Asthma
Paul A. Greenberger
Overview
Asthma is a disease characterized by hyperresponsiveness of bronchi to various stimuli as well as changes in airway resistance, lung volumes, and inspiratory and expiratory flow rates, with symptoms of cough, wheezing, dyspnea, or shortness of breath. In 1991, a National Institutes of Health Expert Panel suggested that asthma was a disease characterized by (a) airway obstruction that is reversible—partially or completely, (b) airway inflammation, and (c) airway hyperresponsiveness (1). In 1997, the Expert Panel 2 report described asthma as follows:
“Asthma is a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role, in particular, mast cells, eosinophils, T lymphocytes, macrophages, neutrophils, and epithelial cells. In susceptible individuals, this inflammation causes recurrent episodes of wheezing, breathlessness, chest tightness, and coughing, particularly at night or in the early morning. These episodes are usually associated with widespread but variable airflow obstruction that is often reversible either spontaneously or with treatment. The inflammation also causes an associated increase in the existing bronchial hyperresponsiveness to a variety of stimuli. Reversibility of airflow limitation may be incomplete in some patients with asthma” (2).
The NIH Expert Panel 3 Report of 2007 confirmed this working definition (3). Asthma has been described by other designations, including allergic bronchitis, asthmatic bronchitis, allergic asthma, atopic asthma, nonallergic asthma, cough equivalent asthma (4), and cardiac asthma (5,6). A central feature of asthma from a physiologic viewpoint is bronchial hyperresponsiveness to stimuli such as histamine or methacholine, a characteristic not shared by patients without asthma. In population screening, such nonspecific hyperresponsiveness has been reported as sensitive but not specific. Surprisingly, in a study of children 7 to 10 years of age, 48% of those with a diagnosis of asthma did not have bronchial hyperresponsiveness (7). Asthma is characterized by wide variations of resistance to airflow on expiration (and inspiration) with remarkable transient increases in certain lung volumes, such as residual volume, functional residual capacity, and total lung capacity.
Asthma is considered, for most patients, a reversible obstructive airway disease as compared with chronic obstructive pulmonary disease (COPD). Many patients with asthma experience symptom-free periods of days, weeks, months, or years in between episodes, whereas chronic symptoms and fixed dyspnea characterize COPD. When daily symptoms of cough, wheezing, and dyspnea have been present for months in a patient with asthma, bronchodilator nonresponsiveness may be present. However, appropriate anti-inflammatory therapy reduces symptoms and improves the quality of life along with improvement in pulmonary function status.
Immunoglobulin E (IgE)-mediated bronchoconstriction can be demonstrated in many patients with asthma, but not all cases of asthma are “allergic.” It is thought that about 80% of patients have allergic asthma. In the Inner-City Asthma Study of children ages 5 to 11 years, 94% of children reacted to at least one allergen (8). Some evidence does exist for IgE
antibodies to respiratory syncytial virus (RSV) (9) and parainfluenza virus (10); however, not all studies are consistent with such a mechanistic explanation of antiviral IgE-mediated asthma. Perhaps, RSV infection allows for TH2 polarization of the immune response and reduced antiviral IFNγ production (11). Serologic tests and nasopharyngeal or sputum cultures were positive for viruses in 23 of 29 (80%) adult patients who reported a recent respiratory tract infection and were hospitalized for asthma (12). Influenza A and rhinovirus were found most often. In children, viral infections (RSV in infants younger than 2 years of age and rhinovirus in children 2 to 16 years of age) were associated with acute wheezing episodes resulting in emergency department treatment or hospitalization (12). It is likely that other viruses or bacteria will be identified as causative of exacerbations of asthma, with use of increasingly sophisticated molecular techniques.
antibodies to respiratory syncytial virus (RSV) (9) and parainfluenza virus (10); however, not all studies are consistent with such a mechanistic explanation of antiviral IgE-mediated asthma. Perhaps, RSV infection allows for TH2 polarization of the immune response and reduced antiviral IFNγ production (11). Serologic tests and nasopharyngeal or sputum cultures were positive for viruses in 23 of 29 (80%) adult patients who reported a recent respiratory tract infection and were hospitalized for asthma (12). Influenza A and rhinovirus were found most often. In children, viral infections (RSV in infants younger than 2 years of age and rhinovirus in children 2 to 16 years of age) were associated with acute wheezing episodes resulting in emergency department treatment or hospitalization (12). It is likely that other viruses or bacteria will be identified as causative of exacerbations of asthma, with use of increasingly sophisticated molecular techniques.
The sudden onset of wheezing dyspnea that occurs within 3 hours of ingestion of aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) (13) is not an IgE-mediated reaction but represents alterations of arachidonic acid metabolism, such as blockage of the cyclooxygenase pathway with shunting of arachidonic acid into the lipoxygenase pathway. Potent lipoxygenase pathway products, such as leukotriene D4 (LTD4), cause acute bronchoconstriction in aspirin- and NSAID-sensitive patients (13–15). Patients with aspirin exacerbated respiratory disease (aspirin intolerant asthma) have a “knock in” condition in that there is increased LTC4 synthase in bronchial and nasal mucosa and elevated urinary concentrations of LTE4, a metabolite of LTD4, even at baseline (13,15). The concentrations of LTE4 rise significantly after ingestion of aspirin or a NSAID in susceptible patients (13–15).
Many patients with asthma may have symptoms precipitated by nonspecific, non–IgE-mediated triggers, such as cold air, air pollutants including ozone (16) and fine particles (<2.5 µm in diameter) (16), exercise, crying or laughing, and changes in barometric pressure. Fortunately, pharmacologic therapy can minimize the effects of these nonspecific triggers. Psychological stress such as from post traumatic stress disorder (17) and violence and sexual or physical abuse (18) also are associated with asthma.
Genetic and Environmental Factors
Genetic and environmental factors are important in terms of development of asthma, but the effects of heredity are much greater (19). Heritability of asthma ranges from 30% to 87% (19,20). In a study of 4,910 4-year-old twins, heredity accounted for 68% and the shared environment accounted for 13% (19). Nonshared environmental factors contributed 19% (19). The authors concluded that “rearing environment, family diet, and air pollutants seem to play a minor role” (19). If the approximate population prevalence for an allergic-type disease in a child is 20%, then having 1 parent with allergies increases the prevalence to 50% (21). If both parents are allergic, there is a 66% chance of the child developing an allergic condition (21). In twin studies, the concordance for asthma in monozygotic twins reared together was similar to that for twins reared apart (22,23). In addition, in a study of 5,864 twins who were evaluated from infancy to age 25 years, the cumulative incidence of asthma was 6% in males and 5.4% in females. If one twin developed asthma, the relative risk of the co-twin developing asthma was 17.9 for identical twins, compared with 2.3 for fraternal twins (23). More than 80% of cases of asthma began by 15 years of age, when nearly all of the study subjects lived in the same home environment (23). These data support a strong genetic effect on development of asthma. Methacholine responsiveness, total serum IgE concentration, and immediate skin test reactivity have been found to be more concordant in monozygotic twins than in dizygotic twins (24), which supports a genetic influence over an environmental influence. Both factors should be considered as contributory, and production of specific antiallergen IgE appears to be affected by environmental and local allergic exposures in the genetically susceptible subject. In Table 19.1, there are some examples of candidate genes for asthma or immunologic aspects involved with asthma (25). Single nucleotide polymorphisms (SNPs) have been associated with asthma (26), and it is likely that more will be identified and then replicated. Examples of SNPs that are associated with asthma include IL4RR551 and IL13+2044GA as they are associated with “Gain of
function” or enhanced biologic activity of IL-4 and IL-13, respectively (26).
function” or enhanced biologic activity of IL-4 and IL-13, respectively (26).
Table 19.1 Examples of Chromosomes Associated with Asthma and Atopy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
The onset of early childhood asthma has been associated with smoking in utero (27) and parental smoking (28). For development of severe asthma in children, cigarette smoking by grandparents also has been identified (29). However, once asthma begins, evidence exists for increased childhood respiratory symptoms from passive smoking (30,31) and added deficits in lung function when there had been in utero smoking (32).
Environmental factors, such as viral infections, have been associated with development of IgE antibodies. Frick et al. (33) demonstrated development of antiallergen IgE in association with increasing antiviral antibodies in a prospective study of high-risk infants whose parents both had allergic diseases. Croup in early childhood has been associated with subsequent development of asthma (34–36), as have RSV, influenza, parainfluenza virus, and metapneumovirus infections (37–39). When infections occur in an at- risk infant, defined when the mother, father, or sibling has asthma, there is a greater incidence of wheezing (35). This association is magnified if there is concomitant parental smoking or sensitization to dust mites (35). These findings demonstrate the overlap between family history, allergic sensitization, and respiratory infections on development of asthma.
Indoor allergen exposures from house dust mites (40), cats (41), and cockroaches (42) have been associated with the development of childhood asthma. New-onset asthma in older men (aged 61 years or older) was associated with detectable serum IgE antibodies to cat allergen but not dust mites, ragweed, or mouse urinary antigen (43). In this study, IgE antibodies to dog dander and cockroach excreta were not measured.
Environmental factors can predispose to development of asthma but also can be associated with a reduced risk of asthma. The concept is that there are beneficial effects of microbes in the home that do not cause any recognizable infection or illness (28,44). The “protective” home environments consist of stables and dairy farms that are part of the family home, which is an L-shape. The home and barn are attached. This observation has been associated with the “hygiene hypothesis” and the purported beneficial effects of certain infections that would shift the TH1/TH2 paradigm toward TH1. Absence of such exposures would permit asthma or atopy. Some specific protective factors have been identified and include small scale pig farming (<10 pigs/farm) but not sheep farming, raw milk consumption, a child’s involvement in frequent haying and staying in animal sheds (45). There remains some controversy about the “hygiene hypothesis” and development of asthma or atopy, but the microbe-rich environments seem to be protective against development of asthma by altering the predominant cytokines generated by CD4+ lymphocytes and interactions with innate immunity and its Toll-like receptors (TLRs) (45). A process that favors asthma includes generation of the helper T-cell subset TH2, which is central to IgE production, as opposed to TH1, which would diminish an “atopic” pattern and contribute to a classic delayed-type hypersensitivity response (type IVa1). In a study of 867 children in Japan who had received Bacille Calmette Guérin (BCG) immunization after birth and at 6 and 12 years of age, the presence of and induration of tuberculosis skin tests were studied in relation to the emergence of atopy (asthma, rhinitis, and atopic dermatitis) (46). By age 12 years, 58% of the children had developed positive (≥10 mm in duration) responses to tuberculin testing, and 36% of children had reported atopic symptoms (46). Asthma symptoms and atopy were associated negatively with positive tuberculin responses, and presence of tuberculin reactivity was associated with remission from asthma by years 6 or 12 (46). The data raised the possibility that the TH1 response produced by BCG immunization resulted in increases in the TH1 cytokines, interferon-γ (IFN-γ), and interleukin-12 (IL-12) and decreases in incidence of asthma, possibly even inducing remissions of atopy. In addition, there were reduced quantities of the TH2 cytokines IL-4, IL-13, and IL-10, compared with the BCG nonresponders, who had more atopy and asthma. Alternatively, these data might be interpreted that children likely to become atopic have a reduced ability to develop TH1 memory lymphocytes after BCG immunization or, by analogy, reduced response to measles vaccination (47). The latter stems from data revealing less atopy when there was a previous episode of measles (47). These studies and the association between RSV and other viral infections and childhood asthma suggest that the critical link may be the predominance of the TH1 cytokines and protective innate immune responses. The latter may be specific such as for TLR 5 for people exposed to pigs and TLRs 6 and 8 for people working with silage (45). The notion that asthma is “an epidemic in the absence of infection” has been suggested (48) and demonstrates how complex asthma and the identification of its origins are.
The effects of air pollution on the early development of asthma remain under investigation although the effects of air pollution from ozone and small particles have been associated with hospitalizations for acute severe asthma (49).
Complexity of Asthma
The cause of asthma remains unknown, although asthma is considered a very complex, heterogeneous, inflammatory disease (50, 51) or syndrome. Some important pathologic findings include a patchy loss of bronchial epithelium, usually associated with eosinophil infiltration (52–54), neutrophilic infiltration (55), lymphocyte infiltration (52), mast cell degranulation (52),
contraction and hypertrophy of bronchial smooth muscles, bronchial mucosa edema and increased blood flow (56), bronchial gland hyperplasia, hypersecretion of thick bronchial mucus, and basement membrane thickening (52,57). Collagen synthesis may result from stimulation or injury to airway epithelial cells (58). A key cell is the myofibroblast, which is a hybrid cell of fibroblast and smooth muscle cell origins. These cells produce types III and I collagen (58). Epithelial cells obtained during bronchoalveolar lavage (BAL) from patients with asthma have been found to be much less viable than in subjects without asthma (59). However, the epithelial cells from patients with asthma produced much more (a) fibronectin, a glycoprotein involved with cell attachment, cell growth, and chemotaxis; and (b) 15- hydroxyeicosatetraenoic acid (15-HETE), a metabolite of arachidonic acid (59). The increased metabolic activity of epithelial cells appears to contribute to airway damage and remodeling. There is subepithelial “fibrosis” that is composed of collagen types I, II, and V, which contributes to the basement membrane thickening of asthma.
contraction and hypertrophy of bronchial smooth muscles, bronchial mucosa edema and increased blood flow (56), bronchial gland hyperplasia, hypersecretion of thick bronchial mucus, and basement membrane thickening (52,57). Collagen synthesis may result from stimulation or injury to airway epithelial cells (58). A key cell is the myofibroblast, which is a hybrid cell of fibroblast and smooth muscle cell origins. These cells produce types III and I collagen (58). Epithelial cells obtained during bronchoalveolar lavage (BAL) from patients with asthma have been found to be much less viable than in subjects without asthma (59). However, the epithelial cells from patients with asthma produced much more (a) fibronectin, a glycoprotein involved with cell attachment, cell growth, and chemotaxis; and (b) 15- hydroxyeicosatetraenoic acid (15-HETE), a metabolite of arachidonic acid (59). The increased metabolic activity of epithelial cells appears to contribute to airway damage and remodeling. There is subepithelial “fibrosis” that is composed of collagen types I, II, and V, which contributes to the basement membrane thickening of asthma.
When bronchial biopsy samples were obtained from 14 patients who had asthma for 1 year or less, increases in numbers of mast cells, eosinophils, lymphocytes, and macrophages were found in the epithelium (60). Deeper in the lamina propria, eosinophils, lymphocytes, macrophages, and plasma cells were present, suggesting that patients with mild asthma, who had not received anti-inflammatory therapy, had marked cellular infiltration in the bronchial mucosa (60).
Human bronchial epithelium from patients with asthma express Fas ligand (Fas L) and Fas on eosinophils and T lymphocytes (61). Activation of Fas by Fas L induces apoptosis. Biopsy samples from patients, who had not received inhaled corticosteroids, had reduced numbers of apoptotic eosinophils and reduced expression Fas L and Bcl-2, which help regulate apoptosis. Conversely, inhaled corticosteroid–treated patients had fewer eosinophils and increased numbers of apoptotic eosinophils (61). In a study of BAL of 12 newly diagnosed and untreated patients with asthma, reduced expression of messenger RNA (mRNA) for both Fas and the Fas receptor (CD95) on CD3+ T lymphocytes was found (62). These findings are consistent with a persisting inflammatory cell infiltrate that characterizes asthma and offers the possibility of targeted anti-inflammatory therapy.
Some physiologic characteristics of asthma include bronchial hyperresponsiveness to stimuli such as histamine (63), methacholine (64), or LTD4 (65) and at least a 12% improvement in forced expiratory volume in 1 second (FEV1) after inhalation of a β2-adrenergic agonist, unless the patient is experiencing acute severe asthma (status asthmaticus) or has had severe, ineffectively treated airway obstruction. There are large changes in lung compliance, depending on severity of the disease.
On a cellular level, during acute episodes of asthma, there are activated or hypodense eosinophils present in increased numbers (66–68) and hyperadhesive eosinophils in the sense of increased binding to VCAM and ICAM (69). Eosinophil products such as major basic protein (MBP) can be identified in sputum (67) and in areas where bronchial epithelium has been denuded (68). Eosinophil cationic protein has been identified in areas of denuded bronchial epithelium. This cationic protein has been reported to be even more cytotoxic than MBP (70). Mast cells in the bronchial lumen and submucosa are activated, and their many cell products are released, whether preformed or synthesized de novo. Mast cells are found to be in close proximity to smooth muscle and their interactions are being investigated regarding the ability of smooth muscle to stretch maximally (71). Indeed, it has been suggested that the mast cells infiltrate smooth muscle causing “mast cell myositis” (71). Macrophages, lymphocytes, and epithelial cells participate as well and when epithelial cells are damaged, the production of the protective PGE2 is reduced. Epithelial cells are able to produce many different effector molecules.
Evidence supports neuroimmunologic abnormalities in asthma, such as the lack of the bronchodilating nonadrenergic noncholinergic (NANC) vasoactive intestinal peptide (VIP) in lung sections from patients with asthma (72) and reduced concentrations of VIP during acute exacerbations of asthma (73). There are increased concentrations of IgG autoantibodies that catalyze the hydrolysis of VIP in women whose asthma became more difficult to control during pregnancy (73). Substance P concentrations in induced sputum have been reported to be markedly elevated, compared with that in controls (74). The concentrations of the tachykinin, neurokinin A, is elevated in bronchoalveolar lavage fluid from patients with asthma compared to normal (75), and the potent vasodilator, calcitonin gene-related peptide, has been detected during late asthmatic reactions (76).
The free radical nitric oxide is detectable in expired air in patients with asthma, and its concentration increases further after allergen challenge (77). Inhaled corticosteroids, such as fluticasone, result in about a 60% reduction in exhaled nitric oxide (eNO) within 6 weeks (78). It has been hypothesized that management of asthma could be improved by using the biomarker, eNO. Nevertheless, when asthma management compared the use of the National Asthma Education and Prevention Program Expert Panel (NAEPP) guidelines combined with measurement of eNO to the guidelines alone, there was no meaningful difference in control of asthma (79). A free radical generated from arachidonic acid, 8-isoprostane, is increased in asthma and reflects ongoing oxidative stress (80). There are progressively greater amounts in expired air as asthma severity increases from mild-to-severe (80).
In addition to the above-described features of asthma, asthma is heterogeneous in its clinical presentations (phenotypes) and responses to pharmacologic treatment. Patients vary in their responses to β2 adrenergic
agonists (81), inhaled corticosteroids (82), leukotriene antagonists (83), and oral corticosteroids (84).
agonists (81), inhaled corticosteroids (82), leukotriene antagonists (83), and oral corticosteroids (84).
These findings demonstrate some but not all of the complexities of asthma, which decades ago was considered a psychological condition. Asthma is not a psychological disorder. Nevertheless, the burden of asthma as a chronic disease, especially when the patient has experienced repeated hospitalizations or emergency department visits, may result in psychological disturbances or abnormal coping styles that coexist with asthma (17,18,85–88).
Incidence and Significance
Asthma affects more than 22 million people in the United States, as of 2005, consisting of 6.5 million children and 15.7 million adults (89). It is estimated that 32.6 million people have at one time been diagnosed with asthma (89). The World Allergy Organization (WAO) has estimated that 300 million people worldwide have asthma, of which half are in developing countries (90). In the United States, using a random digit dialing system, the estimated prevalence of asthma was 8.4% of adults at least 18 years of age (91). The range by various counties across the country was 3% to 13.8% (91). Puerto Rican responders had a prevalence of asthma 80% to 140% higher than non-Hispanic whites (89,91). Native Americans and Alaskan Natives had prevalence rates 40% greater than non-Hispanic whites (89). Acute asthma is the most common childhood medical emergency (92). Disturbingly, the rate of emergency department visits for asthma was 350% higher in black people than in white people (89). Hospitalizations in black people were 240% greater (89). Often, adults and children requiring acute treatment of asthma have not received or are not using optimal anti-inflammatory therapy. In children in the United States, the attack rate (episode in the past 12 months) was 5.9% for boys and 4.5% among girls (89). Morbidity from asthma remains a major issue in that children, ages 5 to 17 years, missed 12.8 million days of school in 2003 and adults, ages 18 years and older, missed 10.1 million work days (89). The rate for hospitalizations for asthma based on the population has remained unchanged during the period from 1980 to 2004 (93) despite vast increments in the knowledge of asthma.
For many countries, the prevalence of “clinical asthma” is greater than the 10.9% reported for the United States (94). For example, Scotland (18.4%), England (15.3), Australia (14.7%), Canada (14.1%), Peru (13.0%), and Brazil (11.4%) had higher rates than the United States (94). “Clinical asthma” in children was reported as 50% of the prevalence data for self-reported wheezing in the past 12 months (94). For adults, the rate for “clinical asthma” was from “breathlessness and wheeze” which was 50% of those reporting current wheeze (94).
Mortality from asthma appears to be decreasing somewhat in that there were 4,055 deaths in 2003, of which 195 were in children (89). There was a disproportionate increase in deaths in Puerto Ricans and black people (89). About 0.5% of hospitalizations for asthma are associated with a death from asthma, ranging from 0.3% in black people to 0.6% in white people more than 5 years of age (95). About one-third of deaths from asthma occur in the hospital (95). In the same study, the typical hospitalization for asthma was 2.7 days with hospital charges of $9,078 in year 2000 (95). Overall, in light of the unchanged per population rate of hospitalizations for asthma from 1980 to 2004 (93), the daunting challenge remains to reduce the number of hospitalizations (and deaths) from asthma.
Intermittent respiratory symptoms may exist for years before the actual diagnosis of asthma is made in patients older than 40 years of age. The diagnosis of asthma may be more likely made in women and nonsmokers, whereas men may be labeled as having chronic bronchitis, when in fact they do not have chronic sputum production for 3 months each year for 2 consecutive years. Asthma may have its onset in the geriatric population and medication nonadherence is found frequently (96). Asthma may begin during or after an upper respiratory tract infection. The prevalence of asthma was found to be 7% or greater, and all cause deaths, but not asthma specifically, have been reported to be increased in geriatric patients with asthma as compared to control patients without asthma (97).
Asthma morbidity can be enormous from a personal and family perspective as well as from the societal aspect. The number of hospitalizations in the United States for asthma increased almost fourfold from 1965 to 1983, with absolute numbers growing from 127,000 to 459,000 per year (98). This number has been stable from 1980 to 2004 based on per population calculation (93). The number of days of school missed from asthma is excessive, as is work absenteeism or presenteeism (present but not fully productive).
Asthma was thought to be related to occupational causes in 2% of the 6 million people with asthma in the United States in 1960. As of 2000, it was estimated that 5% to 15% of newly diagnosed asthma in working adults is caused by an occupational exposure (99). The 2008 Consensus Statement of the American College of Chest Physicians reported that 10% to 15% of asthma cases are related to occupational exposures (100). Terminology includes “work-related asthma” which consists of occupational asthma (de novo asthma or return of previously quiescent asthma) or work-exacerbated asthma (100).
The asthma death rate is over 4,000 annually in the United States (89) which reflects a decline in absolute numbers despite greater U.S. population. The number of fatalities from asthma increased in the United States from 0.8 deaths per 100,000 general population in 1977
to 2.0 in 1989 and still 2.0 in 1997 (101). By 2003, the rate had declined to 1.4 per 100,000 population (89). The fatality rate among Puerto Ricans (4.4/100,000), black people (3.2/100,000 population), American Indians (1.7/100,000 population), and Alaskan natives (2.0/100,000 population) remains higher than among white people (1.2/100,000 population) (89).
to 2.0 in 1989 and still 2.0 in 1997 (101). By 2003, the rate had declined to 1.4 per 100,000 population (89). The fatality rate among Puerto Ricans (4.4/100,000), black people (3.2/100,000 population), American Indians (1.7/100,000 population), and Alaskan natives (2.0/100,000 population) remains higher than among white people (1.2/100,000 population) (89).
A disturbing finding was reported in a study of asthmatics conducted in the Detroit area. Black patients received or filled fewer prescriptions for inhaled corticosteroids and were less likely to be referred to an asthma specialist than Caucasians in the managed care setting in which the study took place. (102) All the patients in this study were enrolled in the same large health maintenance organization; thus, factors such as insurance type or access to medications would not explain the discrepancy in care for black patients as compared to Caucasians.
The costs of asthma include direct costs of medications, hospitalizations, and physician charges in addition to indirect costs for time lost from work (absenteeism) and loss of worker productivity (presenteeism). Some 20% of the patients used 80% of the resources ($2,584, compared with $140 per patient) (103). Some patients have been labeled as the “$100,000 asthmatic patients” because of repeated hospitalization and emergency department visits (104). Emotional costs of asthma are great for the sufferer and the family if asthma is managed ineffectively or if the patient refuses to adhere to appropriate medical advice.
The death of a family member or friend from asthma is shocking; the person may be young, and the fatal attack may not have been anticipated by others or even the patient. It must be kept in mind that with current understanding and treatment of asthma, nearly all fatalities should be avoidable, and asthma need not be a fatal disease. More than half of the deaths from asthma occur outside of the hospital. This observation has led some physicians to conclude that emergency medical services should be improved or even that every patient with asthma should receive a prescription for an albuterol metered-dose inhaler (MDI). One cannot dispute such an argument about emergency services, but it is advisable for the physician managing the patient with asthma to have an emergency plan (action plan) available for the patient or family so that asthma is not managed from a crisis orientation but rather on a preventive basis. Further, an education program or patient instructions can identify what patients should do when their medications are not effective, such as with a change in the level of control or for an exacerbation of asthma.
Anatomy and Physiology
The central function of the lungs is gas exchange with delivery into the blood stream of oxygen and removal of carbon dioxide. The lung is an immunologic organ and has endocrine and drug-metabolizing properties that affect respiration. The lung consists of an alveolar network with capillaries passing near and through alveolar walls and progressively larger intrapulmonary airways, including membranous bronchioles (1 mm or smaller noncartilaginous airways) and larger cartilaginous bronchi and upper airways. Inspired air must reach the gas exchange network of alveoli. The first 16 airway divisions of the lung are considered the conducting zone, whereas subsequent divisions from 17 to 23 are considered transitional and respiratory zones. The conducting zone consists of trachea, bronchi, bronchioles, and terminal bronchioles and produces what is measured as airway resistance. The terminal bronchioles as a rule have diameters as small as 0.5 mm. Respiratory bronchioles, alveolar ducts, and sacs comprise the transitional and respiratory zones (105) and are the sites of gas exchange.
The structures of bronchi and trachea are similar, with cartilaginous rings surrounding the bronchi completely until the bronchi enter the lungs, at which point there are cartilage plates that surround the bronchi. When bronchioles are about 1 mm in diameter, the cartilage plates are not present. Smooth muscle surrounds bronchi and is present until the end of the respiratory bronchioles.
The lining mucous membrane of the trachea and bronchi is composed of pseudostratified ciliated columnar epithelium (Fig. 19.1). Goblet cells are mucin-secreting epithelial cells and are present in airways until their disappearance at the level of terminal bronchioles. In the terminal bronchioles, the epithelium becomes that of cuboidal cells with some cilia, Clara (secretory) cells, and goblet cells until the level of respiratory bronchioles, where the epithelium becomes alveolar in type. Mucus consists of a superficial gel phase composed of glycoproteins and a sol phase consisting of isotonic fluid in contact with the mucous membrane cells. The cilia move in the sol phase proximally to help remove luminal material (debris, cells, mucus) by the ciliary “mucus escalator.” Other cells such as mast cells, alveolar macrophages, polymorphonuclear leukocyte lymphocytes, eosinophils, and airway smooth muscle cells contribute to lung pathology in different ways. Epithelial cells may be thought of in a constant state of “injury” and are not able to be “repaired” completely. There is loss of columnar epithelial cells and the tight junctions. Permeability is increased (106). Primary bronchial epithelial cells from subjects with asthma have been shown to replicate rhinovirus in vitro to several logs, whereas those of normal control subjects were resistant to infection. This resistance was a result of rapid induction of apoptosis and of interferon (IFN)-β in the normal cells, whereas these responses were deficient in asthmatic cells. These studies were recently extended to a novel family of three related proteins, the IFN-γs 1–3, production of which was also deficient in
vitro and related to asthma exacerbation severity in vivo. (106).
vitro and related to asthma exacerbation severity in vivo. (106).
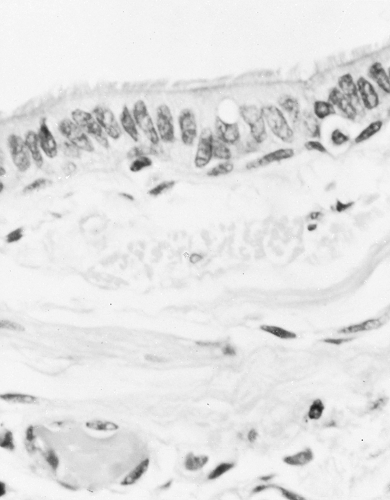 Figure 19.1 Microphotograph of the wall of a normal bronchus. Note the uniform ciliated epithelium and their bands of smooth muscle. (H & E stain, magnification × 250.) |
The bronchial wall is characterized by mucosa, lamina propria, smooth muscle, submucosa, submucosal glands, and then cartilaginous plates. Submucosal glands produce either mucous or serous material depending on their functional type. Mast cells can be identified in the bronchial lumen or between the basement membrane and epithelium. They are “microlocalized” to smooth muscle cells and mucosal glands (107). Mast cells have been recovered from BAL samples but are low in number in these samples (108). Mast cell heterogeneity has been recognized based on contents and functional properties. Briefly, mucosal mast cells are not recognized in a formalin-fixed specimen, but connective tissue mast cells are. Mucosal mast cells are present in the lung and contain tryptase but not chymase, whereas connective tissue mast cells contain tryptase and chymase (109). Mast cells participate in airway remodeling because they activate fibroblasts (109,110) and infiltrate and interact with smooth muscle cells (109,110) causing a “mast cell myositis” of the smooth muscle. Mast cell–derived tryptase is a mitogen for epithelial cells and stimulates synthesis of collagen (109). The mucosal mast cells are stimulated by IL-3, IL-4, and IL-9 (a growth factor for mast cells) (109). The sub-mucosal (connective tissue) mast cells are present in large and small airways and are thought to participate in localized fibrogenesis (109). These mast cells interact with stem cell factor (c-kit ligand) and smooth muscle cells (109). In addition to mast cell generation of histamine, prostaglandin D2 (PGD2), LTD4, and tryptase, they secrete IL-4, which upregulates vascular cell adhesion molecule (VCAM) on vessel endothelial surfaces. Eosinophil entry into tissues is facilitated by VCAM. IL-4 also favors isotype switching within the nucleus to cause production of IgE antibodies. The mast cell has many effects, from mediator release and cytokine production to fibrogenic activity. Their interactions with smooth muscle cells are intriguing in the context of induced “myositis” of the smooth muscle (109).
Neutrophils have been recovered in induced sputum using 3.5% saline in an ultrasonic nebulizer (111) from patients with asthma. The numbers were increased in patients with severe asthma (53%) compared with moderate (49%) and mild (35%) asthma. Sputum from nonatopic, nonasthmatic subjects had 28% neutrophils (111). The concentrations of IL-8, which is chemoattractant for neutrophils and is an angiogenic cytokine, and of myeloperoxidase were increased in sputum from patients with moderate and severe asthma (111). Neutrophils have been identified in some (112) but not all (113) patients with sudden (<3 hours) death from asthma.
Macrophages serve as antimicrobial and pro-inflammatory cells and are accessory cells for presenting antigens. Macrophages are present in patients with asthma but are found in greater numbers in patients with chronic bronchitis. Macrophages have been detected during both early and late bronchial responses to allergens. These cells are metabolically active in that they can generate prostaglandins, leukotrienes, pro-inflammatory cytokines, chemokines, free radicals, and mucus secretagogues.
Increased numbers of eosinophils in bronchial biopsy specimens and sputum can be expected in patients with asthma. It has been estimated that for every 1 eosinophil in peripheral blood, there are 100 to 1,000 in the tissue. Patients with mild asthma have eosinophils detected in bronchial biopsy samples, and eosinophils can be found in postmortem histological sections (112,113). Eosinophils produce major basic protein, eosinophil cationic protein, eosinophil derived neurotoxin, eosinophil peroxidase, free radicals, leukotrienes, and TH2 cytokines. Eosinophils are proinflammatory cells that participate in the pathogenesis of airway remodeling in patients with persistent asthma.
Epithelial cells are shed especially in patients with severe asthma but also in patients with mild asthma. There are a vast number functions and interactions of epithelial cells (114). In addition to being antimicrobial, one of many actions is to produce neutral endopeptidase, which degrades substance P. The loss of functioning epithelium could lead to potentiated effects of this neuropeptide. Similarly, epithelial cells generate smooth muscle–relaxing factors that could be decreased in amount as epithelium is denuded. Epithelial cell fluid obtained during BAL was analyzed for a gelatinase, which is in the family of matrix metalloproteinases (115). Mechanically ventilated patients with asthma were found to have very high quantities of a 92-kDa gelatinase, compared with patients with mild asthma and with ventilated, nonasthmatic subjects (115). This enzyme may damage collagen and elastin and the subepithelial basal lamina region (115). Increased permeability could result because of epithelial cell shedding and alterations of types IV and V collagens that are present in this basement membrane region (115). In this study, mechanically ventilated patients had increased numbers of eosinophils and neutrophils, compared with nonventilated patients with mild asthma (115). There was no difference in numbers of epithelial cells in BAL between patients with mild asthma and the mechanically ventilated patients with asthma, but both groups had twice the percentage as the nonasthmatic subjects, emphasizing that epithelial cell denudation occurs in mild as well as severe asthma.
Innervation
The nervous system and various muscle groups participate in respiration. Table 19.2 lists muscles; their innervation; other respiratory responses, such as smooth muscle cell and bronchial glands; and nonadrenergic, noncholinergic responses. Efferent parasympathetic
(vagal) nerves innervate smooth muscle cells and bronchial glands. The vagus nerve also provides for afferent innervation of three types of sensory responses. The irritant (cough) reflex is rapidly adapting and originates in the trachea and main bronchi. Pulmonary stretch or slowly adapting afferents are also located in the trachea and main bronchi, whereas C fibers are located in small airways and alveolar walls. Afferent stimulation occurs through the carotid body (sensing oxygen tension) and nervous system chemoreceptors in the medulla (sensing hypercapnia).
(vagal) nerves innervate smooth muscle cells and bronchial glands. The vagus nerve also provides for afferent innervation of three types of sensory responses. The irritant (cough) reflex is rapidly adapting and originates in the trachea and main bronchi. Pulmonary stretch or slowly adapting afferents are also located in the trachea and main bronchi, whereas C fibers are located in small airways and alveolar walls. Afferent stimulation occurs through the carotid body (sensing oxygen tension) and nervous system chemoreceptors in the medulla (sensing hypercapnia).
Table 19.2 Examples of Innervation, Muscles, and Respiratory Responses | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
Efferent respiratory responses include cervical and thoracic nervous system innervation of respiratory muscles, such as those listed in Table 19.2. Fortunately, not all respiratory muscles are essential for respiration should a spinal cord injury occur. In addition to efferent parasympathetic innervation of smooth muscle cells and bronchial glands, another source of efferent stimulation is through the nonadrenergic, noncholinergic epithelial sensory nerves. Stimulation of these nerves by epithelial cell destruction that occurs in asthma can trigger release of bronchospastic agonists, such as substance P and neurokinins (A and B), through an antidromic axon reflex. The bronchodilating NANC neurotransmitter, VIP, may oppose effects of other bronchoconstricting agonists, such as substance P. Nitric oxide is a mediator of the NANC system and could offset some of the bronchoconstriction induced by histamine and bradykinin (116). The absence of VIP could contribute to bronchoconstriction.
Smooth muscle cells participate in the Hering-Breuer inflation reflex, in which inspiration leading to inflation of the lung causes bronchodilation. This reflex has been described in animals and humans. The clinical significance in human respiratory disease may be minimal. For example, when a patient with asthma experiences bronchoconstriction when inhaling methacholine or histamine, there is increased airway resistance during a deep inspiration (117). In contrast, patients without asthma and those with rhinitis demonstrate bronchodilation and reduced airway resistance at total lung capacity. During a bronchial challenge procedure in a patient with rhinitis, if the patient performs a FVC maneuver by inhaling to total lung capacity after inhaling the bronchoconstricting agonist in question, the resultant bronchodilation may mask any current airway obstruction. To obviate this possibility, the initial forced expiratory maneuver should be a partial flow volume effort, not a maximal one, which requires maximal inspiration. Otherwise, the dose of agonist necessary to achieve finally a 20% decline in FEV1 will be higher than necessary.
Pathophysiologic Changes in Asthma
From a pathophysiologic perspective, the changes that occur in asthma are multiple, diverse, and complex. Further, some of the abnormalities, such as bronchial hyperresponsiveness and mucus obstruction of bronchi, can be present when patients do not have symptoms. Major pathophysiologic abnormalities in asthma are (a) widespread smooth muscle contraction, (b) mucus hypersecretion, (c) mucosal and submucosal edema, (d) bronchial hyperresponsiveness, and (e) inflammation of airways. Obstruction to airflow during expiration and inspiration results in greater limitation during expiration. Hypertrophy and even hyperplasia of smooth muscle have been recognized in asthma. Smooth muscle contraction occurs in large and or small bronchi. The concept of “airway remodeling” includes inflammation, mucus hypersecretion, subepithelial fibrosis, airway smooth muscle hypertrophy and angiogenesis (3).
Bronchial challenge of patients with asthma by inhalation of histamine demonstrated two abnormal responses compared with patients without asthma (118). First, the patients with asthma have increased sensitivity to histamine (or methacholine) because a smaller-than-normal dose of agonist is usually necessary to produce a 20% decline in FEV1. Second, the maximal response to the agonist in asthma is increased over that which occurs in nonasthmatic, nonrhinitic subjects. In fact, the maximal bronchoconstrictive response (reduction of FEV1) that occurs in the nonasthmatic, nonrhinitis subject, if one occurs at all, reaches a plateau beyond which increases in agonist produce no further bronchoconstriction. In contrast, were it possible (and safe) to give a patient with asthma increasing amounts of an agonist such as histamine, or methacholine, increasing bronchoconstriction would occur. In an analysis of 146 patients with mild asthma who had undergone bronchial provocation challenge with histamine, two patterns were identified (119). The first was the decline of FEV1 and FEV1/FVC without a change in FVC at the dose of histamine causing a 20% decline in FEV1 (PC20). The second pattern, detected at the time of the PC20 response, had reductions in FVC and FEV1 but not FEV1/FVC. It was concluded that the latter subjects experienced excessive bronchoconstriction (119). The authors identified a clinical connection in that there was a moderate correlation between the percentage decline in FVC at the PC20 and patients necessitating prescriptions for oral corticosteroids (but not β2-adrenergic agonists) (119). In the patients who develop a declining FVC and FEV1 after bronchoprovocation challenge, there is a concurrent increase in residual volume, which is detrimental if it continues. In summary from these findings, the ease of bronchoconstriction (PC20) is one parameter, but the extent of bronchoconstriction (drop in FVC), when the patient has reached the PC20, correlated with need for oral corticosteroids.
Hypersecretion of bronchial mucus may be limited or extensive in patients with asthma. Autopsy studies of patients who died from asthma after having symptoms for days or weeks classically reveal extensive mucus
plugging of airways. Large and small airways are filled with viscid mucus that is so thick that the plugs must be cut for examination (120). Reid (120) has described this pattern as consistent with endobronchial mucus suffocation. Other patients have mild amounts of mucus, suggesting that perhaps the fatal asthma episode occurred suddenly (over hours) and that severe bronchial obstruction from smooth muscle contraction contributed to the patient’s death. A virtual absence of mucus plugging, called empty airways or sudden asphyxic asthma, has been reported (120,121). Desquamation of bronchial epithelium can be identified on histological examination (122) or when a patient coughs up clumps of desquamated epithelial cells (creola bodies). Bronchial mucus contains eosinophils, which may be observed in expectorated sputum. Charcot-Leyden crystals (lysophospholipase) are derived from eosinophils and appear as dipyramidal hexagons or needles in sputum. Viscid mucus plugs, when expectorated, can form a cast of the bronchi and are called Curschmann’s spirals.
plugging of airways. Large and small airways are filled with viscid mucus that is so thick that the plugs must be cut for examination (120). Reid (120) has described this pattern as consistent with endobronchial mucus suffocation. Other patients have mild amounts of mucus, suggesting that perhaps the fatal asthma episode occurred suddenly (over hours) and that severe bronchial obstruction from smooth muscle contraction contributed to the patient’s death. A virtual absence of mucus plugging, called empty airways or sudden asphyxic asthma, has been reported (120,121). Desquamation of bronchial epithelium can be identified on histological examination (122) or when a patient coughs up clumps of desquamated epithelial cells (creola bodies). Bronchial mucus contains eosinophils, which may be observed in expectorated sputum. Charcot-Leyden crystals (lysophospholipase) are derived from eosinophils and appear as dipyramidal hexagons or needles in sputum. Viscid mucus plugs, when expectorated, can form a cast of the bronchi and are called Curschmann’s spirals.
In clinically active asthma, mucus hypersecretion is reduced or eliminated after treatment with systemic and then inhaled corticosteroids. Mucus from patients with asthma has tightly bound glycoprotein and oligosaccharide, compared with mucus from patients with chronic bronchitis (123). It remains unknown why the mucus of patients with asthma is so tenacious compared to patients with cystic fibrosis or chronic bronchitis.
The bronchial mucosa is edematous, as is the submucosa, and both are infiltrated with mast cells, activated eosinophils, and CD4+ TH2 lymphocytes (3). Neutrophils can be a manifestation of severe asthma (3). Macrophages and epithelium both amplify the inflammatory responses of asthma (3). Venous dilation, plasma leakage and proliferation of new vessels occur along with the cellular infiltration (3). In addition to its presence on mast cells, basophils, and eosinophils, IgE has been identified in bronchial glands, epithelium, and basement membrane. Because plasma cell staining for IgE was not increased in number, it has been thought that IgE is not produced locally. However, because the lung is recognized as an immunologic organ, further work may show that IgE is produced in the lung.
The mechanism of bronchial hyperresponsiveness in asthma is unknown but is perhaps the central abnormality physiologically. Bronchial hyperresponsiveness occurs in patients with asthma to agonists, such as histamine, methacholine, leukotriene D4, allergens, platelet-activating factor (PAF), PGD2 (short-lived response), adenosine monophosphate, and mannitol. Bronchial hyperresponsiveness is sensitive for asthma if one considers a maximum dose of methacholine of 8 mg/mL, which is necessary to cause a decline in FEV1 of 20%. Patients with active symptomatic asthma often experience such a decline in FEV1 when the dose of methacholine is 2 mg/mL or less. However, bronchial hyperresponsiveness is not specific for asthma because it occurs in patients who have disease other than asthma (Table 19.3).
Table 19.3 Conditions of Patients That May Demonstrate Bronchial Hyperresponsiveness | ||
|---|---|---|
|
Bronchial hyperresponsiveness is measured physiologically by reductions in expiratory flow rates, FEV1, or decreases in specific conductance. Nevertheless, hyperresponsiveness consists of bronchoconstriction, hypersecretion, and hyperemia (mucosal edema). It has been easier to measure airway caliber by changes in FEV1 than to measure changes in bronchial gland secretion, cellular infiltration, or blood vessels (dilation and increased permeability) that also contribute to hyperresponsiveness and cause airways obstruction. Indeed, there has yet to be an “inflammamometer” for asthma. The bronchial responsiveness detected after challenge with histamine or methacholine measures bronchial sensitivity or ease of bronchoconstriction (119). As stated, an additional finding in some patients with asthma is excessive bronchoconstriction, which can be attributable to associated increases in residual volume and possibly more rapid clinical deterioration (119).
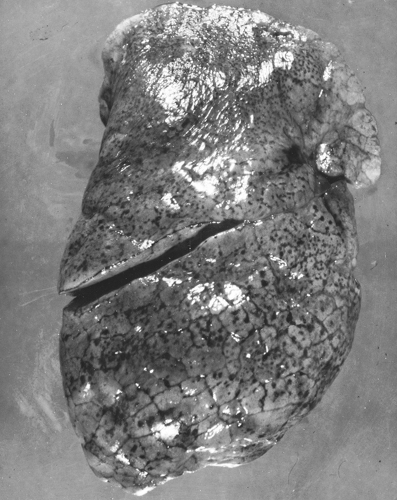
Often, on opening the thorax of a patient who has died from status asthmaticus, the lungs are hyperinflated and do not collapse (Fig. 19.2). Mucus plugging and obstruction of bronchi and bronchioles are present. In some cases, complicating factors, such as atelectasis or acute pneumonia, are identified. On histological examination, there is a patchy loss of bronchial epithelium with desquamation and denudation of mucosal epithelium. Eosinophils are present in areas of absent epithelium, and immunologic staining has revealed evidence of eosinophil MBP at sites of bronchial epithelium desquamation. Activated (EG2-positive) eosinophils are
present in the mucosa, submucosa, and connective tissue. Other histological findings include hyperplasia of bronchial mucus glands, bronchial mucosal edema, smooth muscle hypertrophy, and basement membrane thickening (Fig. 19.3). The latter occurs from the remodeling process from collagen deposition (types I, III, and IV), immunoglobulin deposition and cellular infiltrates as evidence of inflammation. The mucus plugs typically contain eosinophils. Occasionally, bronchial epithelium is denuded, but histologic studies do not identify eosinophils. In some cases, neutrophils have been present (112). Other mechanisms of lung damage are present but not understood completely. Similarly, although many autopsy examinations reveal the classic pattern of mucus plugging (Fig. 19.4) of large and smaller bronchi and bronchioles leading to mucus suffocation or asphyxia as the terminal asthmatic event, some autopsies reveal empty bronchi (113,120,121). Eosinophils have been identified in such cases in airways or in basement membranes, but a gross mechanical explanation, analogous to mucus suffocation, is not present. A third morphologic pattern of patients dying from asthma is that of mild-to-moderate mucus plugging (120).
present in the mucosa, submucosa, and connective tissue. Other histological findings include hyperplasia of bronchial mucus glands, bronchial mucosal edema, smooth muscle hypertrophy, and basement membrane thickening (Fig. 19.3). The latter occurs from the remodeling process from collagen deposition (types I, III, and IV), immunoglobulin deposition and cellular infiltrates as evidence of inflammation. The mucus plugs typically contain eosinophils. Occasionally, bronchial epithelium is denuded, but histologic studies do not identify eosinophils. In some cases, neutrophils have been present (112). Other mechanisms of lung damage are present but not understood completely. Similarly, although many autopsy examinations reveal the classic pattern of mucus plugging (Fig. 19.4) of large and smaller bronchi and bronchioles leading to mucus suffocation or asphyxia as the terminal asthmatic event, some autopsies reveal empty bronchi (113,120,121). Eosinophils have been identified in such cases in airways or in basement membranes, but a gross mechanical explanation, analogous to mucus suffocation, is not present. A third morphologic pattern of patients dying from asthma is that of mild-to-moderate mucus plugging (120).
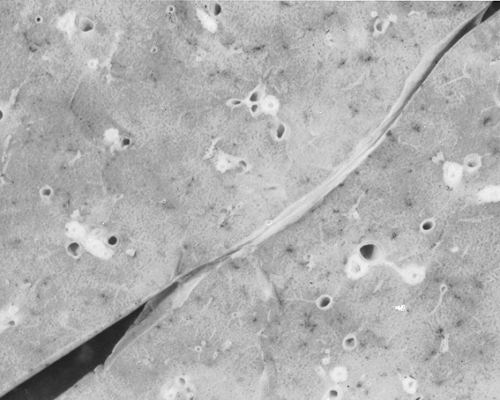 Figure 19.3 Close-up view of pulmonary parenchyma in a case of acute severe asthma. Bronchi are dilated and thickened. |
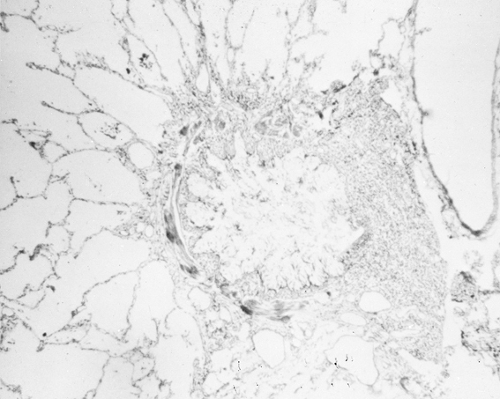 Figure 19.4 Microphotograph of a dilated bronchus filled with a mucous plug. There is hypertrophy of the muscle layer, and the alveolar spaces are dilated. |
Some patients dying from asthma have evidence of myocardial contraction band necrosis, which is different from myocardial necrosis associated with infarction. Contraction bands are present in necrotic myocardial smooth muscle cell bands in asthma and curiously the cells are thought to die in tetanic contraction whereas in cases of fatal myocardial infarction, cells die in relaxation.
In patients who experience acute severe asthma but do not die from it, it can be expected that when the patient presents with an FEV1 of 50% of predicted value, there may be a 10-fold increase in inspiratory muscle work. Pleural pressure becomes more negative, so that as inspiration occurs, the patient is able to apply sufficient radial traction on the airways to maintain their patency. Air can get in more easily than it can be expired, which results in progressively breathing at higher and higher lung volumes. The residual volume (RV) increases several-fold, and functional residual capacity (FRC) expands as well. Expiratory flow rates decrease in large and small airways. The lung hyperinflation is not distributed evenly, and some areas of the lung have a high or low ventilation-perfusion ratio (V/Q). Overall, the hypoxemia that results from acute severe asthma occurs from reduced V/Q, not from shunting of blood. The lung hyperinflation also results in “dynamic autopeep” as the patient attempts to maintain airway caliber by applying some endogenous positive airway pressure.
There is no evidence of chest wall (inspiratory muscle) weakness in patients with asthma. Nevertheless, some patients who have received prolonged courses of daily or twice-daily prednisone or who have been mechanically ventilated with muscle relaxants and corticosteroids can be those who have respiratory muscle fatigue.
After successful treatment of an attack of acute severe asthma, the increases in lung volume may remain present for 6 weeks. The changes are primarily in RV and FRC. Small airways may remain obstructed for weeks or months; in some patients, they do not become normal again. At the same time, it can be expected that the patient has no sensation of dyspnea within 1 week of treatment of acute severe asthma
despite increases in RV and reduced small airways caliber. This divergence between symptom recognition in asthma and physiologic measurements has been demonstrated in ambulatory patients who did not have acute severe asthma (status asthmaticus) (124). When patients with an FEV1 percentage of 60% were studied, 31% overestimated and 17% underestimated the extent of airway obstruction (124). Some patients reported fewer symptoms despite no improvement in FEV1 or peak expiratory flow rate (PEFR). The reduction in trapped gas in the lung can result in symptom reduction even without improvement in expiratory flow rates.
despite increases in RV and reduced small airways caliber. This divergence between symptom recognition in asthma and physiologic measurements has been demonstrated in ambulatory patients who did not have acute severe asthma (status asthmaticus) (124). When patients with an FEV1 percentage of 60% were studied, 31% overestimated and 17% underestimated the extent of airway obstruction (124). Some patients reported fewer symptoms despite no improvement in FEV1 or peak expiratory flow rate (PEFR). The reduction in trapped gas in the lung can result in symptom reduction even without improvement in expiratory flow rates.
Asthma pathophysiology includes poor or impaired symptom perception in some patients and in management of asthma, increased symptom perception in others (125,126). There may be poor sensitivity or discrimination (recognizing improvement or worsening status) (125). Dyspnea has been classified into either (a) inspiratory difficulty, (b) chest tightness, (c) unsatisfied inspiration, or (d) work (126). In Table 19.4, factors that alter the perception of dyspnea in asthma are presented. As the exacerbation of asthma becomes worse, the reduction in inspiratory capacity is associated with increases in functional residual capacity (hyperinflation) and increasing dyspnea (126).
Table 19.4 Factors That Affect the Sensation of Dyspnea in Asthma | |||
|---|---|---|---|
|
Control of Airway Tone
The patency of bronchi and bronchioles is a function of many factors. There is the overriding loss of airway distensibility will less elastic recoil pressures (127). Bronchomotor patency is affected by mediators secreted by mast cells, the autonomic nervous system, the nonadrenergic noncholinergic nervous system, circulating humoral substances, the respiratory epithelium, smooth muscle cells, and effects of cellular infiltration and glandular secretions (Tables 19.5 and 19.6). Even this list is oversimplified because asthma must be considered a very complex condition in terms of airway caliber and tone.
Table 19.5 Selected Mast Cell Mediators and Cytokines and Their Proposed Actions in Asthma | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 19.6 Selected Neuropeptides and Their Proposed Actions in Asthma | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Mediator release caused by mast cell activation results in acute and late bronchial smooth muscle contraction, cellular infiltration, and mucus production. Autonomic nervous stimulation contributes through vagal stimulation. The neurotransmitter for postganglionic parasympathetic nerves is acetylcholine, which causes smooth muscle contraction. Norepinephrine is the neurotransmitter for postganglionic sympathetic nerves. However, there appears to be little if any significant smooth muscle relaxation through stimulation of postganglionic sympathetic nerves. Exogenously administered epinephrine can produce smooth muscle relaxation. Circulating endogenous epinephrine apparently does not serve to produce relaxation of smooth muscles. Sensory nerves in the respiratory epithelium are stimulated and lead to release of a host of neuropeptides that may be potent bronchoconstrictors or bronchodilators. Respiratory epithelium itself may contain bronchi-relaxing factors that may become unavailable when epithelium is denuded. Tables 19.5 and 19.6 list some chemical mediators derived from mast cells and cytokines and neuropeptides that may contribute to pathogenesis of asthma.
Although much attention has been directed at understanding the contribution of IgE and mast cell activation in asthma, triggering or actual regulation of some of the allergic inflammation of asthma may occur because of other cells in lungs of patients. Low-affinity IgE receptors (FcεR II) are present on macrophages, eosinophils, monocytes, B lymphocytes, and platelets. These cells, as well as mast cells in the bronchial mucosa or lumen, can be activated in the absence of classic IgE-mediated asthma.
Bronchial biopsy specimens from patients with asthma demonstrate mucosal mast cells in various stages of activation in patients with and without symptoms. Mast cell hyperreleasibility may occur in asthma, in that bronchoalveolar mast cells recovered during lavage contain and release greater quantities of histamine when stimulated by allergen or anti-IgE in vitro.
Eosinophils are thought to contribute to proinflammatory effects by secretion of damaging cell products, such as MBP, that can result in bronchial epithelial denudation, exposing sensory nerves, and leading to smooth muscle contraction. Eosinophils are proinflammatory in that they cause eosinophil and neutrophil chemotaxis, which produces positive feedback in terms of leukotriene and PAF production from attracted and newly activated eosinophils. The latter can be demonstrated by activation markers such as EG2 and reduced density on centrifugation, so called hypodense eosinophils.
On a cellular level, the control of airway tone is influenced by even more fundamental factors, including IL-1, IL-2, IL-3, IL-4, IL-6, IL-10, IL-12, IL-16, and IFN-γ, among others, that influence lymphocyte development and proliferation. IL-3 and IL-5 are eosinophil growth factors. IL-8, detected in bronchial epithelium, binds to secretory IgA and serves to chemoattract eosinophils that generate PAF and LTC4. IL-8 is also a potent chemotactic substance for neutrophils.
During an acute attack of asthma, there is an increase in inspiratory efforts, which apply greater radial traction to airways. Patients with asthma have great ability to generate increases in inspiratory pressures. Unfortunately, patients who have experienced nearly fatal attacks of asthma have blunted perception of dyspnea and impaired ventilatory responses to hypoxia (128).
Patients with persistent severe asthma have been divided into eosinophil-positive (and macrophage-positive) and eosinophil-negative categories based on results on bronchial biopsy findings (129). Both subgroups of patients were prednisone-dependent (average, 28 mg daily) and had asthma for about 20 years
(129). The residual volume measurements were about 200% of predicted and FEV1 percentage was 56% of predicted in eosinophil-positive and 42% of predicted in eosinophil-negative patients (129). The ratio of the FVC to slow vital capacity was 88%, indicating more airway collapsibility in eosinophil-positive patients, compared with 97% in eosinophil-negative patients. Perhaps the former patients who had somewhat higher FEV1 percentages had more loss of elastic recoil in their lungs, so that their airways collapsed more easily (129). On biopsy assessments, sub-basement membrane thickening was higher in these eosinophil-predominant patients than in eosinophil-negative patients. These findings were associated with eosinophil-predominant patients with severe asthma having an increased number of CD3+ lymphocytes and activated eosinophils (EG2+) in biopsy samples and an increased quantity of β-tryptase in BAL. It is likely that the cellular inflammation and cell products participate in control or perturbation of airway tone, and continued investigations of the many aspects of allergic inflammation should help clarify this difficult issue.
(129). The residual volume measurements were about 200% of predicted and FEV1 percentage was 56% of predicted in eosinophil-positive and 42% of predicted in eosinophil-negative patients (129). The ratio of the FVC to slow vital capacity was 88%, indicating more airway collapsibility in eosinophil-positive patients, compared with 97% in eosinophil-negative patients. Perhaps the former patients who had somewhat higher FEV1 percentages had more loss of elastic recoil in their lungs, so that their airways collapsed more easily (129). On biopsy assessments, sub-basement membrane thickening was higher in these eosinophil-predominant patients than in eosinophil-negative patients. These findings were associated with eosinophil-predominant patients with severe asthma having an increased number of CD3+ lymphocytes and activated eosinophils (EG2+) in biopsy samples and an increased quantity of β-tryptase in BAL. It is likely that the cellular inflammation and cell products participate in control or perturbation of airway tone, and continued investigations of the many aspects of allergic inflammation should help clarify this difficult issue.
Clinical Overview
Clinical Manifestations
Asthma results in coughing, wheezing, dyspnea, sputum production, and shortness of breath. Symptoms vary from patient to patient and within the individual patient depending on the activity of asthma. Some patients experience mild, nonproductive coughing after exercising or exposure to cold air or odors as examples of transient mild bronchoconstriction. The combination of coughing and wheezing with dyspnea is common in patients who have a sudden moderate to severe episode (such as might occur within 3 hours after aspirin ingestion in an aspirin-intolerant patient). Symptoms of asthma may be sporadic and are often present on a nocturnal basis. Some patients with asthma present with a persistent nonproductive cough as a main symptom of asthma (130). Typically, the cough has occurred on a daily basis and may awaken the patient at night. Repetitive spasms of cough from asthma are refractory to treatment with expectorants, antibiotics, antitussives, and opioids. The patient likely may respond to an inhaled β2-adrenergic agonist; if that is unsuccessful, inhaled corticosteroids or the combination may work. At times, oral corticosteroids are necessary to stop the coughing and are very useful as a diagnostic therapeutic trial (130). Pulmonary physiologic studies usually reveal large airway obstruction, as illustrated by reductions in FEV1 with preservation of forced expiratory flow, midexpiratory phase (FEF25%-75%) or small airways function (131). The latter may be reduced in patients with this cough variant form of asthma. Conversely, some patients present with isolated dyspnea as a manifestation of asthma. Some of these patients have small airways obstruction with preservation of function of larger airways. The recognition of variant forms of asthma emphasizes that not all patients with asthma have detectable wheezing on auscultation. The medical history is important, as is a diagnostic-therapeutic trial with antiasthma medications. Pulmonary physiologic abnormalities, such as reduced FEV1 that responds to therapy, or bronchial hyperresponsiveness to methacholine (PC20 <8 mg/mL) can provide additional supportive data.
During an acute, moderately severe episode of asthma or in longer-term ineffectively controlled asthma, patients typically produce clear, yellow, or green sputum that can be viscid. The sputum contains eosinophils, which supports the diagnosis of asthma. If measured, expired nitric oxide concentrations will be elevated. Because either polymorphonuclear leukocytes or eosinophils can cause the sputum to be discolored, it is inappropriate to consider such sputum as evidence of a secondary bacterial infection. Patients with nonallergic asthma also produce eosinophil-laden sputum. An occasional patient with asthma presents with cough syncope, a respiratory arrest that is perceived as anaphylaxis, chest pain, pneumomediastinum, or pneumothorax, or with symptoms of chronic bronchitis or bronchiectasis.
The physical examination may consist of no coughing or wheezing if the patient has stable persistent asthma or if there has not been a recent episode of intermittent asthma. Certainly, patients with variant asthma may not have wheezing or other supportive evidence of asthma. Usually, wheezing is present in other patients and can be associated with reduced expiratory flow rates. A smaller number of patients always have wheezing on even tidal breathing, not just with a forced expiratory maneuver. Such patients may not report symptoms and may or may not have expiratory airflow obstruction when FVC and FEV1 are measured. The physical examination must be interpreted in view of the patient’s clinical symptoms and supplemental tests, such as the chest radiograph or pulmonary function tests. There may be a surprising lack of correlation in some ambulatory patients between symptoms and objective evidence of asthma (physical findings and spirometric values) (132). Attempts at using a biomarker such as exhaled nitric oxide have (133) and have not (79,134) been helpful as a noninvasive marker.
An additional physical finding in patients with asthma is repetitive coughing on inspiration. Although not specific for asthma, it is frequently present in unstable patients. In normal patients, maximal inspiration to total lung capacity results in reduced airway resistance, whereas in patients with asthma, increased resistance occurs with a maximal inspiration. Coughing spasms can be precipitated in patients who otherwise may not be heard to wheeze. This finding is transient and, after
effective therapy, will not occur. The patient with a very severe episode of asthma may be found to have pulsus paradoxus and use of accessory muscles of respiration. Such findings correlate with an FEV1 of less than 1.0 L and air trapping as manifested by hyperinflation of the functional residual capacity and residual volume (135). The most critically ill patients have markedly reduced tidal volumes, and their maximal ventilatory efforts are not much higher than their efforts during tidal breathing. A silent chest with absence of or greatly reduced breath sounds indicates likely alveolar hypoventilation (normal or elevated arterial PCO2) and hypoxemia. Such patients may require intubation or, in most cases, admission to the intensive care unit. Great difficulty in speaking more than a half sentence before needing another inspiration is likely present in such patients.
effective therapy, will not occur. The patient with a very severe episode of asthma may be found to have pulsus paradoxus and use of accessory muscles of respiration. Such findings correlate with an FEV1 of less than 1.0 L and air trapping as manifested by hyperinflation of the functional residual capacity and residual volume (135). The most critically ill patients have markedly reduced tidal volumes, and their maximal ventilatory efforts are not much higher than their efforts during tidal breathing. A silent chest with absence of or greatly reduced breath sounds indicates likely alveolar hypoventilation (normal or elevated arterial PCO2) and hypoxemia. Such patients may require intubation or, in most cases, admission to the intensive care unit. Great difficulty in speaking more than a half sentence before needing another inspiration is likely present in such patients.
Persistent asthma may be occurring in patients who have concurrent gastroesophageal reflux disease (GERD), rhinosinusitis, and allergic or nonallergic rhinitis, all of which can cause a cough or worsen ongoing asthma (136).
Radiographic and Laboratory Studies
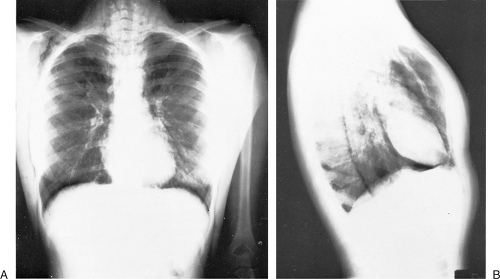
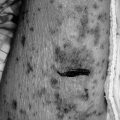
In about 90% of patients, the presentation chest radiograph is considered within normal limits (137). The most frequently found abnormality is hyperinflation. The diaphragm is flattened, and there may be an increase in the anteroposterior diameter and retrosternal air space. The chest radiograph is indicated because it is necessary to exclude other conditions that mimic asthma and to search for complications of asthma. Congestive heart failure, COPD, pneumonia, sarcoidosis, and neoplasms are just some other explanations for acute wheezing dyspnea that may mimic or coexist with asthma. Asthma complications include atelectasis as a result of mucus obstruction of bronchi, mucoid impaction of bronchi (often indicative of allergic bronchopulmonary aspergillosis), pneumomediastinum, and pneumothorax. Atelectasis often involves the middle lobe, which may collapse. The presence of pneumomediastinum or pneumothorax may have associated subcutaneous emphysema with crepitus on palpation of the neck, supraclavicular areas, or face (Figs. 19.5 and 19.6). Sharp pain in the neck or shoulders should be a clue to the presence of a pneumomediastinum in status asthmaticus.
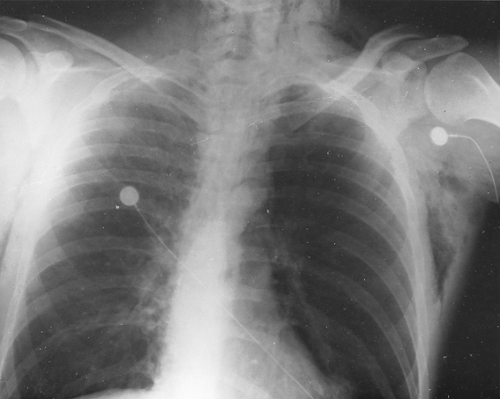 Figure 19.5 Anteroposterior view of the chest of a 41-year-old woman demonstrates hyperinflation of both lungs, with pneumomediastinum and subcutaneous emphysema. |
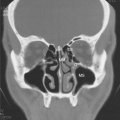
Depending on the patients examined, abnormal findings on sinus CT examination may be frequent. Some findings may include air-fluid levels, indicative of infection; mucoperiosteal thickening, which is consistent with current or previous infection; and opacification of a sinus or presence of nasal polyps (see Chapters 10 and 12). Clinical research studies of acutely ill patients with asthma have been carried out with V/Q scans. These procedures are not indicated in most cases and, in the markedly hypoxemic patient, may be harmful because the technetium-labeled albumin macrospheres injected for the perfusion scan can lower arterial PO2. Ventilation is extremely uneven (138). Perfusion scans reveal abnormalities such that there may or may not be matched V/Q inequalities. In some patients, the V/Q in the superior portions of the lungs has declined from its relatively high value (138). The explanation for such a finding is increased perfusion of upper lobes presumably from reduced resistance relative to lower lobes that receive most of the pulmonary blood flow. Little evidence for shunting exists (138). Of note, even bronchoprovocation challenge with allergen results in 20% increases in ventilation and perfusion with associated evidence of gas trapping (139).
Pulmonary emboli typically do not complicate episodes of acute asthma, but when a pulmonary embolus is suspected, spiral CT examination of the lung may provide the characteristic findings.
In the assessment of the emergency department patient with acute severe wheezing dyspnea, the measurement of arterial PO2, PCO2, and pH can be invaluable. Although hypoxemia is a frequent and expected finding and is identified by measuring the pulse oximetry, the PCO2 provides information on the effectiveness of alveolar ventilation. This latter status will not be assessed if just oxygen saturation is determined. The PCO2 should be decreased initially during the hyperventilation stage of acute asthma. A normal or elevated PCO2 is evidence of alveolar hypoventilation and may be associated with subsequent need for intubation to try to prevent a fatal outcome.
Pulmonary function measurements can help to establish patient status. However, such measurements must be correlated with the physical examination. In the emergency department or ambulatory setting, many physicians determine spirometric values for expiratory flow rates with either PEFR or FEV1. These tests are effort dependent, and patients with acute symptoms
may be unable to perform the maneuver satisfactorily. This finding could be from severe obstruction or patient inability or unwillingness to perform the maneuver appropriately. When properly performed, spirometric measurements can be of significant clinical utility in assessing patient status. For example, as a rule, patients presenting with spirometric determinations of 20% to 25% of predicted value should receive immediate and intensive therapy. Frequent measurements of PEFR or FEV1 in ambulatory patients can establish a range of baseline values for day and night. Declines of more than 20% from usual low recordings or wide swings in PEFF (such as from a best of 400 L/min to 225 L/min) can alert the patient to the need for more intensive pharmacologic therapy. Nevertheless, such measurements can be insensitive in some patients. Pulmonary physiologic values such as PEFR and FEV1 have demonstrated value in clinical research studies, such as in documenting a 12% increase in expiratory flow rates after bronchodilator. Such a response (including a 200-mL increase in FEV1) meets criteria for a bronchodilator response (3). Similarly, in testing for bronchial hyperresponsiveness, a 20% decline in FEV1 is a goal during incremental administration of methacholine or histamine.
may be unable to perform the maneuver satisfactorily. This finding could be from severe obstruction or patient inability or unwillingness to perform the maneuver appropriately. When properly performed, spirometric measurements can be of significant clinical utility in assessing patient status. For example, as a rule, patients presenting with spirometric determinations of 20% to 25% of predicted value should receive immediate and intensive therapy. Frequent measurements of PEFR or FEV1 in ambulatory patients can establish a range of baseline values for day and night. Declines of more than 20% from usual low recordings or wide swings in PEFF (such as from a best of 400 L/min to 225 L/min) can alert the patient to the need for more intensive pharmacologic therapy. Nevertheless, such measurements can be insensitive in some patients. Pulmonary physiologic values such as PEFR and FEV1 have demonstrated value in clinical research studies, such as in documenting a 12% increase in expiratory flow rates after bronchodilator. Such a response (including a 200-mL increase in FEV1) meets criteria for a bronchodilator response (3). Similarly, in testing for bronchial hyperresponsiveness, a 20% decline in FEV1 is a goal during incremental administration of methacholine or histamine.
Some patients may benefit from measuring PEFR daily at home (1–3). Unfortunately, some patients do not continue to measure this PEFR or may fabricate results. Other patients manipulate spirometric measurements to make a convincing case for occupational asthma. Thus, the physician must correlate pulmonary physiologic values with the clinical assessment. A complete set of pulmonary function tests should be obtained in other situations, such as in assessing the degree of reversible versus nonreversible obstruction in patients with heavy smoking histories. The diffusing capacity for carbon monoxide (DLCO) is reduced in the COPD patient but normal or elevated in the patient with asthma. Such tests should be obtained after 2 to 4 weeks of intensive therapy to determine what degree of reversibility exists. In acutely ill patients with asthma, the DLCO may be reduced. Thus, its usefulness in differentiating COPD from asthma will be obscured if the wrong time is chosen to obtain this test. Flow-volume loops will demonstrate intrathoracic obstruction in patients with asthma (140) or extrathoracic obstruction in those with vocal cord dysfunction (VCD) (141) (Fig. 19.7).
The complete blood count should be obtained in the emergency setting. First, the hemoglobin and
hematocrit provide status regarding anemia, which if associated with hypoxemia can compromise oxygen delivery to tissues. Conversely, an elevated hematocrit is consistent with hemoconcentration such as occurs from dehydration or polycythemia. The latter does not occur in asthma in the absence of other conditions. The white blood count may be elevated from infection or systemic corticosteroids, the mechanism of which includes demargination and release from bone marrow. In the absence of prior systemic corticosteroids, the acutely ill patient with allergic or nonallergic asthma often has peripheral blood eosinophilia. For best accuracy, an absolute eosinophil count is required. However, in the management of most patients with asthma, both those with acute symptoms and long-term sufferers, eosinophil counts are not of value. The presence of eosinophilia in patients receiving long-term systemic corticosteroids should suggest nonadherence or possibly rare conditions, such as Churg-Strauss Syndrome, allergic bronchopulmonary aspergillosis, or chronic eosinophilic pneumonia. Usually, the eosinophilia in asthma does not exceed 10% to 20% of the differential. (Some patients with both asthma and atopic dermatitis have persistently elevated absolute eosinophil counts in the absence of idiopathic hyperesoinophilic syndrome or other conditions). Much higher values should suggest an alternative diagnosis (Chapter 35).
hematocrit provide status regarding anemia, which if associated with hypoxemia can compromise oxygen delivery to tissues. Conversely, an elevated hematocrit is consistent with hemoconcentration such as occurs from dehydration or polycythemia. The latter does not occur in asthma in the absence of other conditions. The white blood count may be elevated from infection or systemic corticosteroids, the mechanism of which includes demargination and release from bone marrow. In the absence of prior systemic corticosteroids, the acutely ill patient with allergic or nonallergic asthma often has peripheral blood eosinophilia. For best accuracy, an absolute eosinophil count is required. However, in the management of most patients with asthma, both those with acute symptoms and long-term sufferers, eosinophil counts are not of value. The presence of eosinophilia in patients receiving long-term systemic corticosteroids should suggest nonadherence or possibly rare conditions, such as Churg-Strauss Syndrome, allergic bronchopulmonary aspergillosis, or chronic eosinophilic pneumonia. Usually, the eosinophilia in asthma does not exceed 10% to 20% of the differential. (Some patients with both asthma and atopic dermatitis have persistently elevated absolute eosinophil counts in the absence of idiopathic hyperesoinophilic syndrome or other conditions). Much higher values should suggest an alternative diagnosis (Chapter 35).
Sputum examination reveals eosinophils, eosinophils plus polymorphonuclear leukocytes (asthma and purulent bronchitis or bacterial pneumonia), or absence of eosinophils. In mild asthma, no sputum is produced. In severely ill patients with asthma, the sputum is thick, tenacious, and yellow or green. MBP from eosinophils has been identified in such sputum. Dipyramidal hexagons from eosinophil cytoplasm may be identified and are called Charcot-Leydon crystals. These crystals contain lysophospholipase. Curschmann spirals are expectorated yellow or clear mucus threads that are remnants or casts of small bronchi. Expectorated ciliated and nonciliated bronchial epithelial cells can also be identified that emphasize the patchy loss of bronchial epithelium in asthma. On a related basis, high-molecular-weight neutrophil chemotactic activity has been identified in sera from patients with status asthmaticus (142). This activity declined with effective therapy.
Serum electrolyte abnormalities may be present and should be anticipated in the patient presenting to the emergency department. Recent use of oral corticosteroids can lower the potassium concentration (as can β2-adrenergic agonists) and cause a metabolic alkalosis. Oral corticosteroids may raise the blood glucose in some patients, as can systemic administration of β2-adrenergic agonists. Elevations of atrial natriuretic peptide and antidiuretic hormone can occur in acute asthma or COPD (143). Clinically, few patients have large declines of serum sodium. Because intravenous fluids will be administered, it is necessary to determine the current status of electrolytes and serum chemistry values. After prolonged high-dose corticosteroids, hypomagnesemia or hypophosphatemia may occur.
Rarely, a patient younger than 30 years of age may be thought to have asthma when the underlying condition is α1-antitrypsin deficiency. More commonly, patients with wheezing dyspnea have asthma and cystic fibrosis. The sweat chloride should be elevated markedly in such patients. A properly performed sweat chloride test is essential, as is proper performance of genetic analysis and pancreatic function.
In the outpatient management of asthma, determination of the presence or absence of antiallergen IgE is of value. For decades, skin testing for immediate cutaneous reactivity has been the most sensitive and specific method. Some physicians prefer in vitro tests. One cannot emphasize enough the need for high quality control for both skin testing and in vitro testing. Both tests are subject to misinterpretation. The experienced physician should use either method of demonstration of antiallergen IgE as adjunctive to, rather than a substitute for, the narrative history of asthma. More patients have immediate cutaneous reactivity or detectable in vitro IgE than have asthma that correlates with exposure to the specific allergen.
Complications
Complications from asthma include death, adverse effects of hypoxemia or respiratory failure on other organ systems, growth retardation in children, pneumothorax or pneumomediastinum, rib fractures from severe coughing, cough syncope, and adverse effects of medications or therapeutic modalities used to treat asthma. Some patients develop psychological abnormalities because of the burden of a chronic illness such as asthma. Ineffectively treated asthma in children can result in chest wall abnormalities, such as “pigeon chest,” because of sustained hyperinflation of the chest. Further, the annual rate of decline of FEV1 is increased; for example, it may be 38 mL/year in patients with asthma, compared with 22 mL/year in patients without asthma (144).
In general, long-term asthma does not result in irreversible obstructive lung disease. However, an occasional patient with long-term asthma develops apparently irreversible disease in the absence of cigarette smoking, α1-antitrypsin disease, or other obvious cause (145). Usually, these patients have childhood-onset asthma and are dependent on oral corticosteroids. Intensive therapy with oral and inhaled corticosteroids does not result in a normal FEV1 of 80% of the predicted value as the mean FEV1 was 57% (145). In contrast to the few patients with irreversible asthma, patients with asthma do not become “respiratory cripples,” as might occur from COPD. Nevertheless, pulmonary physiologic studies do not
reveal return of parameters to the expected normal ranges. Asthma patients are not deficient in the antiproteases that can be measured, and they do not have bullous abnormalities on chest radiographs. CT demonstrates gas trapping, especially on expiration, as well as bronchial wall thickening caused by increased smooth muscle mass and elastic and collagenous tissue. Some patients with asthma have evidence of bronchial dilatation on high resolution CT examination (146), but there are few areas of involvement in contrast to that of ABPA (Chapter 24).
reveal return of parameters to the expected normal ranges. Asthma patients are not deficient in the antiproteases that can be measured, and they do not have bullous abnormalities on chest radiographs. CT demonstrates gas trapping, especially on expiration, as well as bronchial wall thickening caused by increased smooth muscle mass and elastic and collagenous tissue. Some patients with asthma have evidence of bronchial dilatation on high resolution CT examination (146), but there are few areas of involvement in contrast to that of ABPA (Chapter 24).
Pneumomediastinum or pneumothorax can occur in patients presenting with acute severe asthma. Neck, shoulder, or chest pain is common, and crepitations can be detected in the neck or supraclavicular fossae. Rupture of distal alveoli results in dissection of air proximally through bronchovascular bundles. The air can then travel superiorly in the mediastinum to the supraclavicular or cervical areas. At times, the air dissects to the face or into the subcutaneous areas over the thorax. Treating the patient’s asthma with systemic corticosteroids is indicated to reduce the likelihood of hyperinflation and continued air leak. Unless the pneumothorax is very large, conservative treatment is effective. Otherwise, thoracostomy with tube placement is necessary.
Fatalities from asthma are unnecessary because asthma is not an inexorably fatal disease. Fatalities do occur, however, and many factors have been suggested as explanations (1–3,50, 85,86,95,104,121,147–150). While some deaths from asthma are unavoidable despite appropriate medical care, a high percentage of deaths from asthma should be considered preventable. Survivors of major asthma events, such as respiratory failure or arrest, patients with pneumomediastinum or pneumothorax on two occasions, and those with repeated status asthmaticus despite oral corticosteroids have potentially fatal asthma and are at higher risk for fatality than other patients with asthma (50,151,152). The NAEPP refers to high risk patients as near fatal asthma (1–3).
Uncontrolled asthma can lead to mucus plugging of airways and frank collapse of a lobe or whole lung segment. The middle lobe can collapse, especially in children. Repeated mucoid impactions should raise the possibility of ABPA or cystic fibrosis.
Cough syncope or cough-associated cyanosis occurs in patients whose respiratory status has deteriorated and in whom acute severe asthma or need for emergency therapy has occurred. In the setting of severe airway obstruction from asthma, during inspiration, intrathoracic pressure is negative because the patient must generate very high negative pressures to apply radial traction on bronchi in an attempt to maintain their patency. During expiration, the patient must overcome severe airway resistance and premature airways collapse. Increases in intrathoracic pressure during expiration with severe coughing, as compared with intra-abdominal pressure, causes a decline in venous return to the right atrium. There may also be increased blood flow to the lung during a short inspiration, but that is accompanied by pooling in the pulmonary vasculature from the markedly elevated negative inspiratory pressure. There will be reduced blood flow to the left ventricle with temporary decreases in cardiac output and cerebral blood flow.
Pulsus paradoxus is present when there is greater than a 10-mm Hg decline in systolic blood pressure during inspiration. It is associated with severe airway obstruction and hyperinflation (153,154). The most frequent electrocardiographic findings during acute asthma are sinus tachycardia followed by right axis deviation, clockwise rotation, prominent R in lead V1 and S in lead V5, and tall peaked P waves consistent with cor pulmonale.
Linear growth retardation can occur from ineffectively controlled asthma and potentially as a complication of high-dosage inhaled corticosteroids. Administration of oral corticosteroids is indicated to prevent repeated hospitalizations and frequent episodes of wheezing dyspnea. The child often responds with a growth spurt. Alternate-day prednisone and recommended doses of inhaled corticosteroids do not result in growth retardation, especially when the prednisone dose is 30 mg on alternate days or less. Even high alternate-day doses in children can be tolerated reasonably well as long as episodes of acute severe asthma are prevented. In contrast, depot corticosteroids given every 2 to 3 weeks in high doses may result in growth retardation. Despite efficacy in asthma, such corticosteroid administration causes hypothalamic-pituitary-adrenal (HPA) suppression (152). The use of depot corticosteroids should be considered only in the most recalcitrant children (or adults) in terms of asthma management. Ineffective parental functioning or poor compliance usually accompanies such cases in which reliable administration of prednisone and inhaled corticosteroids is impossible. The term malignant, potentially fatal asthma has been suggested for such patients who are essentially impossible to manage according to guideline based management (151).
The overuse of short acting β2-adrenergic agonists (>8 inhalations/day and especially 16 inhalations/day) is a risk factor for more severe episodes of asthma and fatalities (148). There has been controversy about scheduled use of long-acting β2-adrenergic agonists, but the data do not support harmful effects (149,150) when used in combination with inhaled corticosteroids.
Psychological Factors
Asthma has evolved from a disorder considered to be psychological to one recognized as extremely complex and of unknown etiology. Psychological stress can cause modest reductions in expiratory flow rates such as occur while watching a terrifying movie (155). Laughing and crying or frank emotional upheaval, such as an argument
with a family member, can result in wheezing. Some patients require additional anti-inflammatory medication to suppress such wheezing. Usually, if the patient has stable baseline respiratory status, acute severe asthma does not result. Nevertheless, some fatal episodes of asthma have been associated with a report of a high level of emotional stress. In an absence of how to quantitate stress and determine whether there is a dose-response effect in asthma, such information must be considered speculative. Specific personality patterns have not been identified in patients with asthma.
with a family member, can result in wheezing. Some patients require additional anti-inflammatory medication to suppress such wheezing. Usually, if the patient has stable baseline respiratory status, acute severe asthma does not result. Nevertheless, some fatal episodes of asthma have been associated with a report of a high level of emotional stress. In an absence of how to quantitate stress and determine whether there is a dose-response effect in asthma, such information must be considered speculative. Specific personality patterns have not been identified in patients with asthma.
The patient with asthma may develop strategies to function with the burden of asthma as a chronic, disruptive, and potentially fatal disease. A variety of behavior patterns have been recognized, including (a) disease denial, with complacency or outright denial of symptoms, wishful thinking, refusal to alert the managing physician about a major change in respiratory symptoms, or personally decreasing medications; (b) using asthma for obvious secondary gain, such as to not attend school or work, or to gain compensation; (c) developing compulsive or manipulative patterns of behavior that restrict the lifestyle of the patient and family members excessively; and (d) resorting to quackery. Some patients display hateful behavior toward physicians and their office staff. Psychiatric care can be of value in some cases (Chapter 43), but patients may refuse appropriate psychiatric referrals. The use of PEFR monitoring devices can be misleading because patients can generate or report truly inaccurate measurements. Obviously, in contrast to theories implying that wheezing dyspnea in patients with asthma was primarily psychologic, the physician must now decide how much of a patient’s symptoms and signs are from asthma and how much might be psychologic as a result of asthma. Indeed, a psychologist, psychiatrist, or social worker may help identify what the patient might lose should asthma symptoms be controlled better.
Major management problems occur when asthma patients also have schizophrenia, delusional behavior, neurosis, depression, or manic-depressive disorders (50,85–88). Suicidal attempts are recognized from unjustified cessation of prednisone or theophylline overdosage. Repeated episodes of life-threatening acute severe asthma are difficult to avoid in the setting of untreated major psychiatric conditions (Chapter 43). The presence of post-traumatic stress disorder (17) and a situation of violence and abuse (18) or serious psychiatric disease may make it difficult or impossible to achieve goals of asthma control according to national or international guidelines.
The presence of factitious asthma indicates significant psychiatric disturbance (156). Initially, there must be trust established between the patient and physician. Abrupt referral of the patient to a psychiatrist can result in an unanticipated suicidal attempt. Psychiatric care can be valuable if the patient is willing to participate in therapy. Abnormal coping styles (88), such as wishful thinking instead of active involvement, impair the quality of life and interfere with optimal control of asthma.
Classification
Some types of asthma with an emphasis on etiology are listed in Table 19.7. It is helpful to categorize the type of asthma because treatment programs vary depending on the type of asthma present. Some patients have more than one type of asthma. The NAEPP Report 3 suggests assessing signs and symptoms of asthma in association with spirometry (3). Asthma severity is classified as intermittent (most of the time, implying mild asthma) or persistent (mild, moderate, or severe). In Table 19.8, a version of this classification system is presented. It can be helpful to determine that patients have “moderate persistent allergic asthma” and use the classifications from Tables 19.7 and 19.8 together when applicable.
Table 19.7 Clinical Classification of Asthma | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 19.8 National Asthma Education and Prevention Program Asthma Classification System: Expert Panel Report 3 | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
Asthma in children may be classified by age of onset and persistence of wheezing. (Chapter 20). Designations include “transient early wheeze” (wheezing with lower respiratory tract illnesses before age 3 years but not thereafter), “late-onset wheeze” (wheezing beginning at or after age 6 years), and “persistent wheeze” (wheeze with lower respiratory tract illnesses before age 3 years and wheeze at age 6 years) (157).
In adults, another approach is that of grouping of patients with asthma into clusters using pre-specified variables including induced sputum eosinophil numbers (158). For example, some patients are classified as “concordant disease” because there is a match between symptoms and eosinophilic inflammation, whereas others are either “discordant symptoms” (excessive symptoms with little sputum eosinophilia that could
characterize obese subjects or hypervigilant people) or “discordant inflammation” (few symptoms but high levels of sputum eosinophilia) (158).
characterize obese subjects or hypervigilant people) or “discordant inflammation” (few symptoms but high levels of sputum eosinophilia) (158).
Allergic Asthma
Allergic asthma is caused by inhalation of allergen that interacts with IgE present in high-affinity receptors (FcεRI) on bronchial mucosal mast cells. Twenty-four hours after allergen bronchoprovocation challenge, bone marrow examination demonstrates increased numbers of eosinophil/basophil progenitor cells (159). These cells have been identified in both early responders and dual responders (159). The inflammatory progenitors then can populate the lungs (and nasal mucosa). There are many dimensions of the immunologic basis for allergic asthma (108).
Allergic asthma often occurs from ages 2 to 4 to 60+ years and has been recognized in the geriatric population (97). The use of the term allergic asthma implies
that a temporal relationship exists between respiratory symptoms (clinical reactivity) and allergen exposure and that antiallergen IgE antibodies can be demonstrated or suspected. Approximately 75% to 90% of patients with persistent asthma have clinical reactivity or at least allergic sensitization depending on the study.
that a temporal relationship exists between respiratory symptoms (clinical reactivity) and allergen exposure and that antiallergen IgE antibodies can be demonstrated or suspected. Approximately 75% to 90% of patients with persistent asthma have clinical reactivity or at least allergic sensitization depending on the study.
Respiratory symptoms may develop within minutes or in an hour after allergen exposure; however, they may not be obvious when there is uninterrupted allergen exposure. Common allergens associated with IgE-mediated asthma include pollens, such as from trees, grasses, and weeds; fungal spores; dust mites; animal dander; and in some settings, animal urine or cockroach excreta. IgE-mediated occupational asthma is considered under the category of occupational asthma. Allergen particle size must be less than 10 μm to penetrate into deeper parts of the lung because larger particles, such as ragweed pollen (19 μm), impact in the oropharynx. However, submicronic, “subpollen” ragweed particles have been described that could reach smaller airways (160). Particles smaller than 1 μm, however, may not be retained in the airways. Fungal spores, such as Aspergillus species, are 2 μm to 3 μm in size, and the major cat allergen (Fel d 1) has allergenic activity from 0.4 μm to 10 μm in size (161). Another study reported that 75% of Fel d 1 was present in particles of at least 5 μm and that 25% of Fel d 1 was present in particles of less than 2.5 μm (162




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree