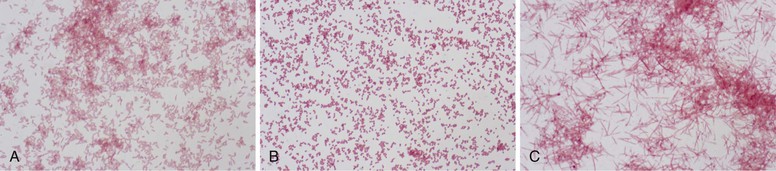
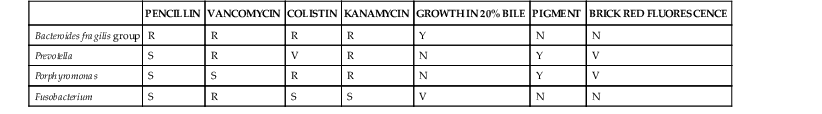
Wendy S. Garrett, Andrew B. Onderdonk The genera Bacteroides, Porphyromonas, Prevotella, and Fusobacterium account for the majority of infections caused by anaerobic gram-negative rods. Bilophila and Sutterella also cause human infections, although they are less frequently encountered in clinical practice. These obligately anaerobic gram-negative bacteria colonize the oropharynx, gastrointestinal tract, and urogenital tract of humans. Several species from some of these genera are useful symbiotic bacteria, facilitating host metabolism and favorably shaping immune responses. However, many of these microbes act opportunistically, causing infections when they gain access to otherwise sterile tissues. The gram-negative anaerobic rods have a predilection for abscess formation, with the most common sites being the oropharynx, abdominal cavity, lungs, and female genital tract. These bacterial species also present clinical challenges because they are often resistant to commonly used antibiotics. Recently, a few species from the Fusobacterium, Bilophila, and Sutterella genera have been associated with either inflammatory bowel disease or colon cancer, although causative roles have yet to be robustly established. The end of the 19th century was a fertile time for the discovery of gram-negative anaerobic rods (GNARs). The recognition of GNARs as important pathogens first occurred in animals in 1884 and is attributed to Loeffler, and Schmorl is credited with identifying GNARs as opportunists in humans in 1891. Identification of GNARs as members of the human endogenous microbiota dates back to 1898, when Halle published his discovery of Fusobacterium colonizing the female genital tract. At the same time, GNARs were also isolated by Veillon and Zuber from clinical material from pelvic, appendiceal, and brain abscesses.1 The Bacteroides spp. as a group were first described in the late 1890s, and for many years Bacteroides was a physiologically disparate genus of pleomorphic, obligately anaerobic, gram-negative rods. Until the early 1960s, competing taxonomic systems and confusion regarding nomenclature made it difficult to determine the role of specific members of this group of organisms during infectious processes. Moore and Holdeman brought some conformity to the phenotypic classification of this group of organisms, which allowed other investigators to identify Bacteroides fragilis as a major abscess-inducing pathogen. With the advent of uniform taxonomic classification and the use of 16S rRNA phylogenetic-driven taxonomic classifications, several species were reclassified. Bacteroides has been further subdivided into two additional genera—Porphyromonas and Prevotella—both of which primarily colonize the oral cavity. The genus Fusobacterium was identified in the early 20th century and is well documented in studies by microbiologists at the Virginia Polytechnic Institute and Pasteur Institute. Bacteroides is a genus of gram-negative, non-spore-forming, obligately anaerobic, rod-shaped bacteria. More than 30 species of Bacteroides have been recognized. The strictest taxonomic definition of Bacteroides limits this census to less than a dozen separate species. Within the Bacteroidaceae family, Bacteroides organisms are distinctive in their DNA guanine-cytosine composition—40 to 48 mol%. The principal products of their saccharolytic metabolism are acetate, succinate, and isovalerate. The saturated anteiso-methyl and isomethyl branched acids are used for their long-chain fatty acid–based identification. Bacteroides organisms express hexose monophosphate shunt–pentose phosphate pathway enzymes. Their sphingolipid-rich membranes also possess menaquinones, particularly MK-10 and MK-11. Bacteroides peptidoglycan contains meso-diaminopimelic acid. Several of the Bacteroides spp. express numerous capsular polysaccharides. These glycoantigens are of interest biologically for their immunomodulatory potential, particularly in the case of B. fragilis polysaccharide A. GNARs are differentiated from one another in the clinical laboratory by use of standard techniques: colony morphology, Gram stain, pigment production visualized in natural light and as fluorescence emission after exposure to ultraviolet (UV) light, and numerous biochemical tests. Short-chain fatty acid analysis by gas-liquid chromatography can also be used for species-level Bacteroides discrimination. The B. fragilis group is of special medical importance for several reasons. It is often the predominant GNAR in polymicrobial infections, and members of this group have the potential to express β-lactamase. B. fragilis group bacteremias are also associated with a high mortality rate: 27%.2 Consequently, identification of the B. fragilis group by the clinical laboratory is often critical for appropriate therapeutic intervention. On blood agar, B. fragilis forms circular, entire, white or gray, 2- to 3-mm colonies that are shiny and smooth. The B. fragilis group can be rather pleomorphic on Gram stain, forming straight rods of varying length, as well as coccobacilli (Fig. 249-1A). When grown in liquid medium, cells develop bipolar vacuoles and show a characteristic “safety pin” appearance. A useful characteristic of the B. fragilis group is its bile tolerance when compared with other GNARs, enabling its growth on Bacteroides bile-esculin agar. In addition, B. fragilis is highly resistant to the antibiotics kanamycin, vancomycin, and colistin. The use of a simple disk diffusion assay for these antibiotics is often part of the identification process for GNARs (Table 249-1). Both Prevotella and Porphyromonas were previously considered to be part of the genus Bacteroides. These pigmented GNARs can be distinguished from one another metabolically as the saccharolytic Prevotella spp. and the asaccharolytic Porphyromonas spp. Approximately 20 Prevotella spp. have been implicated in causing human disease. Prevotella forms circular, convex, 1- to 2-mm, shiny, gray colonies. On Gram stain, they form short gram-negative rods and may assume coccobacilli forms (Fig. 249-1B). Prevotella grows well on laked blood agar with kanamycin and vancomycin (LKV) and has variable resistance to colistin. Although Prevotella spp. are largely regarded as pigmented GNARs, they can be nonpigmented as well. Pigmented Prevotella spp. form brown or black colonies after a week of growth on LKV. Before this brown or black pigment develops, Prevotella may fluoresce a dark red on exposure to a Wood’s lamp (long-wave UV light). By colony morphology and Gram stain, Porphyromonas spp. tend to form smaller colonies and present as shorter rods or coccobacilli on Gram stain but can be difficult to distinguish from Prevotella. Porphyromonas usually grows as pigmented colonies, initially forming gray colonies that darken to black colonies within a week after plating on laked blood agar. Porphyromonas does not grow on LKV media because of its sensitivity to vancomycin, but it is resistant to colistin. Fusobacterium is a genus of obligately anaerobic filamentous gram-negative rods that are members of the phylum Fusobacter, in contrast to Bacteroides, Prevotella, and Porphyromonas, which are members of the phylum Bacteroidetes. On blood agar, Fusobacterium forms pinpoint colonies that can be circular or irregular, with some species, such as Fusobacterium nucleatum, forming umbonate “fried egg” colonies after 3 to 5 days of incubation. Depending on the strain, they can be hemolytic. Fusobacterium spp. can be somewhat variable in their Gram stain and display a range of cellular morphologies from coccoid, pleomorphic spherules (Fusobacterium necrophorum) to rod shaped. Rods can be short with rounded ends or long and thin with pointed ends (F. nucleatum), arrayed end to end (Fig. 249-1C). As a genus, Fusobacterium is sensitive to both kanamycin and colistin and resistant to vancomycin. It can be distinguished by its bile sensitivity and metabolism of threonine to propionate. Most species are indole positive and produce butyric acid during the fermentation of glucose. In adults, bacterial cells outnumber host cells by at least 10 : 1, and the microbiota’s gene count outnumbers that of the human genome by at least 100 : 1. The human microbiome is dominated by anaerobes. Gram-negative anaerobic rods colonize the mucosal surfaces of the oropharynx, gastrointestinal tract, and female urogenital tract, and Bacteroides and Prevotella are among the most abundant genera present. Mutualism and opportunism are important features of the symbiotic relationship between human hosts and colonizing species of the genera Bacteroides, Prevotella, Porphyromonas, and Fusobacterium. The human colon is colonized by 10 trillion to 100 trillion bacteria, making it the largest repository for bacteria in the body. The Bacteroides genus constitutes 30% of the total colonic bacteria. Bacteroides vulgatus, thetaiotaomicron, distasonis, fragilis, and ovatus are the most commonly encountered Bacteroides species in the human colon.3 There is significant variability among humans regarding the colonic colonization, with different B. fragilis group members as assessed by stool culture (Comstock and Onderdonk, unpublished). The colonization of the gastrointestinal tract occurs during descent through the vaginal canal or postnatally for infants delivered by cesarean section. Both breast milk and exposure to environmental factors are important forces in shaping colonization. Many studies over the past several decades have been undertaken to examine this process in detail, with genomic-based technology taking center stage more recently. There has been a recent explosion of interest in clinical correlations between intestinal colonization and human health and disease. The pioneering work of Bengt Björkstén3a and others has examined correlations between infectious disease and atopic and allergic diseases for many decades. One recent study suggests that B. fragilis colonization at younger than 3 weeks of age increases the risk of asthma later in life.4 The basic science behind these clinical observations rests in the hypothesis that certain bacterial factors may alter the balance between T cell–based immune responses. Elegant recent work by the Mazmanian and Kasper laboratories suggests that the B. fragilis zwitterionic polysaccharide A (PSA) may play an essential role in maintaining immunologic equilibrium in the intestine. Using mouse models, these investigators have demonstrated that PSA is a symbiosis factor that balances Th1 and Th2 T-cell subsets in the colon, enhances colonic regulatory T-cell function, and may be a beneficial microbe for the treatment of chronic inflammatory diseases such as inflammatory bowel disease and multiple sclerosis.5 The beneficial immunomodulatory effects of Bacteroides are not exclusive to B. fragilis; B. thetaiotaomicron also has immunomodulatory properties with the potential to dampen proinflammatory responses to commensal, intestinal bacteria. B. thetaiotaomicron targets the RelA subunit of the transcription factor, NF-κB, a master regulator of proinflammatory immune responses. This process is dependent on a nuclear receptor and transcription factor called peroxisome proliferator-activated receptor gamma and independent of the nuclear export receptor CRM1.6 However, the mechanism by which B. thetaiotaomicron drives this process remains to be fully understood. Strains of B. thetaiotaomicron have also been shown to contribute to intestinal inflammation in genetically susceptible mice.7 Bacteroides and other intestinal commensals also contribute to host immunity through the process of colonization resistance, the concept that the entrenched presence of gut symbionts provides protection from invading pathogens. The most central and well-understood function of Bacteroides resides in the mutualistic function of metabolism. The human gut at its core is a bioreactor. The saccharolytic Bacteroides spp. process diverse dietary and host polysaccharides for their own metabolic needs and in doing so aid in human digestion and nutrient liberation for their human hosts. A well-nuanced understanding of the biochemistry and genomics of glycan foraging by B. thetaiotaomicron has emerged over the past several years. In addition, the work of Jeffrey Gordon’s laboratory has drawn attention to the potential role of these bacteria in the human obesity epidemic.7a The female urogenital tract, particularly the vagina, is densely colonized by anaerobes. Although the gram-positive anaerobic lactobacilli are the predominant colonizers, GNARs may also be present in significant numbers. Vaginal colonization is dynamic, with variations not only intrinsic to the female reproductive life cycle from the premenarchal through postmenopausal years but also with significant shift over a given menstrual cycle.8 Pregnancy and parturition result in substantial microbiota population shifts in both the urogenital and the gastrointestinal tract as well.9,10 There is also intraindividual genus-level diversity in colonization with Bacteroides, Fusobacterium, Prevotella, and Porphyromonas. In addition, distinct microbial community differences exist among the labia, the urethral vestibular region, the length of the vagina, and the cervix. Frequent isolates from the vaginal vault include Bacteroides urealyticus and members of the B. fragilis group; Prevotella bivia, disiens, buccalis, melaninogenica, and corporis; Porphyromonas asaccharolytica; and F. nucleatum. Most research on the vaginal microbiota and symbiotic effects has focused on Lactobacillus, with the view that GNARs may contribute to vaginal dysbiosis, negative consequences for fetal outcomes, and host mycotic and bacterial infections. The oropharynx is a diverse niche for both aerobes and anaerobes. Within the mouth, the teeth, gingival crevices, saliva, and posterior pharyngeal structures provide distinctive milieus with diverse pH and oxygen tensions. Gingival scrapings are particularly dense in bacteria, with estimated concentrations on the order of 1011–12 CFU/mL. Similar to the colonization of the lower gastrointestinal tract, colonization of the oropharynx starts at birth. Among the anaerobes, Lactobacillus and Peptostreptococcus are the earliest colonizers. Fusobacterial populations emerge with the eruption of the first teeth and increase with establishment of full juvenile dentition. Prevotella and Porphyromonas colonization emerges after colonization by Fusobacterium. Of the GNARs, Porphyromonas gingivalis and endodontalis; F. nucleatum; Prevotella intermedia, melaninogenica, denticola, and loescheii; and Bacteroides forsythus all commonly populate dental plaque. Poor dentition, gingivitis, and other periodontal diseases correlate with increased numbers of GNARs, as do hospitalization, residence in a long-term care facility, and multiple medical comorbidities. Symbiosis should be viewed as a host-microbe relationship spanning the spectrum from mutualism through opportunism. Most, if not all, symbiotic GNARs have pathogenic potential. B. fragilis merits special consideration because although B. fragilis itself may not be the most abundant GNAR cultured from the gastrointestinal tract, it is the most common GNAR identified in clinical isolates from both blood and abscesses.11 Multiple virulence factors underpin this observation and account for the observed opportunism of Prevotella, Porphyromonas, Fusobacterium, and Bilophila. Virulence factors produced by these bacteria include capsular polysaccharides, outer-membrane proteins, lipopolysaccharide “endotoxins,” attachment factors (fimbriae/pili), toxins, and numerous enzymes. Synergism is another important concept that explains the presence of GNARs in many polymicrobial infections. Several facultative anaerobes and aerobes can provide favorable environments that can promote the growth of GNAR. For example, the production of superoxide dismutase by facultative species protects obligate anaerobes that do not produce this enzyme constitutively against the highly lethal superoxide anion. Without the presence of these other microbes, the oxygen tension would not be favorable for GNARs. In many polymicrobial infections, facultative anaerobes and aerobes function mutualistically for GNARs, providing strong synergy for their expansion, especially for B. fragilis. In turn, the B. fragilis group with its frequent β-lactamase activity can provide a protective environment for normally β-lactam-sensitive facultative anaerobes and aerobes. Endotoxic lipopolysaccharide (LPS) is a powerful mediator of systemic inflammation and a driver of septic shock. LPS can differ in its endotoxic potential. Because of the structure of lipid A in Bacteroides, the LPS does not elicit a strong host inflammatory response. This has been attributed to the absence of C14-2OH fatty acid as part of the lipid A substituent in these species. Porphyromonas and Prevotella also appear to have attenuated LPS for the same reason. Interestingly, the LPS of P. gingivalis may be able to antagonize the proinflammatory effects of other LPS in mixed infections, as suggested by in vitro experiments on primary human monocytes. In contrast, the LPS from Fusobacterium elicits a more inflammatory host response because it has a lipid A that contains C14-2OH fatty acid. B. fragilis produces eight distinct polysaccharides, PSA to PSH, and there is complex phase variation in the expression of these polysaccharides. There is also substantial variability in these polysaccharides across B. fragilis strains.12 Over the past 30 years, the work of Kasper, Onderdonk, Comstock, and Mazmanian has led to a nuanced understanding of the role of these polysaccharides in abscess formation and immunomodulation. The capsular polysaccharide complex facilitates binding of B. fragilis to mesothelial cells lining the peritoneum, providing a nidus for abscess formation. Numerous Porphyromonas and Prevotella spp., including Prevotella oralis and melaninogenica, as well as Fusobacterium, are also encapsulated and are abscessogenic.
Bacteroides, Prevotella, Porphyromonas, and Fusobacterium Species (and Other Medically Important Anaerobic Gram-Negative Bacilli)
Overview
History
Microbiology
Bacteroides
Prevotella and Porphyromonas
Fusobacterium
Symbiosis
Gastrointestinal Tract
Symbiosis and Mutualism in Immunity and Metabolism
Female Urogenital Tract
Oropharynx
Opportunism
Endotoxic Lipopolysaccharide
Capsular Polysaccharides
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Bacteroides, Prevotella, Porphyromonas, and Fusobacterium Species (and Other Medically Important Anaerobic Gram-Negative Bacilli)
249