, et al: MED12 gene mutations in women with uterine myoma. Genetika 2013; 49: pp. 1426-1431
- 130. McGuire MM, Yatsenko A, Hoffner L, et al: Whole exome sequencing in a random sample of North American women with leiomyomas identifies MED12 mutations in majority of uterine leiomyomas. PLoS ONE 2012; 7: pp. e33251
- 131. Li S, Chiang TC, Davis GR, et al: Decreased expression of Wnt7a mRNA is inversely associated with the expression of estrogen receptor-alpha in human uterine leiomyoma. J Clin Endocrinol Metab 2001; 86: pp. 454-457
- 132. Tanwar PS, Lee HJ, Zhang L, et al: Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod 2009; 81: pp. 545-552
- 133. Ono M, Yin P, Navarro A, et al: Paracrine activation of WNT/beta-catenin pathway in uterine leiomyoma stem cells promotes tumor growth. Proc Natl Acad Sci USA 2013; 110: pp. 17053-17058
- 134. Ono M, Yin P, Navarro A, et al: Inhibition of canonical WNT signaling attenuates human leiomyoma cell growth. Fertil Steril 2014; 101: pp. 1441-1449
- 135. Schoenberg Fejzo M, Ashar HR, Krauter KS, et al: Translocation breakpoints upstream of the HMGIC gene in uterine leiomyomata suggest dysregulation of this gene by a mechanism different from that in lipomas. Genes Chromosomes Cancer 1996; 17: pp. 1-6
- 136. Gross KL, Neskey DM, Manchanda N, et al: HMGA2 expression in uterine leiomyomata and myometrium: quantitative analysis and tissue culture studies. Genes Chromosomes Cancer 2003; 38: pp. 68-79
- 137. Markowski DN, Helmke BM, Belge G, et al: HMGA2 and p14Arf: major roles in cellular senescence of fibroids and therapeutic implications. Anticancer Res 2011; 31: pp. 753-761
- 138. Varghese BV, Koohestani F, McWilliams M, et al: Loss of the repressor REST in uterine fibroids promotes aberrant G protein-coupled receptor 10 expression and activates mammalian target of rapamycin pathway. Proc Natl Acad Sci USA 2013; 110: pp. 2187-2192
- 139. Cook JD, Davis BJ, Goewey JA, et al: Identification of a sensitive period for developmental programming that increases risk for uterine leiomyoma in Eker rats. Reprod Sci 2007; 14: pp. 121-136
- 140. Everitt JI, Wolf DC, Howe SR, et al: Rodent model of reproductive tract leiomyomata. Clinical and pathological features. Am J Pathol 1995; 146: pp. 1556-1567
- 141. Crabtree JS, Jelinsky SA, Harris HA, et al: Comparison of human and rat uterine leiomyomata: identification of a dysregulated mammalian target of rapamycin pathway. Cancer Res 2009; 69: pp. 6171-6178
- 142. Wei JJ, Chiriboga L, Arslan AA, et al: Ethnic differences in expression of the dysregulated proteins in uterine leiomyomata. Hum Reprod 2006; 21: pp. 57-67
- 143. Gross KL, Panhuysen CI, Kleinman MS, et al: Involvement of fumarate hydratase in nonsyndromic uterine leiomyomas: genetic linkage analysis and FISH studies. Genes Chromosomes Cancer 2004; 41: pp. 183-190
- 144. Treloar SA, Martin NG, Dennerstein L, et al: Pathways to hysterectomy: insights from longitudinal twin research. Am J Obstet Gynecol 1992; 167: pp. 82-88
- 145. Snieder H, MacGregor AJ, and Spector TD: Genes control the cessation of a woman’s reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab 1998; 83: pp. 1875-1880
- 146. Kurbanova M, Koroleva AG, and Sergeev AS: Genetic-epidemiologic analysis of uterine myoma: assessment of repeated risk. Genetika 1989; 25: pp. 1896-1898
- 147. Van Voorhis BJ, Romitti PA, and Jones MP: Family history as a risk factor for development of uterine leiomyomas. Results of a pilot study. J Reprod Med 2002; 47: pp. 663-669
- 148. Launonen V, Vierimaa O, Kiuru M, et al: Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci USA 2001; 98: pp. 3387-3392
- 149. Tomlinson IP, Alam NA, Rowan AJ, et al: Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 2002; 30: pp. 406-410
- 150. Toro JR, Nickerson ML, Wei MH, et al: Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet 2003; 73: pp. 95-106
- 151. Reed WB, Walker R, and Horowitz R: Cutaneous leiomyomata with uterine leiomyomata. Acta Derm Venereol 1973; 53: pp. 409-416
- 152. Alam NA, Bevan S, Churchman M, et al: Localization of a gene (MCUL1) for multiple cutaneous leiomyomata and uterine fibroids to chromosome 1q42.3-q43. Am J Hum Genet 2001; 68: pp. 1264-1269
- 153. Shveiky D, Rojansky N, Ben Bassat H, et al: Family history of uterine fibroids associated with low level of fumarate hydratase in leiomyomata cells. Eur J Obstet Gynecol Reprod Biol 2009; 146: pp. 234-235
- 154. Stewart L, Glenn GM, Stratton P, et al: Association of germline mutations in the fumarate hydratase gene and uterine fibroids in women with hereditary leiomyomatosis and renal cell cancer. Arch Dermatol 2008; 144: pp. 1584-1592
- 155. Alam NA, Rowan AJ, Wortham NC, et al: Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet 2003; 12: pp. 1241-1252
- 156. Alam NA, Olpin S, Rowan A, et al: Missense mutations in fumarate hydratase in multiple cutaneous and uterine leiomyomatosis and renal cell cancer. J Mol Diagn 2005; 7: pp. 437-443
- 157. Lehtonen R, Kiuru M, Vanharanta S, et al: Biallelic inactivation of fumarate hydratase (FH) occurs in nonsyndromic uterine leiomyomas but is rare in other tumors. Am J Pathol 2004; 164: pp. 17-22
- 158. Catherino WH, Mayers CM, Mantzouris T, et al: Compensatory alterations in energy homeostasis characterized in uterine tumors from hereditary leiomyomatosis and renal cell cancer. Fertil Steril 2007; 88: pp. 1039-1048
- 159. Stewart EA, and Nowak RA: New concepts in the treatment of uterine leiomyomas. Obstet Gynecol 1998; 92: pp. 624-627
- 160. Marsh DJ, Dahia PL, Zheng Z, et al: Germline mutations in PTEN are present in Bannayan-Zonana syndrome. Nat Genet 1997; 16: pp. 333-334
- 161. Dahia PM, Gimm O, Chi H, et al: Absence of germline mutations in MINPP1, a phosphatase encoding gene centromeric of PTEN, in patients with Cowden and Bannayan-Riley-Ruvalcaba syndrome without germline PTEN mutations. J Med Genet 2000; 37: pp. 715-717
- 162. Fackenthal JD, Marsh DJ, Richardson AL, et al: Male breast cancer in Cowden syndrome patients with germline PTEN mutations. J Med Genet 2001; 38: pp. 159-164
- 163. Marsh DJ, Dahia PL, Caron S, et al: Germline PTEN mutations in Cowden syndrome-like families. J Med Genet 1998; 35: pp. 881-885
- 164. Pilarski R, Stephens JA, Noss R, et al: Predicting PTEN mutations: an evaluation of Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome clinical features. J Med Genet 2011; 48: pp. 505-512
- 165. Pilarski R: Cowden syndrome: a critical review of the clinical literature. J Genet Couns 2009; 18: pp. 13-27
- 166. Mehine M, Makinen N, Heinonen HR, et al: Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril 2014; 102: pp. 621-629
- 167. Harrison-Woolrych ML, Charnock-Jones DS, and Smith SK: Quantification of messenger ribonucleic acid for epidermal growth factor in human myometrium and leiomyomata using reverse transcriptase polymerase chain reaction. J Clin Endocrinol Metab 1994; 78: pp. 1179-1184
- 168. Ren Y, Yin H, Tian R, et al: Different effects of epidermal growth factor on smooth muscle cells derived from human myometrium and from leiomyoma. Fertil Steril 2011; 96: pp. 1015-1020
- 169. Tommola P, Pekonen F, and Rutanen EM: Binding of epidermal growth factor and insulin-like growth factor I in human myometrium and leiomyomata. Obstet Gynecol 1989; 74: pp. 658-662
- 170. Mesquita FS, Dyer SN, Heinrich DA, et al: Reactive oxygen species mediate mitogenic growth factor signaling pathways in human leiomyoma smooth muscle cells. Biol Reprod 2010; 82: pp. 341-351
- 171. Tal R, and Segars JH: The role of angiogenic factors in fibroid pathogenesis: potential implications for future therapy. Hum Reprod Update 2014; 20: pp. 194-216
- 172. Harrison-Woolrych ML, Sharkey AM, Charnock-Jones DS, et al: Localization and quantification of vascular endothelial growth factor messenger ribonucleic acid in human myometrium and leiomyomata. J Clin Endocrinol Metab 1995; 80: pp. 1853-1858
- 173. Boehm KD, Daimon M, Gorodeski IG, et al: Expression of the insulin-like and platelet-derived growth factor genes in human uterine tissues. Mol Reprod Dev 1990; 27: pp. 93-101
- 174. Gloudemans T, Prinsen I, Van Unnik JA, et al: Insulin-like growth factor gene expression in human smooth muscle tumors. Cancer Res 1990; 50: pp. 6689-6695
- 175. Vollenhoven BJ, Herington AC, and Healy DL: Messenger ribonucleic acid expression of the insulin-like growth factors and their binding proteins in uterine fibroids and myometrium. J Clin Endocrinol Metab 1993; 76: pp. 1106-1110
- 176. Giudice LC, Irwin JC, Dsupin BA, et al: Insulin-like growth factor (IGF), IGF binding protein (IGFBP), and IGF receptor gene expression and IGFBP synthesis in human uterine leiomyomata. Hum Reprod 1993; 8: pp. 1796-1806
- 177. Gkioka E, Msaouel P, Philippou A, et al: Review: the role of insulin-like growth factor-1 signaling pathways in uterine leiomyoma. In Vivo 2015; 29: pp. 637-649
- 178. Yu L, Saile K, Swartz CD, et al: Differential expression of receptor tyrosine kinases (RTKs) and IGF-I pathway activation in human uterine leiomyomas. Mol Med 2008; 14: pp. 264-275
- 179. Rein MS, Friedman AJ, Pandian MR, et al: The secretion of insulin-like growth factors I and II by explant cultures of fibroids and myometrium from women treated with a gonadotropin-releasing hormone agonist. Obstet Gynecol 1990; 76: pp. 388-394
- 180. Cohen O, Schindel B, and Homburg R: Uterine leiomyomata—a feature of acromegaly. Hum Reprod 1998; 13: pp. 1945-1946
- 181. Nowak RA, Mora S, Diehl T, et al: Prolactin is an autocrine or paracrine growth factor for human myometrial and leiomyoma cells. Gynecol Obstet Invest 1999; 48: pp. 127-132
- 182. Goksu Erol AY, Tokyol C, Ozdemir O, et al: The role of mast cells and angiogenesis in benign and malignant neoplasms of the uterus. Pathol Res Pract 2011; 207: pp. 618-622
- 183. Deleted in review.
- 184. Senturk LM, Sozen I, Gutierrez L, et al: Interleukin 8 production and interleukin 8 receptor expression in human myometrium and leiomyoma. Am J Obstet Gynecol 2001; 184: pp. 559-566
- 185. Matsui S, Yasui T, Uemura H, et al: Induction of circulating monocyte chemoattractant protein-1 in women with gonadotropin-releasing hormone agonist. J Reprod Immunol 2011; 90: pp. 227-234
- 186. Sozen I, Senturk LM, and Arici A: Effect of gonadotropin-releasing hormone agonists on monocyte chemotactic protein-1 production and macrophage infiltration in leiomyomatous uterus. Fertil Steril 2001; 76: pp. 792-796
- 187. Fukuhara K, Kariya M, Kita M, et al: Secreted frizzled related protein 1 is overexpressed in uterine leiomyomas, associated with a high estrogenic environment and unrelated to proliferative activity. J Clin Endocrinol Metab 2002; 87: pp. 1729-1736
- 188. Cermik D, Arici A, and Taylor HS: Coordinated regulation of HOX gene expression in myometrium and uterine leiomyoma. Fertil Steril 2002; 78: pp. 979-984
- 189. Gustavsson I, Englund K, Faxen M, et al: Tissue differences but limited sex steroid responsiveness of c-fos and c-jun in human fibroids and myometrium. Mol Hum Reprod 2000; 6: pp. 55-59
- 190. Yoshida M, Ohtsuru A, Samejima T, et al: Involvement of parathyroid hormone-related peptide in cell proliferation activity of human uterine leiomyomas. Endocr J 1999; 46: pp. 81-90
- 191. Weir EC, Goad DL, Daifotis AG, et al: Relative overexpression of the parathyroid hormone-related protein gene in human leiomyomas. J Clin Endocrinol Metab 1994; 78: pp. 784-789
- 192. Ravakhah K, Gover A, and Mukunda BN: Humoral hypercalcemia associated with a uterine fibroid. Ann Intern Med 1999; 130: pp. 702
- 193. Herring R, and Laji K: Humoral hypercalcaemia of benignancy. A case report. QJM 2008; 101: pp. 329-330
- 194. Dagdelen S, Kalan I, and Gurlek A: Humoral hypercalcemia of benignancy secondary to parathyroid hormone-related protein secreting uterine leiomyoma. Am J Med Sci 2008; 335: pp. 407-408
- 195. Bilici A, Doventas A, Karadag B, et al: Hypercalcemia associated with a uterine leiomyoma: a case report and review of the literature. Gynecol Oncol 2004; 93: pp. 269-271
- 196. Wang T, Zhang X, Obijuru L, et al: A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer 2007; 46: pp. 336-347
- 197. Marsh EE, Lin Z, Yin P, et al: Differential expression of microRNA species in human uterine leiomyoma versus normal myometrium. Fertil Steril 2008; 89: pp. 1771-1776
- 198. Zavadil J, Ye H, Liu Z, et al: Profiling and functional analyses of microRNAs and their target gene products in human uterine leiomyomas. PLoS ONE 2010; 5: pp. e12362
- 199. Georgieva B, Milev I, Minkov I, et al: Characterization of the uterine leiomyoma microRNAome by deep sequencing. Genomics 2012; 99: pp. 275-281
- 200. Cirilo PD, Marchi FA, Barros Filho Mde C, et al: An integrative genomic and transcriptomic analysis reveals potential targets associated with cell proliferation in uterine leiomyomas. PLoS ONE 2013; 8: pp. e57901
- 201. Chuang TD, and Khorram O: miR-200c regulates IL8 expression by targeting IKBKB: a potential mediator of inflammation in leiomyoma pathogenesis. PLoS ONE 2014; 9: pp. e95370
- 202. Pan Q, Luo X, and Chegini N: Differential expression of microRNAs in myometrium and leiomyomas and regulation by ovarian steroids. J Cell Mol Med 2008; 12: pp. 227-240
- 203. Luo X, and Chegini N: The expression and potential regulatory function of microRNAs in the pathogenesis of leiomyoma. Semin Reprod Med 2008; 26: pp. 500-514
- 204. Chuang TD, Luo X, Panda H, et al: miR-93/106b and their host gene, MCM7, are differentially expressed in leiomyomas and functionally target F3 and IL-8. Mol Endocrinol 2012; 26: pp. 1028-1042
- 205. Stewart EA: Clinical practice. Uterine fibroids. N Engl J Med 2015; 372: pp. 1646-1655
- 206. Gliklich RE, Leavy M, Velentgas P, et al: Identification of Future Research Needs in the Comparative Management of Uterine Fibroid Disease.
- 207. Stewart EA: Uterine fibroids: the complete guide. Baltimore: Johns Hopkins University Press, 2007.
- 208. Sener AB, Seckin NC, Ozmen S, et al: The effects of hormone replacement therapy on uterine fibroids in postmenopausal women. Fertil Steril 1996; 65: pp. 354-357
- 209. Carlson KJ, Miller BA, and Fowler FJ: The Maine Women’s Health Study: I. Outcomes of hysterectomy. Obstet Gynecol 1994; 83: pp. 556-565
- 210. Kjerulff KH, Rhodes JC, Langenberg PW, et al: Patient satisfaction with results of hysterectomy. Am J Obstet Gynecol 2000; 183: pp. 1440-1447
- 211. Marret H, Fritel X, Ouldamer L, et al: Therapeutic management of uterine fibroid tumors: updated French guidelines. Eur J Obstet Gynecol Reprod Biol 2012; 165: pp. 156-164
- 212. Stewart EA, Shuster LT, and Rocca WA: Reassessing hysterectomy. Minn Med 2012; 95: pp. 36-39
- 213. Adashi EY: The climacteric ovary as a functional gonadotropin-driven androgen-producing gland. Fertil Steril 1994; 62: pp. 20-27
- 214. Irwin KL, Weiss NS, Lee NC, et al: Tubal sterilization, hysterectomy, and the subsequent occurrence of epithelial ovarian cancer. Am J Epidemiol 1991; 134: pp. 362-369
- 215. Hankinson SE, Hunter DJ, Colditz GA, et al: Tubal ligation, hysterectomy, and risk of ovarian cancer. A prospective study. JAMA 1993; 270: pp. 2813-2818
- 216. Payne TN, and Dauterive FR: A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: surgical outcomes in a community practice. J Minim Invasive Gynecol 2008; 15: pp. 286-291
- 217. Wright JD, Tergas AI, Burke WM, et al: Uterine pathology in women undergoing minimally invasive hysterectomy using morcellation. JAMA 2014; 312: pp. 1253-1255
- 218. Nieboer TE, Johnson N, Lethaby A, et al: Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev 2009; undefined:
- 219. Howard BV, Kuller L, Langer R, et al: Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women’s Health Initiative Observational Study. Circulation 2005; 111: pp. 1462-1470
- 220. Ingelsson E, Lundholm C, Johansson AL, et al: Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J 2011; 32: pp. 745-750
- 221. Blandon RE, Bharucha AE, Melton LJ, et al: Incidence of pelvic floor repair after hysterectomy: a population-based cohort study. Am J Obstet Gynecol 2007; 197: pp. 664.e1-664.e7
- 222. Rocca WA, Grossardt BR, Shuster LT, et al: Hysterectomy, oophorectomy, estrogen, and the risk of dementia. Neurodegener Dis 2012; 10: pp. 175-178
- 223. Thakar R, Ayers S, Clarkson P, et al: Outcomes after total versus subtotal abdominal hysterectomy. N Engl J Med 2002; 347: pp. 1318-1325
- 224. Kuppermann M, Summitt RL, Varner RE, et al: Sexual functioning after total compared with supracervical hysterectomy: a randomized trial. Obstet Gynecol 2005; 105: pp. 1309-1318
- 225. Owusu-Ansah R, Gatongi D, and Chien PF: Health technology assessment of surgical therapies for benign gynaecological disease. Best Pract Res Clin Obstet Gynaecol 2006; 20: pp. 841-879
- 226. Stewart EA, Faur AV, Wise LA, et al: Predictors of subsequent surgery for uterine leiomyomata after abdominal myomectomy. Obstet Gynecol 2002; 99: pp. 426-432
- 227. Spies JB, Spector A, Roth AR, et al: Complications after uterine artery embolization for leiomyomas. Obstet Gynecol 2002; 100: pp. 873-880
- 228. Bonney V: The technique and result of myomectomy. Lancet 1931; 1: pp. 171-177
- 229. Rouzi AA, Al-Noury AI, Shobokshi AS, et al: Abdominal myomectomy versus abdominal hysterectomy for symptomatic and big uterine fibroids. Saudi Med J 2001; 22: pp. 984-986
- 230. Ecker JL, Foster JT, and Friedman AJ: Abdominal hysterectomy or abdominal myomectomy for symptomatic leiomyoma: a comparison of preoperative demography and postoperative morbidity. J Gynecol Surg 1995; 11: pp. 11-18
- 231. Iverson RE, Chelmow D, Strohbehn K, et al: Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol 1996; 88: pp. 415-419
- 232. Sinha R, Hegde A, Mahajan C, et al: Laparoscopic myomectomy: do size, number, and location of the myomas form limiting factors for laparoscopic myomectomy? J Minim Invasive Gynecol 2008; 15: pp. 292-300
- 233. Hackethal A, Westermann A, Tchartchian G, et al: Laparoscopic myomectomy in patients with uterine myomas associated with infertility. Minim Invasive Ther Allied Technol 2011; 20: pp. 338-345
- 234. Jin C, Hu Y, Chen XC, et al: Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol 2009; 145: pp. 14-21
- 235. Mansour FW, Kives S, Urbach DR, et al: Robotically assisted laparoscopic myomectomy: a Canadian experience. J Obstet Gynaecol Can 2012; 34: pp. 353-358
- 236. Advincula AP, Xu X, Goudeau S, et al: Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol 2007; 14: pp. 698-705
- 237. Barakat EE, Bedaiwy MA, Zimberg S, et al: Robotic-assisted, laparoscopic, and abdominal myomectomy: a comparison of surgical outcomes. Obstet Gynecol 2011; 117: pp. 256-265
- 238. Gargiulo AR, Srouji SS, Missmer SA, et al: Robot-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy. Obstet Gynecol 2012; 120: pp. 284-291
- 239. Bedient CE, Magrina JF, Noble BN, et al: Comparison of robotic and laparoscopic myomectomy. Am J Obstet Gynecol 2009; 201: pp. 566.e1-566.e5
- 240. Iavazzo C, Mamais I, and Gkegkes ID: Robotic assisted vs laparoscopic and/or open myomectomy: systematic review and meta-analysis of the clinical evidence. Arch Gynecol Obstet 2016; 294: pp. 5-17
- 241. Gocmen A, Sanlikan F, and Ucar MG: Comparison of robotic-assisted laparoscopic myomectomy outcomes with laparoscopic myomectomy. Arch Gynecol Obstet 2013; 287: pp. 91-96
- 242. Nezhat C, Lavie O, Hsu S, et al: Robotic-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy—a retrospective matched control study. Fertil Steril 2009; 91: pp. 556-559
- 243. Ascher-Walsh CJ, and Capes TL: Robot-assisted laparoscopic myomectomy is an improvement over laparotomy in women with a limited number of myomas. J Minim Invasive Gynecol 2010; 17: pp. 306-310
- 244. Rosenbaum L: N-of-1 policymaking—tragedy, trade-offs, and the demise of morcellation. N Engl J Med 2016; 374: pp. 986-990
- 245. Lee JY, Kim HS, Nam EJ, et al: Outcomes of uterine sarcoma found incidentally after uterus-preserving surgery for presumed benign disease. BMC Cancer 2016; 16: pp. 675
- 246. Yoshiki N, Okawa T, and Kubota T: Single-incision laparoscopic myomectomy with intracorporeal suturing. Fertil Steril 2011; 95: pp. 2426-2428
- 247. Kim YW, Park BJ, Ro DY, et al: Single-port laparoscopic myomectomy using a new single-port transumbilical morcellation system: initial clinical study. J Minim Invasive Gynecol 2010; 17: pp. 587-592
- 248. Lee JH, Choi JS, Jeon SW, et al: Single-port laparoscopic myomectomy using transumbilical GelPort access. Eur J Obstet Gynecol Reprod Biol 2010; 153: pp. 81-84
- 249. Zaima A, and Ash A: Fibroid in pregnancy: characteristics, complications, and management. Postgrad Med J 2011; 87: pp. 819-828
- 250. Dubuisso JB, Fauconnier A, Babaki-Fard K, et al: Laparoscopic myomectomy: a current view. Hum Reprod Update 2000; 6: pp. 588-594
- 251. American College of Obstetricians and Gynecologists : ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol 2008; 112: pp. 387-400
- 252. Kelly BA, Bright P, and Mackenzie IZ: Does the surgical approach used for myomectomy influence the morbidity in subsequent pregnancy? J Obstet Gynaecol 2008; 28: pp. 77-81
- 253. Sudik R, Husch K, Steller J, et al: Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol Reprod Biol 1996; 65: pp. 209-214
- 254. Vercellini P, Maddalena S, De Giorgi O, et al: Determinants of reproductive outcome after abdominal myomectomy for infertility. Fertil Steril 1999; 72: pp. 109-114
- 255. Marchionni M, Fambrini M, Zambelli V, et al: Reproductive performance before and after abdominal myomectomy: a retrospective analysis. Fertil Steril 2004; 82: pp. 154-159
- 256. Obed JY, and Omigbodun A: Rupture of the uterus in patients with previous myomectomy and primary cesarean section scars: a comparison. J Obstet Gynaecol 1996; 16: pp. 16-21
- 257. Harris WJ: Uterine dehiscence following laparoscopic myomectomy. Obstet Gynecol 1992; 80: pp. 545-546
- 258. Dubuisson JB, Chavet X, Chapron C, et al: Uterine rupture during pregnancy after laparoscopic myomectomy. Hum Reprod 1995; 10: pp. 1475-1477
- 259. Pelosi MA, and Pelosi MA: Spontaneous uterine rupture at thirty-three weeks subsequent to previous superficial laparoscopic myomectomy. Am J Obstet Gynecol 1997; 177: pp. 1547-1549
- 260. Hockstein S: Spontaneous uterine rupture in the early third trimester after laparoscopically assisted myomectomy. A case report. J Reprod Med 2000; 45: pp. 139-141
- 261. Parker WH, Iacampo K, and Long T: Uterine rupture after laparoscopic removal of a pedunculated myoma. J Minim Invasive Gynecol 2007; 14: pp. 362-364
- 262. Pitter MC, Gargiulo AR, Bonaventura LM, et al: Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod 2013; 28: pp. 99-108
- 263. Bocca S, Stadtmauer L, and Oehninger S: Uncomplicated full term pregnancy after da Vinci-assisted laparoscopic myomectomy. Reprod Biomed Online 2007; 14: pp. 246-249
- 264. Lonnerfors C, and Persson J: Pregnancy following robot-assisted laparoscopic myomectomy in women with deep intramural myomas. Acta Obstet Gynecol Scand 2011; 90: pp. 972-977
- 265. Zupi E, Piredda A, Marconi D, et al: Directed laparoscopic cryomyolysis: a possible alternative to myomectomy and/or hysterectomy for symptomatic leiomyomas. Am J Obstet Gynecol 2004; 190: pp. 639-643
- 266. Visvanathan D, Connell R, Hall-Craggs MA, et al: Interstitial laser photocoagulation for uterine myomas. Am J Obstet Gynecol 2002; 187: pp. 382-384
- 267. Goldfarb HA: Bipolar laparoscopic needles for myoma coagulation. J Am Assoc Gynecol Laparosc 1995; 2: pp. 175-179
- 268. Arcangeli S, and Pasquarette MM: Gravid uterine rupture after myolysis. Obstet Gynecol 1997; 89: pp. 857
- 269. Berman JM, Guido RS, Garza Leal JG, et al: Three-year outcome of the Halt trial: a prospective analysis of radiofrequency volumetric thermal ablation of myomas. J Minim Invasive Gynecol 2014; 21: pp. 767-774
- 270. Brucker SY, Hahn M, Kraemer D, et al: Laparoscopic radiofrequency volumetric thermal ablation of fibroids versus laparoscopic myomectomy. Int J Gynaecol Obstet 2014; 125: pp. 261-265
- 271. Perez-Lopez FR, Ornat L, Ceausu I, et al: EMAS position statement: management of uterine fibroids. Maturitas 2014; 79: pp. 106-116
- 272. Metwally M, Cheong YC, and Horne AW: Surgical treatment of fibroids for subfertility. Cochrane Database Syst Rev 2012; undefined:
- 273. Wamsteker K, Emanuel MH, and de Kruif JH: Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol 1993; 82: pp. 736-740
- 274. Derman SG, Rehnstrom J, and Neuwirth RS: The long-term effectiveness of hysteroscopic treatment of menorrhagia and leiomyomas. Obstet Gynecol 1991; 77: pp. 591-594
- 275. Rovio PH, Helin R, and Heinonen PK: Long-term outcome of hysteroscopic endometrial resection with or without myomectomy in patients with menorrhagia. Arch Gynecol Obstet 2009; 279: pp. 159-163
- 276. Ubaldi F, Tournaye H, Camus M, et al: Fertility after hysteroscopic myomectomy. Hum Reprod Update 1995; 1: pp. 81-90
- 277. El-Nashar SA, Hopkins MR, Creedon DJ, et al: Prediction of treatment outcomes after global endometrial ablation. Obstet Gynecol 2009; 113: pp. 97-106
- 278. Marjoribanks J, Lethaby A, and Farquhar C: Surgery versus medical therapy for heavy menstrual bleeding. Cochrane Database Syst Rev 2006; undefined:
- 279. Zapata LB, Whiteman MK, Tepper NK, et al: Intrauterine device use among women with uterine fibroids: a systematic review. Contraception 2010; 82: pp. 41-55
- 280. Ravina JH, Herbreteau D, Ciraru-Vigneron N, et al: Arterial embolisation to treat uterine myomata. Lancet 1995; 346: pp. 671-672
- 281. Spies JB: Uterine artery embolization and gynecologuc embolotherapy. Philadelphia: Lippincott Williams & Wilkins, 2005.
- 282. Gupta JK, Sinha AS, Lumsden MA, et al: Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev 2006; undefined:
- 283. Spies JB, Ascher SA, Roth AR, et al: Uterine artery embolization for leiomyomata. Obstet Gynecol 2001; 98: pp. 29-34
- 284. Worthington-Kirsch RL, Popky GL, and Hutchins FL: Uterine arterial embolization for the management of leiomyomas: quality-of-life assessment and clinical response. Radiology 1998; 208: pp. 625-629
- 285. Worthington-Kirsch R, Spies JB, Myers ER, et al: The Fibroid Registry for outcomes data (FIBROID) for uterine embolization: short-term outcomes. Obstet Gynecol 2005; 106: pp. 52-59
- 286. Pron G, Bennett J, Common A, et al: The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril 2003; 79: pp. 120-127
- 287. Walker WJ, and Pelage JP: Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG 2002; 109: pp. 1262-1272
- 288. Watson GM, and Walker WJ: Uterine artery embolisation for the treatment of symptomatic fibroids in 114 women: reduction in size of the fibroids and women’s views of the success of the treatment. BJOG 2002; 109: pp. 129-135
- 289. Rajan DK, Beecroft JR, Clark TW, et al: Risk of intrauterine infectious complications after uterine artery embolization. J Vasc Interv Radiol 2004; 15: pp. 1415-1421
- 290. Goodwin SC, Spies JB, Worthington-Kirsch R, et al: Uterine artery embolization for treatment of leiomyomata: long-term outcomes from the FIBROID Registry. Obstet Gynecol 2008; 111: pp. 22-33
- 291. Edwards RD, Moss JG, Lumsden MA, et al: Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med 2007; 356: pp. 360-370
- 292. Narayan A, Lee AS, Kuo GP, et al: Uterine artery embolization versus abdominal myomectomy: a long-term clinical outcome comparison. J Vasc Interv Radiol 2010; 21: pp. 1011-1017
- 293. Hirst A, Dutton S, Wu O, et al: A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study. Health Technol Assess 2008; 12: pp. 1-248
- 294. Moss JG, Cooper KG, Khaund A, et al: Randomised comparison of uterine artery embolisation (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): 5-year results. BJOG 2011; 118: pp. 936-944
- 295. Gabriel-Cox K, Jacobson GF, Armstrong MA, et al: Predictors of hysterectomy after uterine artery embolization for leiomyoma. Am J Obstet Gynecol 2007; 196: pp. 588.e1-588.e6
- 296. Walker WJ, and Barton-Smith P: Long-term follow up of uterine artery embolisation—an effective alternative in the treatment of fibroids. BJOG 2006; 113: pp. 464-468
- 297. Spies JB, Bruno J, Czeyda-Pommersheim F, et al: Long-term outcome of uterine artery embolization of leiomyomata. Obstet Gynecol 2005; 106: pp. 933-939
- 298. Popovic M, Berzaczy D, Puchner S, et al: Long-term quality of life assessment among patients undergoing uterine fibroid embolization. AJR Am J Roentgenol 2009; 193: pp. 267-271
- 299. Parthipun AA, Taylor J, Manyonda I, et al: Does size really matter? Analysis of the effect of large fibroids and uterine volumes on complication rates of uterine artery embolisation. Cardiovasc Intervent Radiol 2010; 33: pp. 955-959
- 300. Smeets AJ, Nijenhuis RJ, van Rooij WJ, et al: Uterine artery embolization in patients with a large fibroid burden: long-term clinical and MR follow-up. Cardiovasc Intervent Radiol 2010; 33: pp. 943-948
- 301. Deleted in review.
- 302. Hehenkamp WJ, Volkers NA, Donderwinkel PF, et al: Uterine artery embolization versus hysterectomy in the treatment of symptomatic uterine fibroids (EMMY trial): peri- and postprocedural results from a randomized controlled trial. Am J Obstet Gynecol 2005; 193: pp. 1618-1629
- 303. Volkers NA, Hehenkamp WJ, Birnie E, et al: Uterine artery embolization in the treatment of symptomatic uterine fibroid tumors (EMMY trial): periprocedural results and complications. J Vasc Interv Radiol 2006; 17: pp. 471-480
- 304. Spies JB, Cooper JM, Worthington-Kirsch R, et al: Outcome of uterine embolization and hysterectomy for leiomyomas: results of a multicenter study. Am J Obstet Gynecol 2004; 191: pp. 22-31
- 305. Mara M, Fucikova Z, Maskova J, et al: Uterine fibroid embolization versus myomectomy in women wishing to preserve fertility: preliminary results of a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol 2006; 126: pp. 226-233
- 306. Goodwin SC, Bradley LD, Lipman JC, et al: Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril 2006; 85: pp. 14-21
- 307. Siskin GP, Shlansky-Goldberg RD, Goodwin SC, et al: A prospective multicenter comparative study between myomectomy and uterine artery embolization with polyvinyl alcohol microspheres: long-term clinical outcomes in patients with symptomatic uterine fibroids. J Vasc Interv Radiol 2006; 17: pp. 1287-1295
- 308. Smeets AJ, Nijenhuis RJ, Boekkooi PF, et al: Safety and effectiveness of uterine artery embolization in patients with pedunculated fibroids. J Vasc Interv Radiol 2009; 20: pp. 1172-1175
- 309. Margau R, Simons ME, Rajan DK, et al: Outcomes after uterine artery embolization for pedunculated subserosal leiomyomas. J Vasc Interv Radiol 2008; 19: pp. 657-661
- 310. Burn PR, McCall JM, Chinn RJ, et al: Uterine fibroleiomyoma: MR imaging appearances before and after embolization of uterine arteries. Radiology 2000; 214: pp. 729-734
- 311. deSouza NM, and Williams AD: Uterine arterial embolization for leiomyomas: perfusion and volume changes at MR imaging and relation to clinical outcome. Radiology 2002; 222: pp. 367-374
- 312. Vashisht A, Studd J, Carey A, et al: Fatal septicaemia after fibroid embolisation. Lancet 1999; 354: pp. 307-308
- 313. Goodwin SC, and Walker WJ: Uterine artery embolization for the treatment of uterine fibroids. Curr Opin Obstet Gynecol 1998; 10: pp. 315-320
- 314. Payne JF, and Haney AF: Serious complications of uterine artery embolization for conservative treatment of fibroids. Fertil Steril 2003; 79: pp. 128-131
- 315. de Blok S, de Vries C, Prinssen HM, et al: Fatal sepsis after uterine artery embolization with microspheres. J Vasc Interv Radiol 2003; 14: pp. 779-783
- 316. Gajewska M, and Panek G: Malignant neoplasms of the uterus in women treated with uterine artery embolization for presumed leiomyoma—description of three cases. Ginekol Pol 2013; 84: pp. 229-233
- 317. D’Angelo A, Amso NN, and Wood A: Uterine leiomyosarcoma discovered after uterine artery embolisation. J Obstet Gynaecol 2003; 23: pp. 686-687
- 318. Al-Badr A, and Faught W: Uterine artery embolization in an undiagnosed uterine sarcoma. Obstet Gynecol 2001; 97: pp. 836-837
- 319. Joyce A, Hessami S, and Heller D: Leiomyosarcoma after uterine artery embolization. A case report. J Reprod Med 2001; 46: pp. 278-280
- 320. Kainsbak J, Hansen ES, and Dueholm M: Literature review of outcomes and prevalence and case report of leiomyosarcomas and non-typical uterine smooth muscle leiomyoma tumors treated with uterine artery embolization. Eur J Obstet Gynecol Reprod Biol 2015; 191: pp. 130-137
- 321. McLucas B: Pregnancy following uterine artery embolization: an update. Minim Invasive Ther Allied Technol 2013; 22: pp. 39-44
- 322. Ravina JH, Vigneron NC, Aymard A, et al: Pregnancy after embolization of uterine myoma: report of 12 cases. Fertil Steril 2000; 73: pp. 1241-1243
- 323. Goldberg J, Pereira L, Berghella V, et al: Pregnancy outcomes after treatment for fibromyomata: uterine artery embolization versus laparoscopic myomectomy. Am J Obstet Gynecol 2004; 191: pp. 18-21
- 324. Walker WJ, and McDowell SJ: Pregnancy after uterine artery embolization for leiomyomata: a series of 56 completed pregnancies. Am J Obstet Gynecol 2006; 195: pp. 1266-1271
- 325. Pinto Pabon I, Magret JP, Unzurrunzaga EA, et al: Pregnancy after uterine fibroid embolization: follow-up of 100 patients embolized using tris-acryl gelatin microspheres. Fertil Steril 2008; 90: pp. 2356-2360
- 326. Pron G, Mocarski E, Bennett J, et al: Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol 2005; 105: pp. 67-76
- 327. Pisco JM, Duarte M, Bilhim T, et al: Pregnancy after uterine fibroid embolization. Fertil Steril 2011; 95: pp. 1121.e5-1121.e8
- 328. Homer H, and Saridogan E: Uterine artery embolization for fibroids is associated with an increased risk of miscarriage. Fertil Steril 2010; 94: pp. 324-330
- 329. Deleted in review.
- 330. Spies JB, Roth AR, Gonsalves SM, et al: Ovarian function after uterine artery embolization for leiomyomata: assessment with use of serum follicle stimulating hormone assay. J Vasc Interv Radiol 2001; 12: pp. 437-442
- 331. Healey S, Buzaglo K, Seti L, et al: Ovarian function after uterine artery embolization and hysterectomy. J Am Assoc Gynecol Laparosc 2004; 11: pp. 348-352
- 332. Kim HS, Tsai J, Lee JM, et al: Effects of utero-ovarian anastomoses on basal follicle-stimulating hormone level change after uterine artery embolization with tris-acryl gelatin microspheres. J Vasc Interv Radiol 2006; 17: pp. 965-971
- 333. Hehenkamp WJ, Volkers NA, Broekmans FJ, et al: Loss of ovarian reserve after uterine artery embolization: a randomized comparison with hysterectomy. Hum Reprod 2007; 22: pp. 1996-2005
- 334. Trabuco EC, Moorman PG, Algeciras-Schimnich A, et al: Association of ovary-sparing hysterectomy with ovarian reserve. Obstet Gynecol 2016; 127: pp. 819-827
- 335. Al Hilli MM, and Stewart EA: Magnetic resonance-guided focused ultrasound surgery. Semin Reprod Med 2010; 28: pp. 242-249
- 336. Gizzo S, Saccardi C, Patrelli TS, et al: Magnetic resonance-guided focused ultrasound myomectomy: safety, efficacy, subsequent fertility and quality-of-life improvements, a systematic review. Reprod Sci 2014; 21: pp. 465-476
- 337. Stewart EA, Gedroyc WM, Tempany CM, et al: Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol 2003; 189: pp. 48-54
- 338. Funaki K, Fukunishi H, Funaki T, et al: Mid-term outcome of magnetic resonance-guided focused ultrasound surgery for uterine myomas: from six to twelve months after volume reduction. J Minim Invasive Gynecol 2007; 14: pp. 616-621
- 339. Stewart EA, Rabinovici J, Tempany CM, et al: Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril 2006; 85: pp. 22-29
- 340. Stewart EA, Gostout B, Rabinovici J, et al: Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol 2007; 110: pp. 279-287
- 341. Gorny KR, Woodrum DA, Brown DL, et al: Magnetic resonance-guided focused ultrasound of uterine leiomyomas: review of a 12-month outcome of 130 clinical patients. J Vasc Interv Radiol 2011; 22: pp. 857-864
- 342. LeBlang SD, Hoctor K, and Steinberg FL: Leiomyoma shrinkage after MRI-guided focused ultrasound treatment: report of 80 patients. AJR Am J Roentgenol 2010; 194: pp. 274-280
- 343. Funaki K, Fukunishi H, and Sawada K: Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol 2009; 34: pp. 584-589
- 344. Spies JB, Coyne K, Guaou Guaou N, et al: The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol 2002; 99: pp. 290-300
- 345. Fennessy FM, Tempany CM, McDannold NJ, et al: Uterine leiomyomas: MR imaging-guided focused ultrasound surgery—results of different treatment protocols. Radiology 2007; 243: pp. 885-893
- 346. Mindjuk I, Trumm CG, Herzog P, et al: MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol 2015; 25: pp. 1317-1328
- 347. Morita Y, Ito N, Hikida H, et al: Non-invasive magnetic resonance imaging-guided focused ultrasound treatment for uterine fibroids—early experience. Eur J Obstet Gynecol Reprod Biol 2008; 139: pp. 199-203
- 348. Quinn SD, Vedelago J, Gedroyc W, et al: Safety and five-year re-intervention following magnetic resonance-guided focused ultrasound (MRgFUS) for uterine fibroids. Eur J Obstet Gynecol Reprod Biol 2014; 182: pp. 247-251
- 349. Leon-Villapalos J, Kaniorou-Larai M, and Dziewulski P: Full thickness abdominal burn following magnetic resonance guided focused ultrasound therapy. Burns 2005; 31: pp. 1054-1055
- 350. Yoon SW, Seong SJ, Jung SG, et al: Mitigation of abdominal scars during MR-guided focused ultrasound treatment of uterine leiomyomas with the use of an energy-blocking scar patch. J Vasc Interv Radiol 2011; 22: pp. 1747-1750
- 351. Gorny KR, Chen S, Hangiandreou NJ, et al: Initial evaluation of acoustic reflectors for the preservation of sensitive abdominal skin areas during MRgFUS treatment. Phys Med Biol 2009; 54: pp. N125-N133
- 352. Taran FA, Tempany CM, Regan L, et al: Magnetic resonance-guided focused ultrasound (MRgFUS) compared with abdominal hysterectomy for treatment of uterine leiomyomas. Ultrasound Obstet Gynecol 2009; 34: pp. 572-578
- 353. Borah BJ, Carls GS, Moore BJ, et al: Cost comparison between uterine-sparing fibroid treatments one year following treatment. J Ther Ultrasound 2014; 2: pp. 7
- 354. Cain-Nielsen AH, Moriarty JP, Stewart EA, et al: Cost-effectiveness of uterine-preserving procedures for the treatment of uterine fibroid symptoms in the USA. J Comp Eff Res 2014; 3: pp. 503-514
- 355. Zowall H, Cairns JA, Brewer C, et al: Cost-effectiveness of magnetic resonance-guided focused ultrasound surgery for treatment of uterine fibroids. BJOG 2008; 115: pp. 653-662
- 356. O’Sullivan AK, Thompson D, Chu P, et al: Cost-effectiveness of magnetic resonance guided focused ultrasound for the treatment of uterine fibroids. Int J Technol Assess Health Care 2009; 25: pp. 14-25
- 357. Clark NA, Mumford SL, and Segars JH: Reproductive impact of MRI-guided focused ultrasound surgery for fibroids: a systematic review of the evidence. Curr Opin Obstet Gynecol 2014; 26: pp. 151-161
- 358. Bohlmann MK, Hoellen F, Hunold P, et al: High-intensity focused ultrasound ablation of uterine fibroids—potential impact on fertility and pregnancy outcome. Geburtshilfe Frauenheilkd 2014; 74: pp. 139-145
- 359. Rabinovici J, David M, Fukunishi H, et al: Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril 2010; 93: pp. 199-209
- 360. Bouwsma EV, Gorny KR, Hesley GK, et al: Magnetic resonance-guided focused ultrasound surgery for leiomyoma-associated infertility. Fertil Steril 2011; 96: pp. e9-e12
- 361. Orsi F, Arnone P, Chen W, et al: High intensity focused ultrasound ablation: a new therapeutic option for solid tumors. J Cancer Res Ther 2010; 6: pp. 414-420
- 362. Chan AH, Fujimoto VY, Moore DE, et al: In vivo feasibility of image-guided transvaginal focused ultrasound therapy for the treatment of intracavitary fibroids. Fertil Steril 2004; 82: pp. 723-730
- 363. Fruehauf JH, Back W, Eiermann A, et al: High-intensity focused ultrasound for the targeted destruction of uterine tissues: experiences from a pilot study using a mobile HIFU unit. Arch Gynecol Obstet 2008; 277: pp. 143-150
- 364. Chen WZ, Tang LD, Yang WW, et al: Study on the efficacy and safety of ultrasound ablation in treatment of uterine fibroids. Zhonghua Fu Chan Ke Za Zhi 2010; 45: pp. 909-912
- 365. Wang T, Wang W, Chen WZ, et al: Efficacy and safety of focused ultrasound ablation in treatment of submucosal uterine fibroids. Zhonghua Fu Chan Ke Za Zhi 2011; 46: pp. 407-411
- 366. Zhou M, Chen JY, Tang LD, et al: Ultrasound-guided high-intensity focused ultrasound ablation for adenomyosis: the clinical experience of a single center. Fertil Steril 2011; 95: pp. 900-905
- 367. Qin J, Chen JY, Zhao WP, et al: Outcome of unintended pregnancy after ultrasound-guided high-intensity focused ultrasound ablation of uterine fibroids. Int J Gynaecol Obstet 2012; 117: pp. 273-277
- 368. Viswanathan M, Hartmann K, McKoy N, et al: Management of uterine fibroids: an update of the evidence. Evid Rep Technol Assess (Full Rep) 2007; 154: pp. 1-122
- 369. Bradley LD, and Gueye NA: The medical management of abnormal uterine bleeding in reproductive-aged women. Am J Obstet Gynecol 2016; 214: pp. 31-44
- 370. Mutter GL, Bergeron C, Deligdisch L, et al: The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol 2008; 21: pp. 591-598
- 371. Steinauer J, Pritts EA, Jackson R, et al: Systematic review of mifepristone for the treatment of uterine leiomyomata. Obstet Gynecol 2004; 103: pp. 1331-1336
- 372. Donnez J, Tatarchuk TF, Bouchard P, et al: Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 2012; 366: pp. 409-420
- 373. Donnez J, Tomaszewski J, Vazquez F, et al: Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med 2012; 366: pp. 421-432
- 374. Donnez J, Vazquez F, Tomaszewski J, et al: Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril 2014; 101: pp. 1565-1573.e1-e18
- 375. Luyckx M, Squifflet JL, Jadoul P, et al: First series of 18 pregnancies after ulipristal acetate treatment for uterine fibroids. Fertil Steril 2014; 102: pp. 1404-1409
- 376. Stewart EA: Uterine fibroids and evidence-based medicine—not an oxymoron. N Engl J Med 2012; 366: pp. 471-473
- 377. Kapur A, Angomchanu R, and Dey M: Efficacy of use of long-term, low-dose mifepristone for the treatment of fibroids. J Obstet Gynaecol India 2016; 66: pp. 494-498
- 378. Shen Q, Hua Y, Jiang W, et al: Effects of mifepristone on uterine leiomyoma in premenopausal women: a meta-analysis. Fertil Steril 2013; 100: pp. 1722-1726.e1-e10
- 379. Fiscella K, Eisinger SH, Meldrum S, et al: Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size: a randomized controlled trial. Obstet Gynecol 2006; 108: pp. 1381-1387
- 380. Carbonell Esteve JL, Acosta R, Heredia B, et al: Mifepristone for the treatment of uterine leiomyomas: a randomized controlled trial. Obstet Gynecol 2008; 112: pp. 1029-1036
- 381. Engman M, Granberg S, Williams AR, et al: Mifepristone for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Hum Reprod 2009; 24: pp. 1870-1879
- 382. Bagaria M, Suneja A, Vaid NB, et al: Low-dose mifepristone in treatment of uterine leiomyoma: a randomised double-blind placebo-controlled clinical trial. Aust N Z J Obstet Gynaecol 2009; 49: pp. 77-83
- 383. Eisinger SH, Bonfiglio T, Fiscella K, et al: Twelve-month safety and efficacy of low-dose mifepristone for uterine myomas. J Minim Invasive Gynecol 2005; 12: pp. 227-233
- 384. Murphy AA, Kettel LM, Morales AJ, et al: Regression of uterine leiomyomata in response to the antiprogesterone RU 486. J Clin Endocrinol Metab 1993; 76: pp. 513-517
- 385. Hodgen GD: Antiprogestins: the political chemistry of RU486. Fertil Steril 1991; 56: pp. 394-395
- 386. Ioffe OB, Zaino RJ, and Mutter GL: Endometrial changes from short-term therapy with CDB-4124, a selective progesterone receptor modulator. Mod Pathol 2009; 22: pp. 450-459
- 387. Bouchard P, Chabbert-Buffet N, and Fauser BC: Selective progesterone receptor modulators in reproductive medicine: pharmacology, clinical efficacy and safety. Fertil Steril 2011; 96: pp. 1175-1189
- 388. Chwalisz K, Perez MC, Demanno D, et al: Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev 2005; 26: pp. 423-438
- 389. Friedman AJ, Barbieri RL, Doubilet PM, et al: A randomized, double-blind trial of a gonadotropin releasing-hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril 1988; 49: pp. 404-409
- 390. Friedman AJ, Daly M, Juneau-Norcross M, et al: Predictors of uterine volume reduction in women with myomas treated with a gonadotropin-releasing hormone agonist. Fertil Steril 1992; 58: pp. 413-415
- 391. Rein MS, Friedman AJ, Stuart JM, et al: Fibroid and myometrial steroid receptors in women treated with gonadotropin-releasing hormone agonist leuprolide acetate. Fertil Steril 1990; 53: pp. 1018-1023
- 392. Lethaby A, Vollenhoven B, and Sowter M: Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. BJOG 2002; 109: pp. 1097-1108
- 393. Zhang Y, Sun L, Guo Y, et al: The impact of preoperative gonadotropin-releasing hormone agonist treatment on women with uterine fibroids: a meta-analysis. Obstet Gynecol Surv 2014; 69: pp. 100-108
- 394. Friedman AJ, Lobel SM, Rein MS, et al: Efficacy and safety considerations in women with uterine leiomyomas treated with gonadotropin-releasing hormone agonists: the estrogen threshold hypothesis. Am J Obstet Gynecol 1990; 163: pp. 1114-1119
- 395. Friedman AJ: Treatment of leiomyomata uteri with short-term leuprolide followed by leuprolide plus estrogen-progestin hormone replacement therapy for 2 years: a pilot study. Fertil Steril 1989; 51: pp. 526-528
- 396. Thomas EJ: Add-back therapy for long-term use in dysfunctional uterine bleeding and uterine fibroids. Br J Obstet Gynaecol 1996; 103: pp. 18-21
- 397. Moroni RM, Martins WP, Ferriani RA, et al: Add-back therapy with GnRH analogues for uterine fibroids. Cochrane Database Syst Rev 2015; undefined:
- 398. Felberbaum RE, Germer U, Ludwig M, et al: Treatment of uterine fibroids with a slow-release formulation of the gonadotrophin releasing hormone antagonist Cetrorelix. Hum Reprod 1998; 13: pp. 1660-1668
- 399. Gonzalez-Barcena D, Alvarez RB, Ochoa EP, et al: Treatment of uterine leiomyomas with luteinizing hormone-releasing hormone antagonist Cetrorelix. Hum Reprod 1997; 12: pp. 2028-2035
- 400. Flierman PA, Oberye JJ, van der Hulst VP, et al: Rapid reduction of leiomyoma volume during treatment with the GnRH antagonist ganirelix. BJOG 2005; 112: pp. 638-642
- 401. Felberbaum RE, Kupker W, Krapp M, et al: Preoperative reduction of uterine fibroids in only 16 days by administration of a gonadotrophin-releasing hormone antagonist (Cetrotide). Reprod Biomed Online 2001; 3: pp. 14-18
- 402. Song H, Lu D, Navaratnam K, et al: Aromatase inhibitors for uterine fibroids. Cochrane Database Syst Rev 2013; undefined:
- 403. Duhan N, Madaan S, and Sen J: Role of the aromatase inhibitor letrozole in the management of uterine leiomyomas in premenopausal women. Eur J Obstet Gynecol Reprod Biol 2013; 171: pp. 329-332
- 404. Shozu M, Murakami K, Segawa T, et al: Successful treatment of a symptomatic uterine leiomyoma in a perimenopausal woman with a nonsteroidal aromatase inhibitor. Fertil Steril 2003; 79: pp. 628-631
- 405. Varelas FK, Papanicolaou AN, Vavatsi-Christaki N, et al: The effect of anastrazole on symptomatic uterine leiomyomata. Obstet Gynecol 2007; 110: pp. 643-649
- 406. Hilario SG, Bozzini N, Borsari R, et al: Action of aromatase inhibitor for treatment of uterine leiomyoma in perimenopausal patients. Fertil Steril 2009; 91: pp. 240-243
- 407. Parsanezhad ME, Azmoon M, Alborzi S, et al: A randomized, controlled clinical trial comparing the effects of aromatase inhibitor (letrozole) and gonadotropin-releasing hormone agonist (triptorelin) on uterine leiomyoma volume and hormonal status. Fertil Steril 2010; 93: pp. 192-198
- 408. Lingxia X, Taixiang W, and Xiaoyan C: Selective estrogen receptor modulators (SERMs) for uterine leiomyomas. Cochrane Database Syst Rev 2007; undefined:
- 409. Felmingham JE, and Corcoran R: Letter: Rapid enlargement of a uterine fibroid after clomiphene therapy. Br J Obstet Gynaecol 1975; 82: pp. 431-432
- 410. Fuchs-Young R, Howe S, Hale L, et al: Inhibition of estrogen-stimulated growth of uterine leiomyomas by selective estrogen receptor modulators. Mol Carcinog 1996; 17: pp. 151-159
- 411. Walker CL, Burroughs KD, Davis B, et al: Preclinical evidence for therapeutic efficacy of selective estrogen receptor modulators for uterine leiomyoma. J Soc Gynecol Investig 2000; 7: pp. 249-256
- 412. Palomba S, Sammartino A, Di Carlo C, et al: Effects of raloxifene treatment on uterine leiomyomas in postmenopausal women. Fertil Steril 2001; 76: pp. 38-43
- 413. Palomba S, Russo T, Orio F, et al: Effectiveness of combined GnRH analogue plus raloxifene administration in the treatment of uterine leiomyomas: a prospective, randomized, single-blind, placebo-controlled clinical trial. Hum Reprod 2002; 17: pp. 3213-3219
- 414. Palomba S, Orio F, Morelli M, et al: Raloxifene administration in premenopausal women with uterine leiomyomas: a pilot study. J Clin Endocrinol Metab 2002; 87: pp. 3603-3608
- 415. Coutinho EM, and Goncalves MT: Long-term treatment of leiomyomas with gestrinone. Fertil Steril 1989; 51: pp. 939-946
- 416. De Leo V, la Marca A, Morgante G, et al: Administration of somatostatin analogue reduces uterine and myoma volume in women with uterine leiomyomata. Fertil Steril 2001; 75: pp. 632-633
- 417. De Leo V, La Marca A, Vegni V, et al: Quantitative determination of sst2 and sst5 gene expression in uterine leiomyomata and the effect of treatment with somatostatin analogue. Fertil Steril 2003; 80: pp. 1058-1059
- 418. Minakuchi K, Kawamura N, Tsujimura A, et al: Remarkable and persistent shrinkage of uterine leiomyoma associated with interferon alfa treatment for hepatitis. Lancet 1999; 353: pp. 2127-2128
- 419. Shime H, Kariya M, Orii A, et al: Tranilast inhibits the proliferation of uterine leiomyoma cells in vitro through G1 arrest associated with the induction of p21(waf1) and p53. J Clin Endocrinol Metab 2002; 87: pp. 5610-5617
- 420. Zaitseva M, Vollenhoven BJ, and Rogers PA: Retinoic acid pathway genes show significantly altered expression in uterine fibroids when compared with normal myometrium. Mol Hum Reprod 2007; 13: pp. 577-585
- 421. Malik M, Webb J, and Catherino WH: Retinoic acid treatment of human leiomyoma cells transformed the cell phenotype to one strongly resembling myometrial cells. Clin Endocrinol (Oxf) 2008; 69: pp. 462-470
- 422. Blauer M, Rovio PH, Ylikomi T, et al: Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil Steril 2009; 91: pp. 1919-1925
- 423. Deleted in review.
- 424. Zhang D, Al-Hendy M, Richard-Davis G, et al: Antiproliferative and proapoptotic effects of epigallocatechin gallate on human leiomyoma cells. Fertil Steril 2010; 94: pp. 1887-1893
- 425. Weiss G, Maseelall P, Schott LL, et al: Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women’s Health Across the Nation (SWAN). Fertil Steril 2009; 91: pp. 201-206
- 426. McElin TW, and Bird CC: Adenomyosis of the uterus. Obstet Gynecol Annu 1974; 3: pp. 425-441
- 427. Vercellini P, Parazzini F, Oldani S, et al: Adenomyosis at hysterectomy: a study on frequency distribution and patient characteristics. Hum Reprod 1995; 10: pp. 1160-1162
- 428. Raju GC, Naraynsingh V, Woo J, et al: Adenomyosis uteri: a study of 416 cases. Aust N Z J Obstet Gynaecol 1988; 28: pp. 72-73
- 429. Taran FA, Wallwiener M, Kabashi D, et al: Clinical characteristics indicating adenomyosis at the time of hysterectomy: a retrospective study in 291 patients. Arch Gynecol Obstet 2012; 285: pp. 1571-1576
- 430. Templeman C, Marshall SF, Ursin G, et al: Adenomyosis and endometriosis in the California Teachers Study. Fertil Steril 2008; 90: pp. 415-424
- 431. Panganamamula UR, Harmanli OH, Isik-Akbay EF, et al: Is prior uterine surgery a risk factor for adenomyosis? Obstet Gynecol 2004; 104: pp. 1034-1038
- 432. Parazzini F, Vercellini P, Panazza S, et al: Risk factors for adenomyosis. Hum Reprod 1997; 12: pp. 1275-1279
- 433. Ryan GL, Stolpen A, and Van Voorhis BJ: An unusual cause of adolescent dysmenorrhea. Obstet Gynecol 2006; 108: pp. 1017-1022
- 434. Ho ML, Ratts V, and Merritt D: Adenomyotic cyst in an adolescent girl. J Pediatr Adolesc Gynecol 2009; 22: pp. e33-e38
- 435. Taran FA, Weaver AL, Coddington CC, et al: Characteristics indicating adenomyosis coexisting with leiomyomas: a case-control study. Hum Reprod 2010; 25: pp. 1177-1182
- 436. Bergholt T, Eriksen L, Berendt N, et al: Prevalence and risk factors of adenomyosis at hysterectomy. Hum Reprod 2001; 16: pp. 2418-2421
- 437. Munro MG, Critchley HO, Broder MS, et al: FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet 2011; 113: pp. 3-13
- 438. Struble J, Reid S, and Bedaiwy MA: Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol 2016; 23: pp. 164-185
- 439. Ferenczy A: Pathophysiology of adenomyosis. Hum Reprod Update 1998; 4: pp. 312-322
- 440. Ota H, and Tanaka T: Stromal vascularization in the endometrium during adenomyosis. Microsc Res Tech 2003; 60: pp. 445-449
- 441. Ota H, Igarashi S, and Tanaka T: Morphometric evaluation of stromal vascularization in the endometrium in adenomyosis. Hum Reprod 1998; 13: pp. 715-719
- 442. Nishida M: Relationship between the onset of dysmenorrhea and histologic findings in adenomyosis. Am J Obstet Gynecol 1991; 165: pp. 229-231
- 443. Enatsu A, Harada T, Yoshida S, et al: Adenomyosis in a patient with the Rokitansky-Kuster-Hauser syndrome. Fertil Steril 2000; 73: pp. 862-863
- 444. Matsumoto Y, Iwasaka T, Yamasaki F, et al: Apoptosis and Ki-67 expression in adenomyotic lesions and in the corresponding eutopic endometrium. Obstet Gynecol 1999; 94: pp. 71-77
- 445. Ota H, Igarashi S, Hatazawa J, et al: Immunohistochemical assessment of superoxide dismutase expression in the endometrium in endometriosis and adenomyosis. Fertil Steril 1999; 72: pp. 129-134
- 446. Propst AM, Quade BJ, Gargiulo AR, et al: Adenomyosis demonstrates increased expression of the basic fibroblast growth factor receptor/ligand system compared with autologous endometrium. Menopause 2001; 8: pp. 368-371
- 447. Hatazawa J, Ota H, Murata M, et al: Localization of endothelial nitric oxide synthase messenger ribonucleic acid by in situ hybridization in ectopic endometrial tissue in patients with adenomyosis. Reprod Fertil Dev 2000; 12: pp. 283-287
- 448. Lei ZM, Rao CV, Lincoln SR, et al: Increased expression of human chorionic gonadotropin/human luteinizing hormone receptors in adenomyosis. J Clin Endocrinol Metab 1993; 76: pp. 763-768
- 449. Schindl M, Birner P, Obermair A, et al: Increased microvessel density in adenomyosis uteri. Fertil Steril 2001; 75: pp. 131-135
- 450. Kang S, Zhao J, Liu Q, et al: Vascular endothelial growth factor gene polymorphisms are associated with the risk of developing adenomyosis. Environ Mol Mutagen 2009; 50: pp. 361-366
- 451. Yang JH, Wu MY, Chen MJ, et al: Increased matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 secretion but unaffected invasiveness of endometrial stromal cells in adenomyosis. Fertil Steril 2009; 91: pp. 2193-2198
- 452. Wang F, Li H, Yang Z, et al: Expression of interleukin-10 in patients with adenomyosis. Fertil Steril 2009; 91: pp. 1681-1685
- 453. Ishihara H, Kitawaki J, Kado N, et al: Gonadotropin-releasing hormone agonist and danazol normalize aromatase cytochrome P450 expression in eutopic endometrium from women with endometriosis, adenomyosis, or leiomyomas. Fertil Steril 2003; 79: pp. 735-742
- 454. Zhou YF, Mori T, Kudo H, et al: Effects of angiogenesis inhibitor TNP-470 on the development of uterine adenomyosis in mice. Fertil Steril 2003; 80: pp. 788-794
- 455. Brosens JJ, de Souza NM, and Barker FG: Uterine junctional zone: function and disease. Lancet 1995; 346: pp. 558-560
- 456. Wang S, Duan H, Zhang Y, et al: Expression of RhoA and Rho kinase in junctional zone of human adenomyosis and its relationship with dysmenorrheal. Zhonghua Fu Chan Ke Za Zhi 2013; 48: pp. 911-915
- 457. Zhang Y, Yu P, Sun F, et al: Expression of oxytocin receptors in the uterine junctional zone in women with adenomyosis. Acta Obstet Gynecol Scand 2015; 94: pp. 412-418
- 458. Ibrahim MG, Chiantera V, Frangini S, et al: Ultramicro-trauma in the endometrial-myometrial junctional zone and pale cell migration in adenomyosis. Fertil Steril 2015; 104: pp. 1475-1483.e1-e3
- 459. Wang S, Duan H, Zhang Y, et al: Abnormal activation of RhoA/ROCK-I signaling in junctional zone smooth muscle cells of patients with adenomyosis. Reprod Sci 2016; 23: pp. 333-341
- 460. Yamamoto T, Noguchi T, Tamura T, et al: Evidence for estrogen synthesis in adenomyotic tissues. Am J Obstet Gynecol 1993; 169: pp. 734-738
- 461. Ezaki K, Motoyama H, and Sasaki H: Immunohistologic localization of estrone sulfatase in uterine endometrium and adenomyosis. Obstet Gynecol 2001; 98: pp. 815-819
- 462. Kitawaki J: Adenomyosis: the pathophysiology of an oestrogen-dependent disease. Best Pract Res Clin Obstet Gynaecol 2006; 20: pp. 493-502
- 463. Shaikh H, and Khan KS: Adenomyosis in Pakistani women: four year experience at the Aga Khan University Medical Centre, Karachi. J Clin Pathol 1990; 43: pp. 817-819
- 464. Green AR, Styles JA, Parrott EL, et al: Neonatal tamoxifen treatment of mice leads to adenomyosis but not uterine cancer. Exp Toxicol Pathol 2005; 56: pp. 255-263
- 465. Mori T, Kyokuwa M, and Nagasawa H: Animal model of uterine adenomyosis: induction of the lesion in rats by ectopic pituitary isografting. Lab Anim Sci 1998; 48: pp. 64-68
- 466. Mori T, Singtripop T, and Kawashima S: Animal model of uterine adenomyosis: is prolactin a potent inducer of adenomyosis in mice? Am J Obstet Gynecol 1991; 165: pp. 232-234
- 467. Nagasawa H, and Mori T: Stimulation of mammary tumorigenesis and suppression of uterine adenomyosis by temporary inhibition of pituitary prolactin secretion during youth in mice (41492). Proc Soc Exp Biol Med 1982; 171: pp. 164-167
- 468. Ficicioglu C, Tekin HI, Arioglu PF, et al: A murine model of adenomyosis: the effects of hyperprolactinemia induced by fluoxetine hydrochloride, a selective serotonin reuptake inhibitor, on adenomyosis induction in Wistar albino rats. Acta Eur Fertil 1995; 26: pp. 75-79
- 469. Danilovich N, Roy I, and Sairam MR: Emergence of uterine pathology during accelerated biological aging in FSH receptor-haploinsufficient mice. Endocrinology 2002; 143: pp. 3618-3627
- 470. Byun JY, Kim SE, Choi BG, et al: Diffuse and focal adenomyosis: MR imaging findings. Radiographics 1999; 19: pp. S161-S170
- 471. Reinhold C, Tafazoli F, Mehio A, et al: Uterine adenomyosis: endovaginal US and MR imaging features with histopathologic correlation. Radiographics 1999; 19: pp. S147-S160
- 472. Reinhold C, McCarthy S, Bret PM, et al: Diffuse adenomyosis: comparison of endovaginal US and MR imaging with histopathologic correlation. Radiology 1996; 199: pp. 151-158
- 473. Exacoustos C, Manganaro L, and Zupi E: Imaging for the evaluation of endometriosis and adenomyosis. Best Pract Res Clin Obstet Gynaecol 2014; 28: pp. 655-681
- 474. Champaneria R, Abedin P, Daniels J, et al: Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand 2010; 89: pp. 1374-1384
- 475. Katsumata Y, Noda M, Tanaka M, et al: CT appearance of uterine adenomyosis. Rinsho Hoshasen 1989; 34: pp. 227-232
- 476. Levgur M: Diagnosis of adenomyosis: a review. J Reprod Med 2007; 52: pp. 177-193
- 477. Grow DR, and Filer RB: Treatment of adenomyosis with long-term GnRH analogues: a case report. Obstet Gynecol 1991; 78: pp. 538-539
- 478. Hirata JD, Moghissi KS, and Ginsburg KA: Pregnancy after medical therapy of adenomyosis with a gonadotropin-releasing hormone agonist. Fertil Steril 1993; 59: pp. 444-445
- 479. Nelson JR, and Corson SL: Long-term management of adenomyosis with a gonadotropin-releasing hormone agonist: a case report. Fertil Steril 1993; 59: pp. 441-443
- 480. Ozdegirmenci O, Kayikcioglu F, Akgul MA, et al: Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertil Steril 2011; 95: pp. 497-502
- 481. Fong YF, and Singh K: Medical treatment of a grossly enlarged adenomyotic uterus with the levonorgestrel-releasing intrauterine system. Contraception 1999; 60: pp. 173-175
- 482. Fedele L, Bianchi S, Raffaelli R, et al: Treatment of adenomyosis-associated menorrhagia with a levonorgestrel-releasing intrauterine device. Fertil Steril 1997; 68: pp. 426-429
- 483. Bragheto AM, Caserta N, Bahamondes L, et al: Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception 2007; 76: pp. 195-199
- 484. Sheng J, Zhang WY, Zhang JP, et al: The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception 2009; 79: pp. 189-193
- 485. Igarashi M, Abe Y, Fukuda M, et al: Novel conservative medical therapy for uterine adenomyosis with a danazol-loaded intrauterine device. Fertil Steril 2000; 74: pp. 412-413
- 486. Morita M, Asakawa Y, Nakakuma M, et al: Laparoscopic excision of myometrial adenomyomas in patients with adenomyosis uteri and main symptoms of severe dysmenorrhea and hypermenorrhea. J Am Assoc Gynecol Laparosc 2004; 11: pp. 86-89
- 487. Sun AJ, Luo M, Wang W, et al: Characteristics and efficacy of modified adenomyomectomy in the treatment of uterine adenomyoma. Chin Med J 2011; 124: pp. 1322-1326
- 488. Wang PH, Liu WM, Fuh JL, et al: Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril 2009; 92: pp. 876-885
- 489. Wood C: Surgical and medical treatment of adenomyosis. Hum Reprod Update 1998; 4: pp. 323-336
- 490. McCausland V, and McCausland A: The response of adenomyosis to endometrial ablation/resection. Hum Reprod Update 1998; 4: pp. 350-359
- 491. Nijenhuis RJ, Smeets AJ, Morpurgo M, et al: Uterine artery embolisation for symptomatic adenomyosis with polyzene F-coated hydrogel microspheres: three-year clinical follow-up using UFS-QoL questionnaire. Cardiovasc Intervent Radiol 2015; 38: pp. 65-71
- 492. Kim MD, Kim S, Kim NK, et al: Long-term results of uterine artery embolization for symptomatic adenomyosis. AJR Am J Roentgenol 2007; 188: pp. 176-181
- 493. Popovic M, Puchner S, Berzaczy D, et al: Uterine artery embolization for the treatment of adenomyosis: a review. J Vasc Interv Radiol 2011; 22: pp. 901-909
- 494. Smeets AJ, Nijenhuis RJ, Boekkooi PF, et al: Long-term follow-up of uterine artery embolization for symptomatic adenomyosis. Cardiovasc Intervent Radiol 2012; 35: pp. 815-819
- 495. Fukunishi H, Funaki K, Sawada K, et al: Early results of magnetic resonance-guided focused ultrasound surgery of adenomyosis: analysis of 20 cases. J Minim Invasive Gynecol 2008; 15: pp. 571-579
- 496. Rabinovici J, Inbar Y, Eylon SC, et al: Pregnancy and live birth after focused ultrasound surgery for symptomatic focal adenomyosis: a case report. Hum Reprod 2006; 21: pp. 1255-1259
- 497. Vercellini P, Consonni D, Dridi D, et al: Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum Reprod 2014; 29: pp. 964-977
- 498. Harada T, Khine YM, Kaponis A, et al: The impact of adenomyosis on women’s fertility. Obstet Gynecol Surv 2016; 71: pp. 557-568
- 499. Garavaglia E, Audrey S, Annalisa I, et al: Adenomyosis and its impact on women fertility. Iran J Reprod Med 2015; 13: pp. 327-336
- 500. Campo S, Campo V, and Benagiano G: Infertility and adenomyosis. Obstet Gynecol Int 2012; 2012: pp. 786132
- 501. Maheshwari A, Gurunath S, Fatima F, et al: Adenomyosis and subfertility: a systematic review of prevalence, diagnosis, treatment and fertility outcomes. Hum Reprod Update 2012; 18: pp. 374-392
- 502. Thalluri V, and Tremellen KP: Ultrasound diagnosed adenomyosis has a negative impact on successful implantation following GnRH antagonist IVF treatment. Hum Reprod 2012; 27: pp. 3487-3492
- 503. Benaglia L, Cardellicchio L, Leonardi M, et al: Asymptomatic adenomyosis and embryo implantation in IVF cycles. Reprod Biomed Online 2014; 29: pp. 606-611
- 504. Juang CM, Chou P, Yen MS, et al: Adenomyosis and risk of preterm delivery. BJOG 2007; 114: pp. 165-169
- 505. Su T, and Sui L: Expression and significance of p63, aromatase P450 and steroidogenic factor-1 in endometrial polyp. Zhonghua Fu Chan Ke Za Zhi 2014; 49: pp. 604-608
- 506. Maia H, Pimentel K, Silva TM, et al: Aromatase and cyclooxygenase-2 expression in endometrial polyps during the menstrual cycle. Gynecol Endocrinol 2006; 22: pp. 219-224
- 507. Pal L, Niklaus AL, Kim M, et al: Heterogeneity in endometrial expression of aromatase in polyp-bearing uteri. Hum Reprod 2008; 23: pp. 80-84
- 508. Jovanovic AS, Boynton KA, and Mutter GL: Uteri of women with endometrial carcinoma contain a histopathological spectrum of monoclonal putative precancers, some with microsatellite instability. Cancer Res 1996; 56: pp. 1917-1921
- 509. Vanni R, Dal Cin P, Marras S, et al: Endometrial polyp: another benign tumor characterized by 12q13-q15 changes. Cancer Genet Cytogenet 1993; 68: pp. 32-33
- 510. Dal Cin P, Vanni R, Marras S, et al: Four cytogenetic subgroups can be identified in endometrial polyps. Cancer Res 1995; 55: pp. 1565-1568
- 511. Inagaki N, Ung L, Otani T, et al: Uterine cavity matrix metalloproteinases and cytokines in patients with leiomyoma, adenomyosis or endometrial polyp. Eur J Obstet Gynecol Reprod Biol 2003; 111: pp. 197-203
- 512. Xuebing P, TinChiu L, Enlan X, et al: Is endometrial polyp formation associated with increased expression of vascular endothelial growth factor and transforming growth factor-beta1? Eur J Obstet Gynecol Reprod Biol 2011; 159: pp. 198-203
- 513. Hu J, and Yuan R: The expression levels of stem cell markers importin13, c-kit, CD146, and telomerase are decreased in endometrial polyps. Med Sci Monit 2011; 17: pp. BR221-BR227
- 514. Gul A, Ugur M, Iskender C, et al: Immunohistochemical expression of estrogen and progesterone receptors in endometrial polyps and its relationship to clinical parameters. Arch Gynecol Obstet 2010; 281: pp. 479-483
- 515. de Carvalho S, Campaner AB, Lima SM, et al: Differential expression of estrogen and progesterone receptors in endometrial polyps and adjacent endometrium in postmenopausal women. Anal Quant Cytol Histol 2011; 33: pp. 61-67
- 516. Jakab A, Ovari L, Juhasz B, et al: Detection of feeding artery improves the ultrasound diagnosis of endometrial polyps in asymptomatic patients. Eur J Obstet Gynecol Reprod Biol 2005; 119: pp. 103-107
- 517. Chan SS, Tam WH, Yeo W, et al: A randomised controlled trial of prophylactic levonorgestrel intrauterine system in tamoxifen-treated women. BJOG 2007; 114: pp. 1510-1515
- 518. Filho AM, Barbosa IC, Maia H, et al: Effects of subdermal implants of estradiol and testosterone on the endometrium of postmenopausal women. Gynecol Endocrinol 2007; 23: pp. 511-517
- 519. Capmas P, Pourcelot AG, Giral E, et al: Office hysteroscopy: a report of 2402 cases. J Gynecol Obstet Biol Reprod (Paris) 2016; 45: pp. 445-450
- 520. Van den Bosch T, Ameye L, Van Schoubroeck D, et al: Intra-cavitary uterine pathology in women with abnormal uterine bleeding: a prospective study of 1220 women. Facts Views Vis Obgyn 2015; 7: pp. 17-24
- 521. Anastasiadis PG, Koutlaki NG, Skaphida PG, et al: Endometrial polyps: prevalence, detection, and malignant potential in women with abnormal uterine bleeding. Eur J Gynaecol Oncol 2000; 21: pp. 180-183
- 522. Dreisler E, Stampe Sorensen S, Ibsen PH, et al: Prevalence of endometrial polyps and abnormal uterine bleeding in a Danish population aged 20-74 years. Ultrasound Obstet Gynecol 2009; 33: pp. 102-108
- 523. Haimov-Kochman R, Deri-Hasid R, Hamani Y, et al: The natural course of endometrial polyps: could they vanish when left untreated? Fertil Steril 2009; 92: pp. 828.e11-828.e12
- 524. Fabres C, Alam V, Balmaceda J, et al: Comparison of ultrasonography and hysteroscopy in the diagnosis of intrauterine lesions in infertile women. J Am Assoc Gynecol Laparosc 1998; 5: pp. 375-378
- 525. Bueloni-Dias FN, Spadoto-Dias D, Delmanto LR, et al: Metabolic syndrome as a predictor of endometrial polyps in postmenopausal women. Menopause 2016; 23: pp. 759-764
- 526. Reslova T, Tosner J, Resl M, et al: Endometrial polyps. A clinical study of 245 cases. Arch Gynecol Obstet 1999; 262: pp. 133-139
- 527. Onalan R, Onalan G, Tonguc E, et al: Body mass index is an independent risk factor for the development of endometrial polyps in patients undergoing in vitro fertilization. Fertil Steril 2009; 91: pp. 1056-1060
- 528. Rutanen EM, Pekonen F, Nyman T, et al: Insulin-like growth factors and their binding proteins in benign and malignant uterine diseases. Growth Regul 1993; 3: pp. 74-77
- 529. Nappi L, Indraccolo U, Di Spiezio Sardo A, et al: Are diabetes, hypertension, and obesity independent risk factors for endometrial polyps? J Minim Invasive Gynecol 2009; 16: pp. 157-162
- 530. Lecuru F, Metzger U, Scarabin C, et al: Hysteroscopic findings in women at risk of HNPCC. Results of a prospective observational study. Fam Cancer 2007; 6: pp. 295-299
- 531. Kalin A, Merideth MA, Regier DS, et al: Management of reproductive health in Cowden syndrome complicated by endometrial polyps and breast cancer. Obstet Gynecol 2013; 121: pp. 461-464
- 532. Runowicz CD, Costantino JP, Wickerham DL, et al: Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR). Am J Obstet Gynecol 2011; 205: pp. 535.e1-535.e5
- 533. Cohen I: Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol 2004; 94: pp. 256-266
- 534. Miranda SP, Traiman P, Candido EB, et al: Expression of p53, Ki-67, and CD31 proteins in endometrial polyps of postmenopausal women treated with tamoxifen. Int J Gynecol Cancer 2010; 20: pp. 1525-1530
- 535. Althuis MD, Sexton M, Langenberg P, et al: Surveillance for uterine abnormalities in tamoxifen-treated breast carcinoma survivors: a community based study. Cancer 2000; 89: pp. 800-810
- 536. McGurgan P, Taylor LJ, Duffy SR, et al: Does tamoxifen therapy affect the hormone receptor expression and cell proliferation indices of endometrial polyps? An immunohistochemical comparison of endometrial polyps from postmenopausal women exposed and not exposed to tamoxifen. Maturitas 2006; 54: pp. 252-259
- 537. Chin J, Konje JC, and Hickey M: Levonorgestrel intrauterine system for endometrial protection in women with breast cancer on adjuvant tamoxifen. Cochrane Database Syst Rev 2009; undefined:
- 538. Maia H, Barbosa IC, Marques D, et al: Hysteroscopy and transvaginal sonography in menopausal women receiving hormone replacement therapy. J Am Assoc Gynecol Laparosc 1996; 4: pp. 13-18
- 539. Akkad AA, Habiba MA, Ismail N, et al: Abnormal uterine bleeding on hormone replacement: the importance of intrauterine structural abnormalities. Obstet Gynecol 1995; 86: pp. 330-334
- 540. Bakour SH, Gupta JK, and Khan KS: Risk factors associated with endometrial polyps in abnormal uterine bleeding. Int J Gynaecol Obstet 2002; 76: pp. 165-168
- 541. Elliott J, Connor ME, and Lashen H: The value of outpatient hysteroscopy in diagnosing endometrial pathology in postmenopausal women with and without hormone replacement therapy. Acta Obstet Gynecol Scand 2003; 82: pp. 1112-1119
- 542. Van Bogaert LJ: Clinicopathologic findings in endometrial polyps. Obstet Gynecol 1988; 71: pp. 771-773
- 543. Clevenger-Hoeft M, Syrop CH, Stovall DW, et al: Sonohysterography in premenopausal women with and without abnormal bleeding. Obstet Gynecol 1999; 94: pp. 516-520
- 544. Wu HH, Schuetz MJ, and Cramer H: Significance of benign endometrial cells in Pap smears from postmenopausal women. J Reprod Med 2001; 46: pp. 795-798
- 545. Hassa H, Tekin B, Senses T, et al: Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol 2006; 194: pp. 718-721
- 546. Savelli L, De Iaco P, Santini D, et al: Histopathologic features and risk factors for benignity, hyperplasia, and cancer in endometrial polyps. Am J Obstet Gynecol 2003; 188: pp. 927-931
- 547. Bakour SH, Khan KS, and Gupta JK: The risk of premalignant and malignant pathology in endometrial polyps. Acta Obstet Gynecol Scand 2000; 79: pp. 317-320
- 548. Papadia A, Gerbaldo D, Fulcheri E, et al: The risk of premalignant and malignant pathology in endometrial polyps: should every polyp be resected? Minerva Ginecol 2007; 59: pp. 117-124
- 549. Rios SS, Andrade RV, Pereira RW, et al: Microsatellite instability in endometrial polyps. Eur J Obstet Gynecol Reprod Biol 2010; 153: pp. 193-197
- 550. Lee SC, Kaunitz AM, Sanchez-Ramos L, et al: The oncogenic potential of endometrial polyps: a systematic review and meta-analysis. Obstet Gynecol 2010; 116: pp. 1197-1205
- 551. Elfayomy AK, and Soliman BS: Risk factors associated with the malignant changes of symptomatic and asymptomatic endometrial polyps in premenopausal women. J Obstet Gynaecol India 2015; 65: pp. 186-192
- 552. Litta P, Di Giuseppe J, Moriconi L, et al: Predictors of malignancy in endometrial polyps: a multi-institutional cohort study. Eur J Gynaecol Oncol 2014; 35: pp. 382-386
- 553. Costa-Paiva L, Godoy CE, Antunes A, et al: Risk of malignancy in endometrial polyps in premenopausal and postmenopausal women according to clinicopathologic characteristics. Menopause 2011; 18: pp. 1278-1282
- 554. Wang JH, Zhao J, and Lin J: Opportunities and risk factors for premalignant and malignant transformation of endometrial polyps: management strategies. J Minim Invasive Gynecol 2010; 17: pp. 53-58
- 555. Chavez NF, Garner EO, Khan W, et al: Does the introduction of new technology change population demographics? Minimally invasive technologies and endometrial polyps. Gynecol Obstet Invest 2002; 54: pp. 217-220
- 556. Seto MT, Ip PP, Ngu SF, et al: Positive predictive value of endometrial polyps in Pipelle aspiration sampling: a histopathological study of 195 cases. Eur J Obstet Gynecol Reprod Biol 2016; 203: pp. 12-15
- 557. Ahmadi F, Zafarani F, Haghighi H, et al: Application of 3D ultrasonography in detection of uterine abnormalities. Int J Fertil Steril 2011; 4: pp. 144-147
- 558. Ayida G, Kennedy S, Barlow D, et al: Contrast sonography for uterine cavity assessment: a comparison of conventional two-dimensional with three-dimensional transvaginal ultrasound; a pilot study. Fertil Steril 1996; 66: pp. 848-850
- 559. Salim S, Won H, Nesbitt-Hawes E, et al: Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol 2011; 18: pp. 569-581
- 560. Inoue T, Kitajima M, Taniguchi K, et al: Three-dimensional saline-infusion sonohysterography is useful for the identification of endometrial polyp. J Obstet Gynaecol Res 2016; 42: pp. 855-859
- 561. Dominick S, Hickey M, Chin J, et al: Levonorgestrel intrauterine system for endometrial protection in women with breast cancer on adjuvant tamoxifen. Cochrane Database Syst Rev 2015; undefined:
- 562. Fu Y, and Zhuang Z: Long-term effects of levonorgestrel-releasing intrauterine system on tamoxifen-treated breast cancer patients: a meta-analysis. Int J Clin Exp Pathol 2014; 7: pp. 6419-6429
- 563. Wan YL, and Holland C: The efficacy of levonorgestrel intrauterine systems for endometrial protection: a systematic review. Climacteric 2011; 14: pp. 622-632
- 564. Lieng M, Istre O, Sandvik L, et al: Prevalence, 1-year regression rate, and clinical significance of asymptomatic endometrial polyps: cross-sectional study. J Minim Invasive Gynecol 2009; 16: pp. 465-471
- 565. DeWaay DJ, Syrop CH, Nygaard IE, et al: Natural history of uterine polyps and leiomyomata. Obstet Gynecol 2002; 100: pp. 3-7
- 566. Cravello L, Stolla V, Bretelle F, et al: Hysteroscopic resection of endometrial polyps: a study of 195 cases. Eur J Obstet Gynecol Reprod Biol 2000; 93: pp. 131-134
- 567. Preutthipan S, and Herabutya Y: Hysteroscopic polypectomy in 240 premenopausal and postmenopausal women. Fertil Steril 2005; 83: pp. 705-709
- 568. Yang JH, Chen CD, Chen SU, et al: Factors influencing the recurrence potential of benign endometrial polyps after hysteroscopic polypectomy. PLoS ONE 2015; 10: pp. e0144857
- 569. AlHilli MM, Nixon KE, Hopkins MR, et al: Long-term outcomes after intrauterine morcellation vs hysteroscopic resection of endometrial polyps. J Minim Invasive Gynecol 2013; 20: pp. 215-221
- 570. Henriquez DD, van Dongen H, Wolterbeek R, et al: Polypectomy in premenopausal women with abnormal uterine bleeding: effectiveness of hysteroscopic removal. J Minim Invasive Gynecol 2007; 14: pp. 59-63
- 571. Lieng M, Istre O, Sandvik L, et al: Clinical effectiveness of transcervical polyp resection in women with endometrial polyps: randomized controlled trial. J Minim Invasive Gynecol 2010; 17: pp. 351-357
- 572. Bosteels J, Kasius J, Weyers S, et al: Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database Syst Rev 2015; undefined:
- 573. Shokeir TA, Shalan HM, and El-Shafei MM: Significance of endometrial polyps detected hysteroscopically in eumenorrheic infertile women. J Obstet Gynaecol Res 2004; 30: pp. 84-89
- 574. Lee I, Luo X, Cui XT, et al: Highly sensitive single polyaniline nanowire biosensor for the detection of immunoglobulin G and myoglobin. Biosens Bioelectron 2011; 26: pp. 3297-3302
- 575. Ben-Nagi J, Miell J, Yazbek J, et al: The effect of hysteroscopic polypectomy on the concentrations of endometrial implantation factors in uterine flushings. Reprod Biomed Online 2009; 19: pp. 737-744
- 576. Mollo A, Stile A, Alviggi C, et al: Endometrial polyps in infertile patients: do high concentrations of interferon-gamma play a role? Fertil Steril 2011; 96: pp. 1209-1212
- 577. Elbehery MM, Nouh AA, Mohamed ML, et al: Insulin-like growth factor binding protein-1 and glycodelin levels in uterine flushing before and after hysteroscopic polypectomy. Clin Lab 2011; 57: pp. 953-957
- 578. Perez-Medina T, Bajo-Arenas J, Salazar F, et al: Endometrial polyps and their implication in the pregnancy rates of patients undergoing intrauterine insemination: a prospective, randomized study. Hum Reprod 2005; 20: pp. 1632-1635
- 579. Lass A, Williams G, Abusheikha N, et al: The effect of endometrial polyps on outcomes of in vitro fertilization (IVF) cycles. J Assist Reprod Genet 1999; 16: pp. 410-415
- 580. Isikoglu M, Berkkanoglu M, Senturk Z, et al: Endometrial polyps smaller than 1.5 cm do not affect ICSI outcome. Reprod Biomed Online 2006; 12: pp. 199-204
- 581. Afifi K, Anand S, Nallapeta S, et al: Management of endometrial polyps in subfertile women: a systematic review. Eur J Obstet Gynecol Reprod Biol 2010; 151: pp. 117-121
- 582. Wren BG: Dysfunctional uterine bleeding. Aust Fam Physician 1998; 27: pp. 371-377
- 583. Ferenczy A: Pathophysiology of endometrial bleeding. Maturitas 2003; 45: pp. 1-14
- 584. Rogers PA, and Abberton KM: Endometrial arteriogenesis: vascular smooth muscle cell proliferation and differentiation during the menstrual cycle and changes associated with endometrial bleeding disorders. Microsc Res Tech 2003; 60: pp. 412-419
- 585. Patterson-Keels LM, Selvaggi SM, Haefner HK, et al: Morphologic assessment of endometrium overlying submucosal leiomyomas. J Reprod Med 1994; 39: pp. 579-584
- 586. Confino E: Abnormal uterine bleeding, a new terminology is needed. Fertil Steril 2007; 87: pp. 479-480
- 587. Fraser IS, Critchley HO, and Munro MG: Abnormal uterine bleeding: getting our terminology straight. Curr Opin Obstet Gynecol 2007; 19: pp. 591-595
- 588. Munro MG, Critchley HO, and Fraser IS: The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril 2011; 95: pp. 2204-2208
- 589. Deleted in review.
- 590. Munro MG, Critchley HO, and Fraser IS: The flexible FIGO classification concept for underlying causes of abnormal uterine bleeding. Semin Reprod Med 2011; 29: pp. 391-399
- 591. Critchley HO, Munro MG, Broder M, et al: A five-year international review process concerning terminologies, definitions, and related issues around abnormal uterine bleeding. Semin Reprod Med 2011; 29: pp. 377-382
- 592. Shankar M, Lee CA, Sabin CA, et al: von Willebrand disease in women with menorrhagia: a systematic review. BJOG 2004; 111: pp. 734-740
- 593. Seravalli V, Linari S, Peruzzi E, et al: Prevalence of hemostatic disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol 2013; 26: pp. 285-289
- 594. Claessens EA, and Cowell CA: Acute adolescent menorrhagia. Am J Obstet Gynecol 1981; 139: pp. 277-280
- 595. Hale GE, Hughes CL, Burger HG, et al: Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase (LOOP) events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause 2009; 16: pp. 50-59
- 596. Hickey M, and Fraser I: Human uterine vascular structures in normal and diseased states. Microsc Res Tech 2003; 60: pp. 377-389
- 597. Critchley HO, and Maybin JA: Molecular and cellular causes of abnormal uterine bleeding of endometrial origin. Semin Reprod Med 2011; 29: pp. 400-409
- 598. Whitaker L, and Critchley HO: Abnormal uterine bleeding. Best Pract Res Clin Obstet Gynaecol 2016; 34: pp. 54-65
- 599. Smith SK, Abel MH, Kelly RW, et al: A role for prostacyclin (PGi2) in excessive menstrual bleeding. Lancet 1981; 1: pp. 522-524
- 600. Smith SK, Abel MH, Kelly RW, et al: Prostaglandin synthesis in the endometrium of women with ovular dysfunctional uterine bleeding. Br J Obstet Gynaecol 1981; 88: pp. 434-442
- 601. Gleeson NC: Cyclic changes in endometrial tissue plasminogen activator and plasminogen activator inhibitor type 1 in women with normal menstruation and essential menorrhagia. Am J Obstet Gynecol 1994; 171: pp. 178-183
- 602. Hewett P, Nijjar S, Shams M, et al: Down-regulation of angiopoietin-1 expression in menorrhagia. Am J Pathol 2002; 160: pp. 773-780
- 603. Blumenthal RD, Taylor AP, Goldman L, et al: Abnormal expression of the angiopoietins and Tie receptors in menorrhagic endometrium. Fertil Steril 2002; 78: pp. 1294-1300
- 604. Gultekin M, Diribas K, Buru E, et al: Role of a non-hormonal oral anti-fibrinolytic hemostatic agent (tranexamic acid) for management of patients with dysfunctional uterine bleeding. Clin Exp Obstet Gynecol 2009; 36: pp. 163-165
- 605. Wellington K, and Wagstaff AJ: Tranexamic acid: a review of its use in the management of menorrhagia. Drugs 2003; 63: pp. 1417-1433
- 606. Bonnar J, and Sheppard BL: Treatment of menorrhagia during menstruation: randomised controlled trial of ethamsylate, mefenamic acid, and tranexamic acid. BMJ 1996; 313: pp. 579-582
- 607. Xiao B, Wu SC, Chong J, et al: Therapeutic effects of the levonorgestrel-releasing intrauterine system in the treatment of idiopathic menorrhagia. Fertil Steril 2003; 79: pp. 963-969
- 608. Lethaby AE, Cooke I, and Rees M: Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev 2005; undefined:
- 609. Irvine GA, and Cameron IT: Medical management of dysfunctional uterine bleeding. Baillieres Best Pract Res Clin Obstet Gynaecol 1999; 13: pp. 189-202
- 610. Roy SN, and Bhattacharya S: Benefits and risks of pharmacological agents used for the treatment of menorrhagia. Drug Saf 2004; 27: pp. 75-90
- 611. El-Nashar SA, Hopkins MR, Feitoza SS, et al: Global endometrial ablation for menorrhagia in women with bleeding disorders. Obstet Gynecol 2007; 109: pp. 1381-1387
- 612. El-Nashar SA, Hopkins MR, Creedon DJ, et al: Efficacy of bipolar radiofrequency endometrial ablation vs thermal balloon ablation for management of menorrhagia: a population-based cohort. J Minim Invasive Gynecol 2009; 16: pp. 692-699
- 613. Khan Z, El-Nashar SA, Hopkins MR, et al: Efficacy and safety of global endometrial ablation after cesarean delivery: a cohort study. Am J Obstet Gynecol 2011; 205: pp. 450.e1-450.e4
- 614. Schenker JG: Etiology of and therapeutic approach to synechia uteri. Eur J Obstet Gynecol Reprod Biol 1996; 65: pp. 109-113
- 615. Deans R, and Abbott J: Review of intrauterine adhesions. J Minim Invasive Gynecol 2010; 17: pp. 555-569
- 616. Hooker AB, Lemmers M, Thurkow AL, et al: Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update 2014; 20: pp. 262-278
- 617. Tam WH, Lau WC, Cheung LP, et al: Intrauterine adhesions after conservative and surgical management of spontaneous abortion. J Am Assoc Gynecol Laparosc 2002; 9: pp. 182-185
- 618. Eriksen J, and Kaestel C: The incidence of uterine atresia after post-partum curettage. A follow-up examination of 141 patients. Dan Med Bull 1960; 7: pp. 50-51
- 619. Poujade O, Grossetti A, Mougel L, et al: Risk of synechiae following uterine compression sutures in the management of major postpartum haemorrhage. BJOG 2011; 118: pp. 433-439
- 620. Rasheed SM, Amin MM, Abd Ellah AH, et al: Reproductive performance after conservative surgical treatment of postpartum hemorrhage. Int J Gynaecol Obstet 2014; 124: pp. 248-252
- 621. Rathat G, Do Trinh P, Mercier G, et al: Synechia after uterine compression sutures. Fertil Steril 2011; 95: pp. 405-409
- 622. Ibrahim MI, Raafat TA, Ellaithy MI, et al: Risk of postpartum uterine synechiae following uterine compression suturing during postpartum haemorrhage. Aust N Z J Obstet Gynaecol 2013; 53: pp. 37-45
- 623. Gambadauro P, Gudmundsson J, and Torrejon R: Intrauterine adhesions following conservative treatment of uterine fibroids. Obstet Gynecol Int 2012; 2012: pp. 853269
- 624. Taskin O, Sadik S, Onoglu A, et al: Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J Am Assoc Gynecol Laparosc 2000; 7: pp. 351-354
- 625. Kodaman PH, and Arici A: Intra-uterine adhesions and fertility outcome: how to optimize success? Curr Opin Obstet Gynecol 2007; 19: pp. 207-214
- 626. Fedele L, Vercellini P, Viezzoli T, et al: Intrauterine adhesions: current diagnostic and therapeutic trends. Acta Eur Fertil 1986; 17: pp. 31-37
- 627. Polishuk WZ, Anteby SO, and Weinstein D: Puerperal endometritis and intrauterine adhesions. Int Surg 1975; 60: pp. 418-420
- 628. Abiodun OM, Balogun OR, and Fawole AA: Aetiology, clinical features and treatment outcome of intrauterine adhesion in Ilorin, Central Nigeria. West Afr J Med 2007; 26: pp. 298-301
- 629. Efetie ER, Umezulike AC, and Okafor UV: Clinical and demographic characteristics of women with intrauterine adhesion in abuja, Nigeria. Obstet Gynecol Int 2012; 2012: pp. 435475
- 630. Gaya SA, Adamu IS, Yakasai IA, et al: Review of intrauterine adhesiolysis at the Aminu Kano Teaching Hospital, Kano, Nigeria. Ann Afr Med 2012; 11: pp. 65-69
- 631. Shaheen R, Subhan F, and Tahir F: Epidemiology of genital tuberculosis in infertile population. J Pak Med Assoc 2006; 56: pp. 306-309
- 632. Singh N, Sumana G, and Mittal S: Genital tuberculosis: a leading cause for infertility in women seeking assisted conception in North India. Arch Gynecol Obstet 2008; 278: pp. 325-327
- 633. Khanna A, and Agrawal A: Markers of genital tuberculosis in infertility. Singapore Med J 2011; 52: pp. 864-867
- 634. Yu D, Wong YM, Cheong Y, et al: Asherman syndrome—one century later. Fertil Steril 2008; 89: pp. 759-779
- 635. Schenker JG, and Margalioth EJ: Intrauterine adhesions: an updated appraisal. Fertil Steril 1982; 37: pp. 593-610
- 636. Levison JH, Barbieri RL, Katz JT, et al: Clinical problem-solving. Hard to conceive. N Engl J Med 2010; 363: pp. 965-970
- 637. AAGL Advancing Minimally Invasive Gynecology Worldwide : AAGL practice report: practice guidelines for management of intrauterine synechiae. J Minim Invasive Gynecol 2010; 17: pp. 1-7
- 638. Dykes TA, Isler RJ, and McLean AC: MR imaging of Asherman syndrome: total endometrial obliteration. J Comput Assist Tomogr 1991; 15: pp. 858-860
- 639. Letterie GS, and Haggerty MF: Magnetic resonance imaging of intrauterine synechiae. Gynecol Obstet Invest 1994; 37: pp. 66-68
- 640. Soares SR, Barbosa dos Reis MM, and Camargos AF: Diagnostic accuracy of sonohysterography, transvaginal sonography, and hysterosalpingography in patients with uterine cavity diseases. Fertil Steril 2000; 73: pp. 406-411
- 641. Magos A: Hysteroscopic treatment of Asherman’s syndrome. Reprod Biomed Online 2002; 4: pp. 46-51
- 642. Valle RF, and Sciarra JJ: Intrauterine adhesions: hysteroscopic diagnosis, classification, treatment, and reproductive outcome. Am J Obstet Gynecol 1988; 158: pp. 1459-1470
- 643. Capella-Allouc S, Morsad F, Rongieres-Bertrand C, et al: Hysteroscopic treatment of severe Asherman’s syndrome and subsequent fertility. Hum Reprod 1999; 14: pp. 1230-1233
- 644. Preutthipan S, and Linasmita V: A prospective comparative study between hysterosalpingography and hysteroscopy in the detection of intrauterine pathology in patients with infertility. J Obstet Gynaecol Res 2003; 29: pp. 33-37
- 645. Siegler AM, and Valle RF: Therapeutic hysteroscopic procedures. Fertil Steril 1988; 50: pp. 685-701
- 646. Revaux A, Ducarme G, and Luton D: Prevention of intrauterine adhesions after hysteroscopic surgery. Gynecol Obstet Fertil 2008; 36: pp. 311-317
- 647. Di Spiezio Sardo A, Spinelli M, Bramante S, et al: Efficacy of a polyethylene oxide-sodium carboxymethylcellulose gel in prevention of intrauterine adhesions after hysteroscopic surgery. J Minim Invasive Gynecol 2011; 18: pp. 462-469
- 648. Myers EM, and Hurst BS: Comprehensive management of severe Asherman syndrome and amenorrhea. Fertil Steril 2012; 97: pp. 160-164
- 649. Orhue AA, Aziken ME, and Igbefoh JO: A comparison of two adjunctive treatments for intrauterine adhesions following lysis. Int J Gynaecol Obstet 2003; 82: pp. 49-56
- 650. Li L, Nai M, Gao G, et al: Comparison among measures to prevent intrauterine adhesions after artificial abortion. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2016; 41: pp. 975-978
- 651. Di Spiezio Sardo A, Calagna G, Scognamiglio M, et al: Prevention of intrauterine post-surgical adhesions in hysteroscopy. A systematic review. Eur J Obstet Gynecol Reprod Biol 2016; 203: pp. 182-192
- 652. Santamaria X, Cabanillas S, Cervello I, et al: Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod 2016; 31: pp. 1087-1096
- 653. Singh N, Mohanty S, Seth T, et al: Autologous stem cell transplantation in refractory Asherman’s syndrome: a novel cell based therapy. J Hum Reprod Sci 2014; 7: pp. 93-98
- 654. Johary J, Xue M, Zhu X, et al: Efficacy of estrogen therapy in patients with intrauterine adhesions: systematic review. J Minim Invasive Gynecol 2014; 21: pp. 44-54
- 655. Iacovides S, Avidon I, and Baker FC: What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update 2015; 21: pp. 762-778
- 656. Campbell MA, and McGrath PJ: Use of medication by adolescents for the management of menstrual discomfort. Arch Pediatr Adolesc Med 1997; 151: pp. 905-913
- 657. Wilson CA, and Keye WR: A survey of adolescent dysmenorrhea and premenstrual symptom frequency. A model program for prevention, detection, and treatment. J Adolesc Health Care 1989; 10: pp. 317-322
- 658. Klein JR, and Litt IF: Epidemiology of adolescent dysmenorrhea. Pediatrics 1981; 68: pp. 661-664
- 659. Johnson J: Level of knowledge among adolescent girls regarding effective treatment for dysmenorrhea. J Adolesc Health Care 1988; 9: pp. 398-402
- 660. Burnett MA, Antao V, Black A, et al: Prevalence of primary dysmenorrhea in Canada. J Obstet Gynaecol Can 2005; 27: pp. 765-770
- 661. Andersch B, and Milsom I: An epidemiologic study of young women with dysmenorrhea. Am J Obstet Gynecol 1982; 144: pp. 655-660
- 662. Ortiz MI: Primary dysmenorrhea among Mexican university students: prevalence, impact and treatment. Eur J Obstet Gynecol Reprod Biol 2010; 152: pp. 73-77
- 663. Ortiz MI, Rangel-Flores E, Carrillo-Alarcon LC, et al: Prevalence and impact of primary dysmenorrhea among Mexican high school students. Int J Gynaecol Obstet 2009; 107: pp. 240-243
- 664. Polat A, Celik H, Gurates B, et al: Prevalence of primary dysmenorrhea in young adult female university students. Arch Gynecol Obstet 2009; 279: pp. 527-532
- 665. Hillen TI, Grbavac SL, Johnston PJ, et al: Primary dysmenorrhea in young Western Australian women: prevalence, impact, and knowledge of treatment. J Adolesc Health 1999; 25: pp. 40-45
- 666. Latthe P, Mignini L, Gray R, et al: Factors predisposing women to chronic pelvic pain: systematic review. BMJ 2006; 332: pp. 749-755
- 667. Widholm O, and Kantero RL: A statistical analysis of the menstrual patterns of 8,000 Finnish girls and their mothers. Acta Obstet Gynecol Scand Suppl 1971; 14: pp. 1-36
- 668. Deleted in review.
- 669. Iacovides S, Baker FC, Avidon I, et al: Women with dysmenorrhea are hypersensitive to experimental deep muscle pain across the menstrual cycle. J Pain 2013; 14: pp. 1066-1076
- 670. Dawood MY: Primary dysmenorrhea: advances in pathogenesis and management. Obstet Gynecol 2006; 108: pp. 428-441
- 671. Altunyurt S, Gol M, Sezer O, et al: Primary dysmenorrhea and uterine blood flow: a color Doppler study. J Reprod Med 2005; 50: pp. 251-255
- 672. Dmitrovic R: Transvaginal color Doppler study of uterine blood flow in primary dysmenorrhea. Acta Obstet Gynecol Scand 2000; 79: pp. 1112-1116
- 673. Akerlund M: Vascularization of human endometrium. Uterine blood flow in healthy condition and in primary dysmenorrhoea. Ann N Y Acad Sci 1994; 734: pp. 47-56
- 674. Bieglmayer C, Hofer G, Kainz C, et al: Concentrations of various arachidonic acid metabolites in menstrual fluid are associated with menstrual pain and are influenced by hormonal contraceptives. Gynecol Endocrinol 1995; 9: pp. 307-312
- 675. Durham PL, Vause CV, Derosier F, et al: Changes in salivary prostaglandin levels during menstrual migraine with associated dysmenorrhea. Headache 2010; 50: pp. 844-851
- 676. Akdemir N, Cinemre H, Bilir C, et al: Increased serum asymmetric dimethylarginine levels in primary dysmenorrhea. Gynecol Obstet Invest 2010; 69: pp. 153-156
- 677. Marjoribanks J, Ayeleke RO, Farquhar C, et al: Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev 2015; undefined:
- 678. Marjoribanks J, Proctor M, Farquhar C, et al: Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev 2010; undefined:
- 679. Eccles R, Holbrook A, and Jawad M: A double-blind, randomised, crossover study of two doses of a single-tablet combination of ibuprofen/paracetamol and placebo for primary dysmenorrhoea. Curr Med Res Opin 2010; 26: pp. 2689-2699
- 680. Budoff PW: Use of mefenamic acid in the treatment of primary dysmenorrhea. JAMA 1979; 241: pp. 2713-2716
- 681. Milsom I, Minic M, Dawood MY, et al: Comparison of the efficacy and safety of nonprescription doses of naproxen and naproxen sodium with ibuprofen, acetaminophen, and placebo in the treatment of primary dysmenorrhea: a pooled analysis of five studies. Clin Ther 2002; 24: pp. 1384-1400
- 682. Wong CL, Farquhar C, Roberts H, et al: Oral contraceptive pill for primary dysmenorrhoea. Cochrane Database Syst Rev 2009; undefined:
- 683. Edelman AB, Gallo MF, Jensen JT, et al: Continuous or extended cycle vs. cyclic use of combined oral contraceptives for contraception. Cochrane Database Syst Rev 2005; undefined:
- 684. Machado RB, de Melo NR, and Maia H: Bleeding patterns and menstrual-related symptoms with the continuous use of a contraceptive combination of ethinylestradiol and drospirenone: a randomized study. Contraception 2010; 81: pp. 215-222
- 685. Dmitrovic R, Kunselman AR, and Legro RS: Continuous compared with cyclic oral contraceptives for the treatment of primary dysmenorrhea: a randomized controlled trial. Obstet Gynecol 2012; 119: pp. 1143-1150
- 686. Roumen FJ: The contraceptive vaginal ring compared with the combined oral contraceptive pill: a comprehensive review of randomized controlled trials. Contraception 2007; 75: pp. 420-429
- 687. Audet MC, Moreau M, Koltun WD, et al: Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive: a randomized controlled trial. JAMA 2001; 285: pp. 2347-2354
- 688. Williamson M, and Bulmer P: Using the Mirena intrauterine system to treat severe primary dysmenorrhoea in an adolescent. J Obstet Gynaecol 2010; 30: pp. 206-207
- 689. Lindh I, and Milsom I: The influence of intrauterine contraception on the prevalence and severity of dysmenorrhea: a longitudinal population study. Hum Reprod 2013; 28: pp. 1953-1960
- 690. Igwea SE, Tabansi-Ochuogu CS, and Abaraogu UO: TENS and heat therapy for pain relief and quality of life improvement in individuals with primary dysmenorrhea: a systematic review. Complement Ther Clin Pract 2016; 24: pp. 86-91
- 691. Akin MD, Weingand KW, Hengehold DA, et al: Continuous low-level topical heat in the treatment of dysmenorrhea. Obstet Gynecol 2001; 97: pp. 343-349
- 692. Akin M, Price W, Rodriguez G, et al: Continuous, low-level, topical heat wrap therapy as compared to acetaminophen for primary dysmenorrhea. J Reprod Med 2004; 49: pp. 739-745
- 693. Vaziri F, Hoseini A, Kamali F, et al: Comparing the effects of aerobic and stretching exercises on the intensity of primary dysmenorrhea in the students of universities of Bushehr. J Family Reprod Health 2015; 9: pp. 23-28
- 694. Daley AJ: Exercise and primary dysmenorrhoea: a comprehensive and critical review of the literature. Sports Med 2008; 38: pp. 659-670
- 695. Israel RG, Sutton M, and O’Brien KF: Effects of aerobic training on primary dysmenorrhea symptomatology in college females. J Am Coll Health 1985; 33: pp. 241-244
- 696. Yang NY, and Kim SD: Effects of a yoga program on menstrual cramps and menstrual distress in undergraduate students with primary dysmenorrhea: a single-blind, randomized controlled trial. J Altern Complement Med 2016; 22: pp. 732-738
- 697. Cho SH, and Hwang EW: Acupuncture for primary dysmenorrhoea: a systematic review. BJOG 2010; 117: pp. 509-521
- 698. Rakhshaee Z: Effect of three yoga poses (cobra, cat and fish poses) in women with primary dysmenorrhea: a randomized clinical trial. J Pediatr Adolesc Gynecol 2011; 24: pp. 192-196
- 699. Armour M, Dahlen HG, and Smith CA: More than needles: the importance of explanations and self-care advice in treating primary dysmenorrhea with acupuncture. Evid Based Complement Alternat Med 2016; 2016: pp. 3467067
- 700. Smith CA, Crowther CA, Petrucco O, et al: Acupuncture to treat primary dysmenorrhea in women: a randomized controlled trial. Evid Based Complement Alternat Med 2011; 2011: pp. 612464
- 701. Ou MC, Hsu TF, Lai AC, et al: Pain relief assessment by aromatic essential oil massage on outpatients with primary dysmenorrhea: a randomized, double-blind clinical trial. J Obstet Gynaecol Res 2012; 38: pp. 817-822
- 702. Lee CH, Roh JW, Lim CY, et al: A multicenter, randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of a far infrared-emitting sericite belt in patients with primary dysmenorrhea. Complement Ther Med 2011; 19: pp. 187-193
- 703. Pattanittum P, Kunyanone N, Brown J, et al: Dietary supplements for dysmenorrhoea. Cochrane Database Syst Rev 2016; undefined:
- 704. Barnard ND, Scialli AR, Hurlock D, et al: Diet and sex-hormone binding globulin, dysmenorrhea, and premenstrual symptoms. Obstet Gynecol 2000; 95: pp. 245-250
- 705. Abdul-Razzak KK, Ayoub NM, Abu-Taleb AA, et al: Influence of dietary intake of dairy products on dysmenorrhea. J Obstet Gynaecol Res 2010; 36: pp. 377-383
- 706. Rahbar N, Asgharzadeh N, and Ghorbani R: Effect of omega-3 fatty acids on intensity of primary dysmenorrhea. Int J Gynaecol Obstet 2012; 117: pp. 45-47
- 707. Ziaei S, Faghihzadeh S, Sohrabvand F, et al: A randomised placebo-controlled trial to determine the effect of vitamin E in treatment of primary dysmenorrhoea. BJOG 2001; 108: pp. 1181-1183
- 708. Ziaei S, Zakeri M, and Kazemnejad A: A randomised controlled trial of vitamin E in the treatment of primary dysmenorrhoea. BJOG 2005; 112: pp. 466-469
- 709. Ulmsten U: Calcium blockade as a rapid pharmacological test to evaluate primary dysmenorrhea. Gynecol Obstet Invest 1985; 20: pp. 78-83
- 710. Sandahl B, Ulmsten U, and Andersson KE: Trial of the calcium antagonist nifedipine in the treatment of primary dysmenorrhoea. Arch Gynecol 1979; 227: pp. 147-151
- 711. Andersson KE, and Ulmsten U: Effects of nifedipine on myometrial activity and lower abdominal pain in women with primary dysmenorrhoea. Br J Obstet Gynaecol 1978; 85: pp. 142-148
- 712. Proctor ML, and Murphy PA: Herbal and dietary therapies for primary and secondary dysmenorrhoea. Cochrane Database Syst Rev 2001; undefined:
- 713. Moya RA, Moisa CF, Morales F, et al: Transdermal glyceryl trinitrate in the management of primary dysmenorrhea. Int J Gynaecol Obstet 2000; 69: pp. 113-118
- 714. Norman J: Nitric oxide and the myometrium. Pharmacol Ther 1996; 70: pp. 91-100
- 715. Dmitrovic R, Kunselman AR, and Legro RS: Sildenafil citrate in the treatment of pain in primary dysmenorrhea: a randomized controlled trial. Hum Reprod 2013; 28: pp. 2958-2965
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree