First author
Year
No. of patients
IFR (%)
FNR (%)
SLNB oncologically safe?
Xing [19]
2006
1273
90
12
√
Hunt [18]
2009
3736
97.4
5.9
√
Kelly [20]
2009
1799
89.6
8.4
√
Classe [21]
2009
135
94.6
9.4
√
The meta-analysis of Xing and colleagues [19] included 21 studies with a total of 1,273 patients, who had previously undergone SLNB after PST followed by ALND during the surgical removal of the primary breast tumour. Overall, an identification rate of 90% and a false-negative rate of 12% for SLNB were found. They reported a negative predictive value of 90% and an overall accuracy of 94%. In 5 of the 21 included studies, a within-study comparison of IFRs and FNRs in patients with and without PST was performed. Neither for the IFR nor for the FNR could statistically significant differences be found; therefore, SLNB was considered to be oncologically safe even in the neoadjuvant setting.
In 2009 a large trial led by Hunt and colleagues confirmed these findings. In 3,736 patients with clinically lymph node-negative disease, axillary status was confirmed by palpation and US or computed tomography. Regarding IFR and FNR, a minimal difference was found in patients with (IFR 97.4%, FNR 5.9%) and without PST (IFR 98.7%, FNR 4.1%). Consequently, SLNB seems to be an accurate staging procedure. Between patients with and without PST, no significant differences were observed regarding local, regional and distant recurrence rates [18].
Another comprehensive meta-analysis was published in 2009 by Kelly and colleagues. They included 24 trials with a total of 1,799 patients with clinically lymph node-negative breast cancer, who underwent SLNB after PST. With an observed IFR of 89.6% and a FNR of 8.4%, they recommended SLNB as an accurate staging alternative to ALND [20].
The German AGO recommendations for patients with advanced breast cancer suggested that a SLNB in a cN0 situation is a feasible procedure. If no SLN can be identified, ALND should not be performed. However, if suspicious axillary lymph nodes are found, an exploratory axillary dissection is indicated [22, 23].
The S3 German guidelines stated that patients with cN0 disease should undergo SLNB; however, the guidelines are one of the few which recommend the procedure before PST is administered [24].
In summary, SLNB is the standard of care for axillary staging in patients with clinically node-negative breast cancer before and after PST. Taking the above-mentioned arguments regarding the timing of SLNB into account, there are advantages to performing SLNB after PST.
25.3 Management of the Axilla in Patients with Initial Node-Positive Disease Downstaged to Clinical Node Negativity After PST (cN1/cN0)
Several authors state that ALND remains the standard procedure in patients with initially lymph node-positive breast cancer. The rationale for this notion is the high false-negative rates of SLNB in this setting and a low SLNB identification rate [25–27]. Shen and colleagues investigated 69 patients with histologically proven axillary lymph node metastases and observed a false-negative rate of 25%. In conclusion, SLNB in this study population was found to be an inaccurate tool for staging the axillary nodes [25].
In contrast, Classe and colleagues suggested that SLNB was an accurate staging tool after PST. They found a FNR of 15% in patients with cN+ disease who underwent SLNB after PST [21].
To clarify the accuracy of SLNB in patients with initial node-positive disease, the results of three prospective, multicentre studies are presented below:
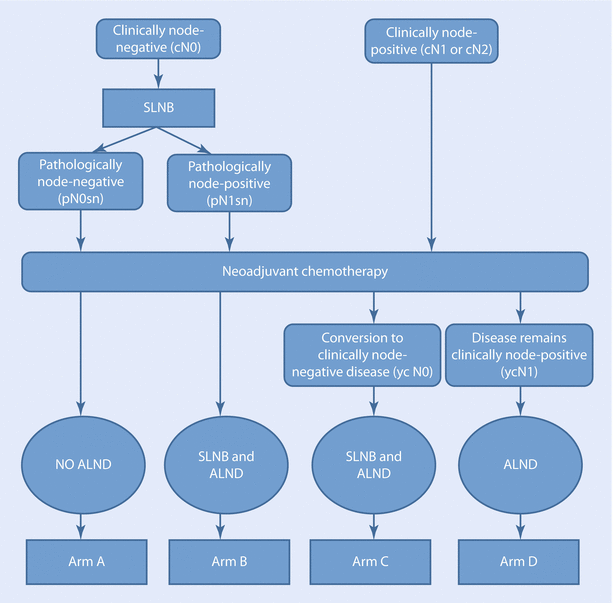
The SENTINA study is a four-armed study with the primary endpoint “accuracy of SLNB after PST” measured by the false-negative rate in patients with initially node-positive disease who convert under PST to clinically node negative.
Secondary outcomes were the IFR of the SLNB before and after PST, the IFR and the FNR of a SLNB after PST when a positive SLN was already removed prior to PST. ◘ Figure 25.1 shows the trial design of the SENTINA study. In patients with cN1, who underwent a second SLNB after PST (arm B), a very high FNR of 51.6% was found. In comparison, in those patients who converted from cN+ to ycN− (arm C), the FNR was 14.2%. The highest IFR (99.1%) was found in patients who underwent SLNB before PST (arms A and B). In those who converted from cN+ to ycN0 and SLNB had been performed after PST (arm C), an IFR of 80.1% was achieved. The lowest IFR was reported when a second SLNB was performed after PST. In this case the SLN could only be identified in 60.8%. The SLNB seems to be a less reliable tool for staging when performed after PST.
The ACOSOG Z1071 study (Alliance) focused on the assessment of the reliability of the SLNB after PST in patients with initially node-positive breast cancer [29]. Distinguishing between N1 and N2 disease, at least one SLN could be found in 92.9% in patients with N1 breast cancer and in 89.5% in patients with N2 nodal status. An overall FNR of 12.6% was reported. However, if some factors, which are mentioned below, are taken into consideration, a lower rate may be achieved.
The SN FNAC study assessed the accuracy of SLNB after PST in patients with biopsy-proven node-positive breast cancer [8]. They reported an IFR of 87.6% and a FNR of 8.4%.
The results of these studies are shown in ◘ Table 25.2.
Table 25.2
Study results of SENTINA, ALLIANCE and SN FNAC trials
Study | SENTINA [28] | ALLIANCE [29] | SN FNAC [8] | |
|---|---|---|---|---|
pN1/ycN0 | pN1/ypN0 | cN1/ypN0 | cN1/ypN0 | |
Procedure before PST | SLNB | X | X | FNA or core biopsy |
Procedure after PST | SLNB + ALND | SLNB + ALND | SLNB + ALND | SLNB + ALND |
No. of patients | 360 | 592 | 649 | 153 |
IFR (%) | 60.8 | 80.1 | 92.9 | 87.6 |
FNR (%) | 51.6 | 14.2 | 12.6 | 8.4 |
The panel of the 2015 NCCN Guidelines group also recommended ALND for patients with histologically proven lymph node metastasis at presentation. Patients who do not undergo ALND are supposed to have an increased risk for locoregional recurrence in the axilla [27]. An update of the American Society of Clinical Oncology clinical practice guideline in 2014 stated that patients undergoing PST might be offered SLNB. If the biopsy is performed after PST, a higher FNR must be expected. Due to insufficient data, no unambiguous statements were issues [26]. In contrast to the NCCN Guidelines, the panel of the St. Gallen International Expert Consensus 2017 considered SLNB to be feasible in patients with clinically node-positive breast cancer who were downstaged during PST. However, in consideration of the high FNR after PST, it was recommended that at least three SLNs be examined.
Nevertheless, advances in systemic breast cancer therapies and the above-mentioned rates of axillary pCR during PST provide hope that even node-positive disease will be convertible for many patients, and therefore SLNB potentially gains in importance in these cases. To increase the accuracy and feasibility of SLNB in patients with node-positive disease prior to PST, it is important to identify the factors that influence identification rates and false-negative rates of SLNB in this setting.
25.4 Technical Considerations
Several trials have shown that the IFR of the SLN may be favourably influenced by the selection of suitable mapping agents. The SENTINA trial reported that the use of blue dye in addition to the application of a radiocolloid was associated with a higher IFR when the SLNB was performed after PST. When the biopsy was performed before PST, no difference in the IFR was found between single-agent mapping (radiocolloid alone) and combined mapping (radiocolloid and blue dye).
The study protocol specified the use of a radiocolloid as mapping agent because it is assumed to be an objective, highly reproducible and measurable technique. They further implied that the use of blue dye is less reproducible and that the classification of whether a lymph node turns blue is subjective. Nevertheless, in 7.3% of patients who converted from clinically node positive to node negative under PST, no SLN could be detected, although lymphoscintigraphy revealed a radiocolloid hot spot. The technically more challenging procedure after PST is thought to be caused by fibrosis and structural changes in lymphatic vessels [28].
The SNFNAC trial didn’t describe the influence of dual mapping on the IFR, but the authors reported a lower FNR of 5.2% if radiocolloid and dye were used. If only single mapping was performed by using an isotope, the FNR increased to 16.0% [8].
Boughey and colleagues (2015) published a multivariate logistic regression model to assess the factors, which influenced the IFR of SLN in the ACOSOG Z1071 study in 2013. No significant influence on the IFR of the SLN was found for patient’s age, body mass index, tumour subtype, type of breast surgery, nodal response during PST and length of chemotherapy. However, it was shown that the method of SLN mapping was the only factor that affected the identification of the SLN. If dual mapping was used, a significant higher IFR was found than if only a single mapping agent was used [30]. Once again, fibrosis of the axilla after PST was proposedds as a possible reason. Further, the different molecular sizes and the varying time of transmission in the lymphatic tissue of both mapping agents could be responsible for the increased IFR if both agents were used [29].
One of the factors that might be able to improve the accuracy of SLNB is the number of harvested SLNs. The SENTINA trial demonstrated that there was a significant relation between the number of resected SLNs and the FNR. In patients with one removed SLN, the FNR was 24.3%. In patients with two removed SLNs, a FNR of 18.5% was found, whereas in those with more than three harvested SLNs, a FNR of less than 10% was found [28].
The ACOSOG Z0171 trial reported an overall FNR of 12.6%, which was higher than their predefined primary endpoint of 10%. When two SLNs were removed, the FNR reached 19.6%, while it decreased to 8.3% in patients with three removed SLNs [21].
Similar results were described by Boileau and colleagues in the SNFPST trial. If two or more SLNs were harvested, the FNR decreased to 4.9%. If less than two SLNs were removed, the FNR increased to 18.2–20.8% [7, 8]. Van Nijnatten and colleagues reported in their meta-analysis a FNR of 15.1% (12.7–17.6%). After a subgroup analysis, it could be shown that the FNR significantly increased when only one SLN was harvested and that it decreased when more than two SLNs were removed (23.9% vs. 10.4%) [31].
The pathological examination technique of harvested SLNs also influences the accuracy of SLNB. According to the results of a meta-analysis, Fu and colleagues reported a lower FNR of the SLNB after PST in patients with initially node-positive disease if IHC was combined with haematoxylin and eosin (H&E) staining than if H&E staining was used alone (8.7% vs. 16.0%) [32]. The use of mandatory immunohistochemistry was also supported by the results of the SN FNAC trial. They found a low FNR of 8.4% if IHC was used in patients with biopsy-proven lymph node-positive breast cancer [8]. In contrast, a recently published meta-analysis [33] didn’t find any significant differences in FNRs of the SLNB between studies using H&E alone and those using H&E in combination with IHC (FNR 11% (95% CI, 4%–18%) vs. 4% (95% CI, 1%–7%), p = 0.241). Further studies to determine if the routine combination of IHC and H&E has a clinical relevance for patients undergoing PST are needed.
A retrospective analysis from of the Alliance Z1071 trial showed that clip placement during initial node biopsy can reduce the FNR of SLNB after PST. In patients with cN1 disease, who underwent SLNB where at least two SLNs were removed and the clip was found within the SLNB specimen, a FNR of 6.8% could be achieved. The ability to identify former suspicious nodes and to extend the SLNB by the additional removal of clip-marked nodes may increase the accuracy of this procedure [34]. Further evaluation of the effect of clip placement in suspicious nodes prior to PST on the SLNB as a staging tool is required.
In conclusion, SLNB seems to be an appropriate way to stage axillary lymph nodes even in patients with initially node-positive disease that converts to node negative under the influence of PST. Nevertheless, long-term data regarding oncologic outcomes is still pending. However, there are some factors that have to be taken into account to improve the accuracy of this procedure in this particular setting: To achieve a lower FNR, at least three SLNs should be removed, and the use of dual-tracer mapping and immunohistochemistry are mandatory. Clip placement in clinically positive nodes prior to PST and additional resection of those clipped nodes during SLN surgery may also help to decrease the FNR.
25.5 Management of the Axilla in Patients with Micrometastases and Isolated Tumour Cells
The prognostic impact of micrometastasis (pN1mi) and isolated tumour cells (pN0[i+]) seems to be different in patients without PST from patients with PST. In patients undergoing primary surgery, ALND is not recommended if only micrometastases or isolated tumour cells are identified in the SN specimen. In the absence of PST, IHC is still not mandatory if initial H&E staining is negative. However, in patients undergoing PST, minimal nodal disease after PST represents remnant tumour cells that did not respond to systemic treatment adequately [35, 36]. The presence of remaining metastatic axillary nodes is significantly associated with a decreasing DFS [10]. As a result, ALND should remain the standard procedure in patients with any size of axillary lymph node metastases after PST. Even micrometastases or isolated tumour cells are considered to be node positive. It should be noted that the use of IHC – while not being routine practice in most centres – facilitates the detection of micrometastases and isolated tumour cells in SLNs, which were negative on initial H&E staining [8].
Boileau and colleagues reported that finding sentinel lymph node metastases of any size, including micrometastases or isolated tumour cells, should be considered as node positive after PST, which was one of the most important results of their trial because, thus, they were able to achieve a FNR of <10%. If micrometastases and isolated tumour cells were considered “node positive”, the FNR was 8.4%. If isolated tumour cells (<0.2 mm) were considered “negative”, the FNR increased to 13.3%, whereas it increased to 16.9% even if micrometastases (>0.2–2 mm) were considered as negative. Although no statistically significant correlation was found between the size of SLN metastases and the rate of positive non-SLNs, they observed a rate of positive non-SLNs after PST of 57% in patients with isolated tumour cells, of 38% in patients with micrometastases and of 56% in patients with metastases exceeding 2 mm, respectively [8]. Due to these findings, micrometastases and isolated tumour cells should be considered as node-positive disease after PST.
In the neoadjuvant setting (i.e. after PST), any size of lymph node metastases should be considered as node positive. Based on the currently available data, ALND represents the standard treatment for the axilla in patients with macrometastases, micrometastases and isolated tumour cells in the SLN after PST. Nevertheless, further studies have to evaluate the prognostic value of isolated tumour cells in the neoadjuvant setting.
25.6 Timing
With respect to the optimal timing of SLNB in the context of PST, unanimous recommendations can be found in the literature: If SLNB is performed before PST, similar FNR and IFR can be found as in primary surgery patients without PST. The SENTINA trial, for example, found an IFR of 99.1% when the SLNB was scheduled before PST. In comparison, after PST, a significantly lower IFR of 80.1% was observed [28]. Other trials confirmed the finding of lower IFRs and higher FNRs when biopsy is performed after PST [18, 19, 28, 29]. These findings are commonly used arguments to support SLNB before PST. Furthermore, the assessment of pathological axillary status before PST might also influence pre-surgical therapeutic strategies such as recommending immediate versus delayed implant reconstruction where knowledge of the need to give chest wall radiotherapy is important, for example.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree