Abstract
The term assisted reproduction incorporates a wide range of technologies that are used to enhance the probability of achieving a pregnancy after the collection and direct handling of oocytes, sperm, and the resulting embryos outside the body. The mainstay of these technologies is in vitro fertilization and embryo transfer (IVF-ET), in which aspirated oocytes are fertilized, followed by the transcervical replacement of an embryo(s) into the uterine cavity. The ET can be performed in the same cycle as controlled ovarian stimulation (COS), or in a subsequent cycle using cryopreserved embryos, thereby allowing fertility preservation, genetic testing, optimization of embryo-endometrial synchrony, minimization of risk of ovarian hyperstimulation syndrome (OHSS), and/or transfer of supernumerary embryos. Historically, other techniques such as gamete or zygote intrafallopian tube transfer (GIFT, ZIFT) were also performed, which limited exposure of gametes and embryos to the in vitro environment. However, as our understanding of the in vitro conditions necessary to support normal fertilization and preimplantation embryo development has improved considerably, GIFT and ZIFT have been rendered obsolete. Therefore this chapter focuses exclusively on IVF-ET and its adjunct technologies. Specifically, we will focus on the clinical and laboratory indications regarding the implementation and outcomes following the use of autologous and donor gametes, gestational carriers, and cryopreserved embryos. These techniques, along with the gamete and embryo micromanipulations discussed in another chapter, including intracytoplasmic sperm injection (ICSI), assisted hatching (AH), and preimplantation genetic testing (PGT), are collectively referred to as the assisted reproductive technologies (ART).
Keywords
Assisted reproduction, embryo, ICSI, implantation, infertility, in vitro fertilization, preimplantation genetic testing, pregnancy
Introduction
The term assisted reproduction incorporates a wide range of technologies that are used to enhance the probability of achieving a pregnancy after the collection and direct handling of oocytes, sperm, and the resulting embryos outside the body. The mainstay of these technologies is in vitro fertilization and embryo transfer (IVF-ET), in which aspirated oocytes are fertilized, followed by the transcervical replacement of an embryo(s) into the uterine cavity. The ET can be performed in the same cycle as controlled ovarian stimulation (COS), or in a subsequent cycle using cryopreserved embryos, thereby allowing fertility preservation, genetic testing, optimization of embryo-endometrial synchrony, minimization of risk of ovarian hyperstimulation syndrome (OHSS), and/or transfer of supernumerary embryos. Historically, other techniques such as gamete or zygote intrafallopian tube transfer (GIFT, ZIFT) were also performed, which limited exposure of gametes and embryos to the in vitro environment. However, as our understanding of the in vitro conditions necessary to support normal fertilization and preimplantation embryo development has improved considerably, GIFT and ZIFT have been rendered all but obsolete. Therefore this chapter focuses exclusively on IVF-ET and its adjunct technologies. Specifically, we will focus on the clinical and laboratory indications regarding the implementation and outcomes following the use of autologous and donor gametes, gestational carriers, and cryopreserved embryos. These techniques, along with the gamete and embryo micromanipulations discussed in Chapter 32 , including intracytoplasmic sperm injection (ICSI), assisted hatching (AH), and preimplantation genetic testing (PGT), are collectively referred to as the assisted reproductive technologies (ART).
Brief History of Assisted Reproductive Technologies
Assisted reproduction is nearly 130 years old, beginning with the attempts of Schenck to achieve fertilization in vitro and the successful transfer of embryos from a donor to a recipient rabbit by Heape. In 1959, Chang successfully fertilized a rabbit oocyte in vitro. In the human, successful capacitation of sperm in vitro and the fertilization of human oocytes matured in vitro were followed by the insight that preovulatory oocytes were optimal for IVF. These exploratory steps culminated in 1978 with a term birth resulting from IVF of a single preovulatory human oocyte obtained from a natural menstrual cycle, transferred to the uterus at the eight-cell stage.
Within the 10 years following this initial success, the first pregnancies were achieved from oocyte donation, gestational surrogacy, and cryopreserved eggs and embryos. In the following years, further landmark accomplishments included the synthesis of recombinant human FSH, along with the development of ICSI for male-factor infertility and PGT for single-gene defects and aneuploidy screening. The current era is focused on refining single-cell molecular techniques for diagnostic and potentially therapeutic purposes prior to ET. Where our field heads in the future will be driven not only by continued technological advancements, but also by efforts to understand the basic underpinnings of developmental biology and what it means to be human. Indeed, as ART becomes the standard treatment for infertility, the pace of knowledge transfer from bench to bedside has become alarmingly fast, challenging our concepts of self, family, and society.
Physiology of Sexual Reproduction
- ◆
Genetic diversity is generated through homologous recombination and random assortment of chromosomes.
- ◆
Meiosis produces haploid (1n, 1c) gametes from diploid (2n, 4c) progenitor cells.
- ◆
Binding of sperm to ZP3 initiates the acrosome reaction.
- ◆
Interaction between sperm Izumo1 and its cognate Juno receptor on the oolemma is essential for fertilization.
- ◆
After fusion of the sperm with the oolemma, the sperm-derived phospholipase C (PLC)-zeta mobilizes intracellular Ca 2+ stores to induce oocyte activation.
- ◆
The cells of the human embryo are likely totipotent through the cleavage stage; the first overt indication of differentiation is the morphologic distinction of inner cell mass cells from the outer cells, progenitors of the trophectoderm.
- ◆
Implantation requires adequate and timely signaling between the blastocyst and the uterine epithelium.
- ◆
Implantation depends on three fundamental principles:
- ◆
A developmentally competent blastocyst
- ◆
A receptive endometrium (the “window of implantation”)
- ◆
Synchronicity between the blastocyst and the endometrium
- ◆
In sexual reproduction, diploid progenitor cells divide by the process of meiosis to produce unique haploid cells that can fuse at fertilization to yield a new, totally unique diploid organism. Genetic diversity is maximized during gametogenesis, through genetic recombination, which occurs between homologous chromosomes of maternal and paternal origin during the prophase of meiosis I; such exchange of genetic material creates new combinations of haplotypes. Therefore one advantage of sexual reproduction is that the random recombination of genetic material increases the range of traits displayed by members of the species. This diversity increases the chances of success of the species in adapting to an ever-changing environment.
Gametes
In most species that reproduce by sexual reproduction, two types of gametes are produced. The egg, or ovum, is large and nonmotile. The sperm or spermatozoa are small and motile. The developing egg is referred to as an oocyte and is one of the largest cells in the body. A mature human oocyte measures approximately 110 µm in diameter. In contrast, the diameter of a human sperm head is 2 to 3 µm.
The generation of the germ cells is achieved through meiosis, which consists of two divisions: meiotic division I and meiotic division II ( Fig. 31.1 ). Prior to entry into prophase of meiotic division I, the progenitor cell undergoes duplication of deoxyribonucleic acid (DNA) so that each duplicated chromosome (2n) consists of two sister chromatids (4 copies, 4c) bound together at the centromere by cohesions (46 duplicated chromosomes). During progression through prophase I, homologous pairs of chromosomes align in bivalents, and genetic recombination occurs at chiasmata in the synaptonemal complex. This “crossing-over” between nonsister chromatids is critical to producing genetic diversity in the gametes.
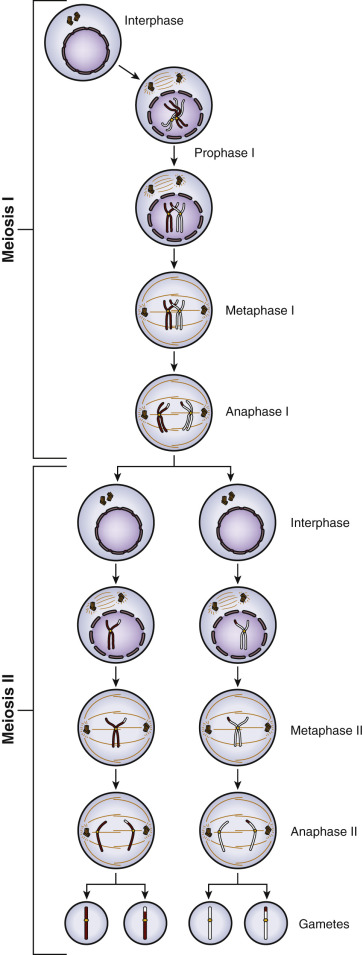
In meiotic division I, also known as the reduction division, one homologue (consisting of linked sister chromatids) of each chromosome pair is distributed to each daughter cell, such that each is now 1n, 2c. No DNA replication occurs in the second meiotic division, and the strands of the sister chromatids are separated to the derivative haploid cells, such that each is now 1n, 1c. Thus homologous chromosomes segregate in the first meiotic division, and sister chromatids in the second (see Fig. 31.1 ).
Oocyte
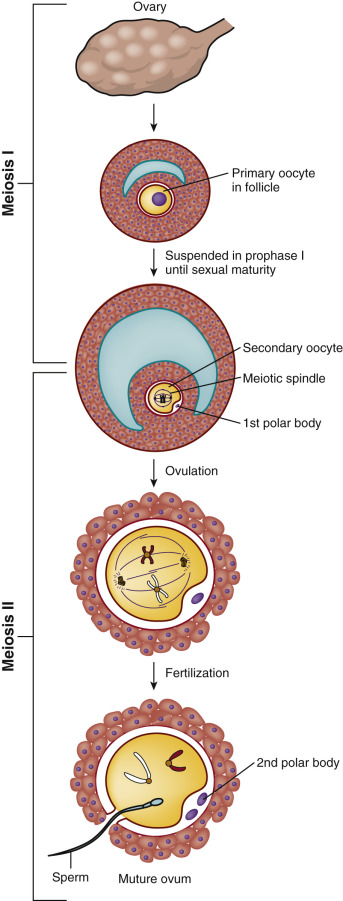
The process of oocyte formation, known as oogenesis , begins when the primordial germ cells (PGCs) migrate from the extraembryonic endoderm of the yolk sac into the undifferentiated genital ridge and become oogonia (see Chapter 8 ). The oogonia proliferate by mitotic division, become invested with a single layer of granulosa cells, and differentiate into primary oocytes, the female progenitor cells. After duplication of its complement of DNA during interphase and entry into the meiotic process, the primary oocyte becomes arrested at diplotene of prophase I, and then enters a prolonged state of meiotic arrest. The arrested, so-called germinal vesicle (GV) stage oocyte ( Fig. 31.2A ) synthesizes a coat of glycoproteins, the zona pellucida , and remains arrested until the follicle in which it is enclosed either degenerates (atresia) or is recruited into the pool of growing follicles, undergoes growth, and ultimately is selected to become the dominant follicle.
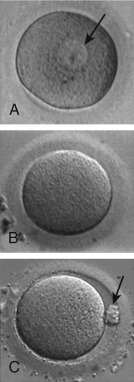
Under the influence of the luteinizing hormone (LH) surge, the fully grown GV-stage oocyte in the dominant follicle resumes meiosis and progresses through metaphase I (see Fig. 31.2B ) and anaphase I, to reach telophase I. Upon extrusion of the first polar body at telophase I, the oocyte completes meiosis I to become a secondary oocyte. The secondary oocyte then rapidly proceeds to metaphase II, after which it undergoes a second period of meiotic arrest (see Fig. 31.2C ). Progression of the oocyte from prophase I to metaphase II takes approximately 36 hours and is termed “meiotic maturation.” The oocyte is ovulated at metaphase II, the stage at which it is normally fertilized. The progression through meiotic maturation from prophase I to metaphase II, and then to telophase II and extrusion of the second polar body following fertilization, is shown in Fig. 31.3 . Thus elimination of one chromosome of each homologous pair through extrusion of the first polar body at telophase I results in a 1n, 2c oocyte, whereas elimination of one of the sister chromatids of each retained homologue through extrusion of the second polar body at telophase II results in a 1n, 1c oocyte.
A normal mature haploid human oocyte has a complement of 23 chromosomes. However, meiotic errors occur resulting in a high incidence of aneuploid oocytes. Such errors typically occur during the first meiotic division and involve a variety of mis-segregations, including nondysjunction of homologous chromosomes and premature separation of sister chromatids. Resulting oocytes either possess too few chromosome copies and are referred to as hypohaploid, or have too many copies and are termed hyperhaploid. Maternal age is the main factor associated with the occurrence of aneuploidy, which in turn accounts for the majority of failed conceptions (reviewed by Hassold et al. ). The root cause of such age-related aneuploidy appears to relate to abnormalities in morphology of the meiotic spindle, as well as a decrease in chromosome cohesion caused by an increase in interkinetochore distance between sister chromatids.
Regardless of chromosome number, an oocyte that has reached metaphase II (i.e., completed nuclear maturation) has not necessarily achieved cytoplasmic maturation. Although the molecular processes underlying acquisition of cytoplasmic maturity remain poorly understood, it is clear that cytoplasmic maturation is critical for the oocyte to undergo “activation” at fertilization. Inadequate or absent oocyte activation leads to fertilization failure and/or abnormal syngamy and early developmental programming.
Sperm
In contrast to the egg, sperm are among the smallest cells in mammals. Sperm are highly specialized for the primary purpose of transporting DNA to an oocyte. Sperm consist of four key functional components:
- 1.
The acrosome, derived from the Golgi apparatus, which contains enzymes that aid in digesting and penetrating the cumulus oophorus.
- 2.
The nucleus, which contains highly compacted and epigenetically modified chromatin.
- 3.
The midpiece, which includes the centrosome that is key in human embryogenesis, as well as mitochondria, which provide the energy source for sperm.
- 4.
The tail, which contains the axoneme and the dynein motor proteins that are responsible for the generation of motility.
The mature sperm has no ribosomes or endoplasmic reticulum and no active transcription or translation of messenger ribonucleic acids (mRNAs). However, small RNAs are transported within the sperm and may have a role in embryogenesis.
Spermatogenesis differs from oogenesis functionally and temporally ( Fig. 31.4 ; see Chapter 23 ). In the male embryo, PGCs migrate to the testis and undergo epigenetic reprogramming that includes erasure and resetting of imprinted genes, after which the PGCs enter a state of arrest until puberty. Under the influence of testosterone and other hormones, the spermatogonia divide mitotically and generate two pools of derivative cells. The cells of one pool continue to divide mitotically and serve as the spermatogonial stem cells. The second pool of cells will enter meiosis and become primary spermatocytes (46 duplicated chromosomes in human).
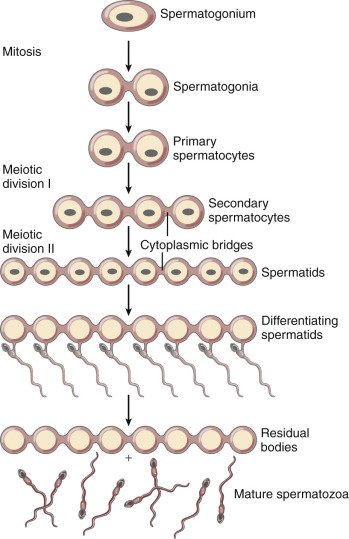
The primary spermatocytes proceed through the first meiotic division and then become secondary spermatocytes (22 duplicated autosomal chromosomes, plus a duplicated X chromosome or a duplicated Y chromosome). After the second meiotic division, the secondary spermatocytes become spermatids (haploid number of single chromosomes), which then differentiate into mature sperm. The process of meiotic reduction of the spermatogonia, as well as maturation of the spermatids (spermiogenesis), occurs inside the seminiferous tubule, with the precursor cells located at the outer border of the tubule and the mature sperm in the lumen of the tubule.
The developing sperm cells undergo nuclear division but do not complete cytoplasmic division until near the end of sperm differentiation (see Fig. 31.4 ). Consequently, the developing germ cells are connected to supporting Sertoli cells by cytoplasmic bridges in a syncytium, which allows the diploid spermatogonium to produce proteins and cellular materials for the haploid sperm.
During the late stages of spermiogenesis, greater than 90% of the histones bound to sperm DNA are selectively removed and replaced by protamines, small basic molecules that facilitate tight packaging of the chromatin for sperm transport. This process is known as the histone to protamine exchange. Recent studies have demonstrated that the remaining 5% to 10% of the genome bound to histones have a combination of unique epigenetic marks, DNA demethylation and bivalent histone modifications, that appear to “poise” key developmental genes from the sperm for rapid activation in the zygote. These changes are found in diverse species and suggest a role for the sperm genome to facilitate early embryonic gene expression, a hypothesis also supported by recent data from IVF patients exhibiting abnormal embryogenesis.
Upon entry into the epididymis, the sperm undergo sequential steps of differentiation, largely related to the acquisition of motility, and the ability to bind to and penetrate the oocyte. Recent transcriptomic and proteomic studies have revealed that specific variations of gene expression occur in the different epididymal segments, and that proteins thought to be directly related to sperm maturation processes are differentially expressed in sperm derived from the different segments of the epididymis. Interestingly, the expression of the proteins is altered in some infertile men. Thus sperm from the caput epididymis are generally unable to undergo natural fertilization, but gain the ability to fertilize by the time they reach the cauda epididymis. Sperm from all regions of the epididymis, and also the testes, are capable of undergoing fertilization via ICSI.
During ejaculation, the sperm are exposed to stabilizing factors from the seminal fluid, which preclude the ability of the sperm to undergo the acrosome reaction. The sperm undergo a process of “capacitation” as they progress through the female reproductive tract that includes removal of cholesterol from the membranes and an influx of calcium, ultimately resulting in a more fluid and less stable plasma membrane that facilitates the acrosome reaction in response to the zona pellucida proteins of the oocyte plasma membrane. Sperm capacitation can be facilitated in the laboratory prior to ART or artificial insemination by sperm processing methods that remove the sperm from seminal plasma. Defects of capacitation have been associated with reduced fertilization potential, resulting in male infertility, but can be overcome by ICSI.
Sperm penetration of the cumulus cell layers surrounding the egg is facilitated through hyperactivated motility, a pattern of vigorous nonlinear motility that can be stimulated by cumulus cell progesterone secretion. The sperm motility and hyaluronidase secretion allow the sperm to move through the cumulus extracellular matrix to reach the zona pellucida.
On reaching the zona pellucida, the capacitated sperm then undergo the acrosome reaction , a process that is essential for fertilization. The acrosome is a large secretory cap on the sperm head that contains proteases and hyaluronidases. In the acrosome reaction, the outer acrosome membrane fuses with the plasma membrane of the sperm and the contents of the acrosome are emptied. In many species, the acrosome reaction is initiated by the glycoproteins of the zona pellucida and can be accelerated by progesterone.
Fertilization
The process of fertilization involves at least two key initial steps: interaction and penetration of the zona pellucida by the sperm, followed by fusion of the sperm and oocyte membranes ( Fig. 31.5 ). Although the mechanisms that allow the human sperm and zona pellucida of the oocyte to interact are not fully characterized, use of antibodies and antagonists to candidate molecules has greatly advanced understanding in this area. Prevailing data suggest that a dynamic array of zona pellucida receptor-sperm ligand interactions regulate sperm-zona binding. In the mouse, the zona pellucida contains three glycoproteins (ZP1, ZP2, and ZP3), but the human zona has a fourth glycoprotein, ZP4. ZP2 and ZP3 have a filament structure, and ZP1 appears to link ZP2 and ZP3 in a complex three-dimensional array, because absence of either ZP2 or ZP3 prevents assembly of the zona matrix. In the human, capacitated sperm can be induced to undergo the acrosome reaction by ZP1, ZP2, and ZP4, although downstream signaling events may be different with ZP3 compared with ZP1 or ZP4. In an early study, purified ZP3 blocked the ability of sperm to bind to the zona pellucida in a dose-dependent manner, implying that ZP3 is the zona pellucida sperm receptor, and data from several other studies have implied that O-linked carbohydrate moieties underlie the interaction of ZP3 and the sperm receptor. The nature of this carbohydrate ligand has been further identified as the sialyl-Lewis sequence, a member of the selectin family. Nevertheless, the biologic complexity of the sperm-egg recognition process appears to reflect participation by a series of sperm proteins (see Reid et al. ), and involve sperm binding to ZP1, ZP3, and ZP4, but not ZP2, which appears only to bind previously acrosome-reacted sperm.
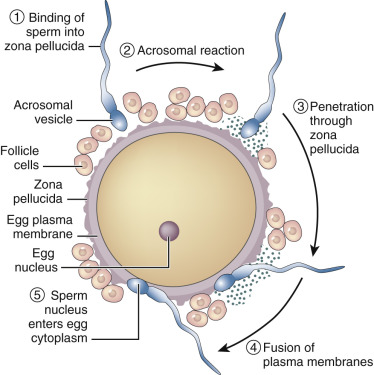
The search for the sperm receptor to the ZPs has been difficult, and it appears that since knockout studies of putative receptors have generally not precluded binding, there is likely a small number of proteins on the sperm surface that appear to be responsible for interaction with ZP3. These proteins include fertilin, galactosyltransferase, and cyritestin. βl,4-Galactosyltransferase on the sperm surface appears to be important for the ZP3-induced acrosome reaction. Fertilin and cyritestin are members of the ADAM ( a d isintegrin a nd m etalloprotease) family of proteins, which can bind to integrins but also have proteolytic activity. The sperm of mice lacking cyritestin (ADAM3) have defective binding to the zona pellucida. Fertilin, a heterodimeric sperm surface protein composed of α and β subunits, was originally thought to be involved in fusion of sperm with the egg plasma membrane (the oolemma). However, mice lacking fertilin β (ADAM2) show defects in zona binding. Other studies have implicated the involvement of a new candidate enzyme, mouse sperm lysozyme-like protein (mSLLP1), in sperm-oolemmal binding. This protein is located in the equatorial segment of acrosome-reacted sperm in both mouse and human, and appears to bind to the entire oolemma, except in that region where sperm normally do not bind, overlying the meiotic spindle.
Binding of the sperm to ZP3 initiates the acrosome reaction through the action of the core protein of ZP3, causing a fusion of the acrosomal and sperm plasma membranes and the release of hydrolytic enzymes within the acrosome, including preacrosin. Preacrosin is activated by binding to ZP2, and the resulting acrosin, as well as flagellar beating, are key in digestion of the zona pellucida and forward progression to the oocyte plasma membrane. After completion of the acrosome reaction, the sperm lose their affinity for ZP3, and the continued attachment of the sperm to the oocyte appears to be dependent on ZP2.
Sperm binding and fusion to the oolemma is a complex process that involves the transmembrane protein PH-30, a protein found in the remnant equatorial region of the former acrosome. Another key protein involved in binding and fusion is called Izumo1, which is a cell-surface ligand expressed by capacitated sperm. Izumo1 is the specific binding partner for Juno, a folate receptor expressed on the oolema. Binding of Izumo1 to Juno is not only essential for normal fertilization, but also conserved across mammals and is species-specific. Interestingly, both the male Izumo1 knockout mouse and the female Juno mouse are sterile. Once the sperm fuses with the oolemma, a depolarization of the oolemma occurs that acts as the primary block to polyspermy. Temporally, this occurs as Juno is shed from the oolema membrane into the extracellular space. Shortly thereafter, the inositol phospholipid cell-signaling pathway is activated, and submembrane cortical granules release their contents. The contents of the cortical granules change the glycoprotein coat of the zona pellucida, preventing sperm binding by hydrolyzing the oligosaccharides of ZP3 and by the proteolytic cleavage of ZP2. In the mouse at least, this proteolytic cleavage is achieved by exocytosis of the cortical granule metalloendoprotease, ovastacin. This process of zona hardening creates a secondary block to polyspermy.
After fusion of the sperm with the oolemma, the sperm PLC isoform PLC-zeta mobilizes the Ca 2+ signal that induces egg activation and embryo development. Accordingly, abnormalities in PLC-zeta negatively affect fertilization rates and possibly embryo development, confirming the key role of this factor in triggering oocyte activation. This finding suggests that certain types of infertility could be caused by failure of the sperm cell to properly activate the oocyte due to a defective PLC-zeta factor.
Recent studies have begun to demonstrate the important role that sperm may play in postfertilization events. Once in the oocyte, sperm chromatin and DNA integrity are necessary to ensure normal embryo development. It is now clear that DNA damage in spermatozoa, in the form of single and double-stranded DNA breaks, has a negative influence on blastocyst development and ICSI outcome. Similarly, the role of the “poised” sperm epigenome in normal embryonic development has become better understood, and recent studies highlight this area as a focus of future potential advances in understanding postfertilization defects. Lastly, it is now understood that centrosome integrity is critical for successful fertilization and embryo development, and data indicate that the replacement of defective centrosomes, which are responsible for specific types of male infertility, with functional donor sperm centrosomes may restore normal functionality.
One nascent area of interest is the role of sperm RNAs on postfertilization events. The sperm cell contains various forms of RNA (e.g., mRNA, miRNA, siRNA), as well as more than 2000 proteins with unknown roles. Data are emerging that indicate sperm-derived micro RNAs may be involved in normal embryogenesis.
Preimplantation Embryo
Sperm penetration into the oocyte triggers the oocyte to complete meiosis by rapid progression from metaphase II to telophase II. As discussed previously, telophase II is characterized by elimination of one of the sister chromatids of each chromosome through extrusion of the second polar body. The retained set of oocyte chromatids undergoes decondensation, followed by decondensation of the set of sperm chromatids, each to form a pronucleus. The zygote is therefore characterized by the presence of two pronuclei and two polar bodies ( Fig. 31.6A ).
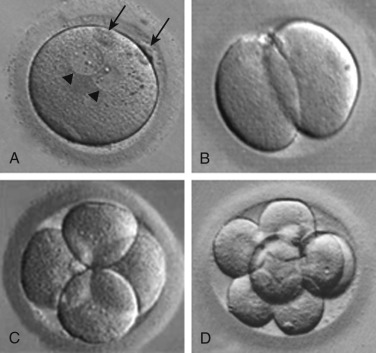
Following migration to the center of the oocyte, and then apposition of the two pronuclei, pronuclear membrane breakdown occurs, the maternal and paternal chromosomes intermingle at syngamy, and the diploid zygote is formed. Shortly thereafter, the chromosomes undergo condensation and the paired homologues (one maternal and one paternal) align on the metaphase plate of the first mitotic spindle, in preparation for the first cleavage division. The embryo then progresses through several cleavage divisions (see Fig. 31.6B–D ), giving rise to an embryo composed of multiple cells or blastomeres.
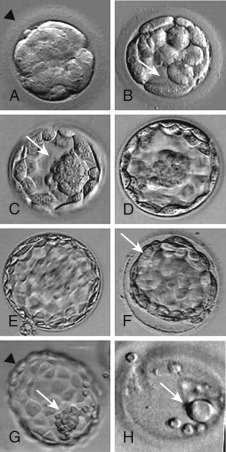
In human embryos, the blastomeres are totipotent up to around the eight-cell stage. Beyond the eight-cell stage, the cells begin to undergo differentiation. Compaction ensues to form the morula stage ( Fig. 31.7A ), as blastomeres adhere because of secretion of cell adhesion molecules such as E-cadherin. Tight junctions form between blastomere membranes, and pockets of fluid begin to accumulate among the blastomeres. Concomitant with onset of coalescence of these small fluid collections to form a fluid-filled cavity (the blastocoele), the first signs of overt cellular differentiation occur; the surface cells begin to undergo a change in shape from spherical to squamous epithelial-like to form trophectoderm cells, while a small cluster of inner cells retain their spherical appearance, ultimately to form the inner cell mass (see Fig. 31.7B ). The trophectoderm cells form the trophoblast, which gives rise to the extraembryonic structures, such as the placenta, and the inner cell mass gives rise to the embryo. Following gradual enlargement of the blastocelic cavity, which causes expansion of the blastocyst (see Fig. 31.7C–E ), the blastocyst undergoes pulsation as it contracts (see Fig. 31.7F ) and reexpands, ultimately to escape from the zona pellucida in preparation for implantation (see Fig. 31.7G and H ).
Strictly speaking, the embryonic stage consists of the period from the development of the primitive streak through the initial steps in the development of all the major organs. As defined in the human, the embryonic stage begins approximately 14 days after fertilization. In assisted reproduction, most authorities use the term “embryo” to describe the conceptus from the first cleavage through the initial stages of organ development. This convention will be followed in the remainder of this chapter.
Implantation
The molecular events underlying the process of implantation in humans are not completely understood (see Chapter 9 ). The human embryo enters the uterus late on day 3 after fertilization at the cleavage stage . Within 5 to 6 days of fertilization, the embryo reaches the blastocyst stage and begins to hatch from the zona pellucida, thereby allowing the outer covering of syncytial trophoblast cells to interact directly with the uterine epithelium. The trophectoderm nearest the inner cell mass, the polar trophoblast, plays an important role in the interaction of the blastocyst and the endometrium. The interaction of the blastocyst with the endometrium involves phases of apposition, stable adhesion, and invasion.
The blastocyst can attach to the endometrium only during a critical window of implantation corresponding to menstrual cycle days 19 to 24. This “implantation window” is characterized by a transition from the proliferative to secretory phase of the endometrium, mediated primarily by pulsatile secretion of progesterone from the corpus luteum. Stromal edema becomes predominant during this receptive phase, accompanied by the ultrastructural appearance of pinopodes. These surface epithelial cells are devoid of microvilli and may participate in fluid absorption from the uterine cavity, thus bringing the blastocyst in closer contact with the underlying endometrium.
Although the signals that control the attachment of the blastocyst to the uterine epithelium are not well characterized, transcriptomic analyses have shown that several growth factors, cytokines, integrins, and adhesion molecules are expressed in the trophectoderm and endometrium at the time of implantation. Indeed, transcriptomic profiling of endometrial biopsies by an endometrial receptivity array may be able to distinguish a receptive state from a pre- or postreceptive state, in which the window of implantation has been displaced. While preliminary studies that assess the clinical utility of actively managing a displaced window of implantation in patients with a nonreceptive endometrial biopsy indicate that pregnancy rates may be rescued by altering the day of progesterone start in an IVF cycle, this approach awaits further validation in randomized controlled trials (RCTs).
Differential secretion of some chemokines and growth factors attract the blastocyst to the pinopodes, while the repellent activity of the glycoprotein, MUC-1, appears to play an important role in repulsion of the blastocyst away from less desirable implantation areas. Active, local removal of the antiadhesive MUC-1 by the metalloproteinase ADAM17 at the site of implantation is required for proper embryo adhesion. Moreover, gene and protein expression studies have revealed that endometrial glands and stroma have distinct mRNA signatures that depend on the day of the cycle. Interestingly, COS in general leads to disruptions in the transcriptional activity of genes involved in endometrial receptivity, and these distributions are altered by different COS protocols. These alterations in endometrial receptivity may partly explain the reduced birth weight in neonates conceived following fresh ET (i.e., under the influence of COS) versus that of neonates conceived following transfer of thawed embryos (i.e., into a “prepped” uterus). Indeed, for this and other reasons detailed later in the “Elective Freeze-All” section, segmentation of the IVF cycle into two phases, which temporally dissociates COS from ET by at least one menstrual period, has become an increasingly utilized approach in contemporary IVF.
After attachment of the blastocyst to the uterine epithelium, the syncytiotrophoblast invades the endometrium, and by 12 days after fertilization, the conceptus is completely embedded in the decidualized stroma. Once this contact is established, human chorionic gonadotropin (hCG), secreted by the syncytiotrophoblast, can be detected in the maternal circulation. Through autocrine and paracrine activity, hCG modulates the expression of key factors such as prokineticin-1 and its G-protein coupled receptor, PROKR, which control the depth of trophoblastic invasion and the formation of plugs within the spiral arteries. These endovascular plugs of extravillous trophoblast are responsible for maintaining the physiologic hypoxia of the first trimester. As hCG decreases late in the first trimester, so does the expression of prokineticin-1 by the syncytiotrophoblast, and the trophoblastic plugs in the spiral arteries are remodeled, ultimately bringing the chorionic villi in direct communication with the maternal blood to establish hemochorial placentation. While the molecular events guiding embryo apposition, adhesion, and invasion have begun to be elucidated, much remains to be learned about how implantation proceeds normally, and what mechanisms underlie disease states such as recurrent implantation failure, preeclampsia, fetal growth restriction, and placenta accreta.
Pre–In Vitro Fertilization Evaluation
As detailed in Chapters 22 and 23 , testing to determine the etiology of infertility will help predict the likelihood of healthy pregnancy and delivery with various treatments, including IVF. Prior to IVF, the basic evaluation must include appropriate infectious disease and genetic testing, ovarian reserve testing, a uterine cavity evaluation, and semen analysis. Testing options for this evaluation are summarized in Table 31.1 . As described later, the etiology of infertility has implications as to the prognosis for pregnancy and live birth with IVF.
| Infectious Disease Screening * |
|
| Genetic Testing |
|
| Ovarian Reserve Testing |
|
| Uterine Cavity Evaluation |
|
| Semen Analysis |
|
* For cycles using donor gametes or a gestational carrier, CMV, HTLV-1, and HTLV-2 are likewise required by the US Food and Drug Administration, in addition to a physical exam and itemized questionnaire about recent travel and high-risk behaviors.
Indications for In Vitro Fertilization
- ◆
The most common indications for IVF are male-factor infertility and diminished ovarian reserve (DOR).
- ◆
Success rates following IVF are not only dependent on female patient age, but also on the primary infertility diagnosis.
Conventional indications for IVF are tubal factor infertility, male factor infertility, DOR, endometriosis, and unexplained infertility. IVF is now recommended for essentially all infertility conditions that have not been successfully treated by other modalities and, indeed, is often the preferred first-line treatment by many patients. For example, IVF has been advocated as a potential first-line treatment of polycystic ovary syndrome (PCOS), when oral agents such as clomiphene or letrozole fail to lead to ovulation or pregnancy, in order to avoid the increased risk of ovarian hyperstimulation and multiple gestation, associated with gonadotropin therapy. Similarly, a good prognosis patient who would have a high-risk pregnancy should she conceive a multiple pregnancy may be best treated with IVF and transfer of a single embryo, rather than with other modalities such as gonadotropin stimulation and IUI, in which the multiple pregnancy rate is higher, and generally poorly predictable.
Other indications for IVF include elective and medically indicated fertility preservation, PGT to avoid transmission of a heritable condition, and gestational surrogacy in specific settings (e.g., absolute uterine factor due to congenital or acquired absence of a functional uterus, those with a serious medical condition that precludes safe pregnancy, and members of the lesbian, gay, bisexual, and transgender [LGBT] communities). Indeed, as the technology has improved, the application of IVF has broadened.
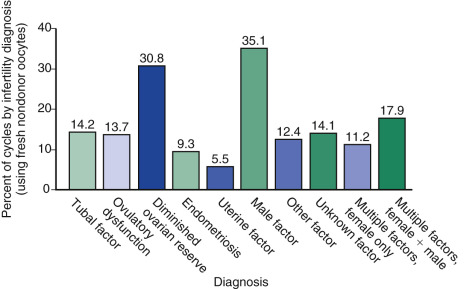
The 2014 ART National Summary Report from the Centers for Disease Control (CDC) describes the infertility diagnoses for patients undergoing autologous IVF cycles ( Fig. 31.8 ). The most common indication for IVF was male factor (35.8%), followed by DOR (30.8%), and then multiple male and female factors (17.9%). Nevertheless, other diagnoses such as tubal factor, endometriosis, and PCOS are common indications, as well.
Tubal Factor Infertility
IVF was initially developed as a treatment for tubal factor infertility. Tubal occlusion is typically diagnosed with a hysterosalpingogram, but may also be identified with laparoscopic chromopertubation or hysterosalpingo-contrast sonography. It should be noted that proximal tubal occlusion often can be artifactual due to tubal spasm, and the diagnosis should be confirmed with either repeat imaging or laparoscopic chromopertubation. For cases of true proximal occlusion, fluoroscopic-guided tubal recanalization is an option. Tubal reanastomosis also can restore tubal patency, and provide reasonable live birth rates, in particular among patients with prior surgical sterilization. The decision to proceed with tubal reconstruction versus IVF in these cases is complex, and should factor in patient age, other causes of infertility, desired family size, and cost of treatment.
For patients with distal tubal occlusion, surgical repair and IVF are both possible therapeutic options, although no prospective trials have been reported, comparing their relative efficacies. Clinical experience indicates that IVF provides higher delivery and lower ectopic rates compared with surgery. However, surgery is reasonable in women less than 35 years, with no other causes of infertility and mild tubal disease; pregnancy rates in other patient populations are poor. It is clear that the presence of communicating hydrosalpinges (defined as fluid-filled tubes on ultrasound, not merely occluded fallopian tubes) is detrimental to IVF outcomes. There are several meta-analyses of IVF outcomes in the setting of hydrosalpinges. A 2010 Cochrane review indicates that unilateral or bilateral removal of hydrosalpinges, or interruption of the hydrosalpinges, result in two- to fourfold higher delivery rates in IVF compared with no intervention. Initial concern about the possibility that interruption of collateral blood vessels to the ovary at the time of salpingectomy may reduce ovarian reserve has not borne out in larger studies and meta-analysis. A 2016 meta-analysis that compared IVF cycles immediately prior to salpingectomy for ectopic or hydrosalpinx to cycles following surgery found no differences in total gonadotropin dose, peak estradiol, number of oocytes obtained, or clinical pregnancy between the groups (18 studies, n = 1482).
IVF following placement of transcervical sterilization devices (e.g., Essure) has been undertaken in a few small reports, with births recorded. One study treated 20 women with the Essure device bilaterally or unilaterally, based on hydrosalpinx location. There were 12 live births, with one complicated by premature rupture of membranes and the other by placenta previa. In our practice we have seen coils of the Essure device protruding into the endometrial cavity. In our opinion, data are insufficient to recommend the Essure rather than laparoscopic salpingectomy or interruption as first-line treatment for hydrosalpinges prior to IVF. Likewise, women who have documented tubal patency following tubal surgery, and who are less than 35 years and fail to conceive after 12 months, should undergo IVF, whereas those who are ≥35 years and who fail to conceive after 6 months should proceed to IVF.
Endometriosis
For women presenting with infertility and early-stage endometriosis, IVF has not been definitively shown to be superior to other available treatments, such as expectant management, human menopausal gonadotropin (hMG) with intrauterine insemination (IUI), or surgical treatment (see Chapter 30 ). However, several nonrandomized studies suggest that IVF treatment results in a higher pregnancy rate per cycle than conception attempts after surgical treatment, hMG with IUI, clomiphene treatment with IUI, or expectant management. For example, one retrospective study of 313 women with endometriosis and infertility evaluated cumulative pregnancy rates following gonadotropin IUI versus IVF. The pregnancy rate after one cycle of IVF was significantly higher than that of 6 cycles of gonadotropin IUI (47% vs. 41%; P < .05). After stratification by disease stage, the benefit of IVF was more pronounced, and women with stage IV endometriosis and age greater than 38 years were much more likely to conceive from IVF than from IUI treatment.
Patients often ask whether surgical treatment of early endometriosis prior to IVF improves the likelihood of pregnancy. There are no randomized trials to address this issue. However, a retrospective study evaluating the utility of diagnostic laparoscopy prior to IVF demonstrated that treatment of stage I or II endometriosis ( n = 399) was associated with higher implantation and live birth rates per oocyte retrieval compared with diagnostic laparoscopy alone ( n = 262) in patients with peritoneal endometriosis (implantation: 30.9% vs. 23.9%, P = .02; live birth: 27.7% vs. 20.6%, P = .004).
Women with advanced stage endometriosis have lower ovarian reserve than women without endometriosis. A meta-analysis of 33 studies demonstrated that women with endometriomas had a twofold higher cycle cancellation rate and significantly fewer oocytes retrieved than women without endometriomas; however, the overall live birth rate was not different among the groups. Surgical resection of an endometrioma can have a further temporary detrimental effect on ovarian reserve, as measured by serum AMH pre- and postoperatively. Surgery can likewise impair ovarian responsiveness to gonadotropins. In an RCT of ovarian cystectomy for endometrioma followed by IVF versus immediate IVF, the surgery group required a longer duration of COS (14.0 vs. 10.8 days; P = .001), higher total gonadotropin dose (4575 IU FSH vs. 3675 IU FSH; P = .001), and had fewer mature oocytes retrieved (7.8 vs. 8.6; P = .03). There was no difference in fertilization, implantation, or pregnancy rates. In the most extreme cases, bilateral ovarian cystectomies for endometriomas have been associated with a 2.5% rate of ovarian failure. Accordingly, unless pathologic confirmation of a complex ovarian cyst is necessary, or the location of an endometrioma would prevent safe oocyte retrieval, observation of advanced endometriosis in favor of immediate IVF has become an increasingly acceptable approach. If an endometrioma is encountered during oocyte retrieval, it should not be purposefully aspirated due to risk of abscess formation.
For patients with all stages of endometriosis who are planning to undergo IVF, a meta-analysis of three RCTs including 165 infertile women with endometriosis who were randomized to receive either 3 to 6 month pretreatment with depot gonadotropin-releasing hormone (GnRH) agonist versus no treatment demonstrated that GnRH agonist treatment may significantly increase the odds of clinical pregnancy and live birth.
Male Factor
Male factor infertility is a broad category that ranges from minimally abnormal semen parameters to nonobstructive azoospermia. Because abnormal semen analysis values are only suggestive of male infertility and have low predictive power, follow-up assays may be necessary to evaluate fertilization ability. In general, men with severe semen abnormalities are best treated with ICSI (see Chapters 23 ). Severe oligoasthenospermia (less than 1.5 million motile sperm per ejaculate) and severe isolated teratospermia are associated with poor pregnancy rates in standard IVF. Typically ICSI is indicated for men with fewer than 10 million sperm/mL or less than 5 million/mL after processing. Prior to undergoing ICSI, men with less than 5 million sperm per mL in unwashed ejaculate should have a karyotype and Y chromosome microdeletion assessment, because the incidence of karyotypic and genetic abnormalities is high in this group. In a study of 1935 men with severe male factor infertility (1214 with nonobstructive azoospermia and 721 with severe oligoasthenospermia), the incidence of karyotypic abnormalities was 16.4% and 5.8%, respectively. The incidence of Y chromosome microdeletions was 9.5% and 1.9%, respectively. While other studies have reported lower rates of abnormal karyotypes and questioned the value of routine screening, the preponderance of data suggest continued screening, and patients with normal karyotypes are generally good candidates for IVF. ICSI in cases of severe male factor infertility results in fertilization and pregnancy rates comparable to those seen in standard IVF (see Chapter 32 ).
DNA damage in sperm has been shown to be associated with male infertility and decreased outcomes in couples undergoing IVF, although the data remain controversial due to variations in testing procedures and conflicting data. In general, testing for DNA damage may be useful in some cases of infertility, including couples with a history of recurrent miscarriage or poor IVF outcomes, but general guidelines recommend selective testing rather than routine screening of infertile men. A recent meta-analysis reports that evaluation of chromatin structural abnormalities, as measured by protamine abnormalities, is associated with male subfertility and closely related to sperm DNA damage. This line of sperm evaluation may be of utility in the future, but has not been widely accepted presently. Lastly, sperm epigenetic assays may offer novel insight into poor IVF outcome, but are not yet validated for routine screening.
Idiopathic Infertility
For 10 to 17% of infertile couples, a thorough evaluation reveals no identifiable cause of infertility. Data reported to Society for Assisted Reproductive Technologies (SART) in 2014 show that 14.1% of IVF/ICSI cycles initiated were for a primary infertility diagnosis of “unknown factor” infertility (see Fig. 31.8 ).
Many couples with idiopathic infertility become pregnant after a stepwise treatment approach (see Chapter 30 ), which may include superovulation with clomiphene IUI, followed by gonadotropin IUI and then IVF. Two RCTs have been performed to determine the most effective treatment approach for achieving live birth among patients initiating care for unexplained infertility. In the Fast Track and Standard Treatment Trial (FASTT), 503 women ages 21 to 39 years with at least 12 months of unexplained infertility were randomized to one of two treatment groups: a conventional approach with 3 cycles of clomiphene IUI, followed by 3 cycles of gonadotropin IUI, then up to 6 cycles of IVF; or an accelerated approach that omitted the gonadotropin IUI cycles. Delivery rates per cycle of treatment were 7.6% for clomiphine IUI, 9.8% for gonadotropin IUI, and 30.7% for IVF. Couples allocated to the accelerated arm were pregnant on average 3 months earlier than those allocated to the conventional arm (8 vs. 11 months), and the cost per delivery was $9800 less for the accelerated arm. The Forty and Over Treatment Trial (FORT-T) randomized 154 women ages 38 to 42 years with at least 6 months of unexplained infertility to either 2 cycles of clomiphene IUI or 2 cycles of gonadotropin IUI, then up to 6 cycles of IVF, or directly to IVF. The cumulative clinical pregnancy rates after the first 2 cycles were 21.6%, 17.3%, and 49.0%, respectively. Importantly, 84.2% of all live births in the study were achieved with IVF, and significantly fewer treatment cycles were required in the immediate IVF group. Accordingly, among patients less than 38 years with unexplained infertility, a fast-track to IVF may be the most effective approach in terms of cost and time spent in treatment; among patients 38 years of age or older, proceeding directly to IVF is reasonable based on the available evidence.
Polycystic Ovary Syndrome and Anovulation
Traditionally, infertile women with PCOS in whom both clomiphene and gonadotropin ovulation induction failed had few remaining treatment options, except surgical procedures like ovarian diathermy to reduce thecal androgen production. However, evidence has accumulated that IVF is often effective for such patients. A meta-analysis of 9 studies comparing IVF outcomes in 458 PCOS patients, as defined by the Rotterdam criteria, to 694 matched non-PCOS patients found that PCOS patients required on average 1.2 days longer for stimulation, and had higher cycle cancellation rates, higher oocyte yields, lower fertilization rates, but equivalent clinical pregnancy rates per ET. As the incidence of oocyte immaturity is high among PCOS patients, the similar clinical pregnancy rates reported in this study are reassuring.
PCOS patients are at high risk of OHSS, in particular those with an AMH greater than 3.5 ng/mL or an antral follicle count (AFC) greater than 16. This risk may be mitigated by use of GnRH antagonists rather than agonists for pituitary suppression, coupled with a GnRH agonist trigger instead of hCG to induce final oocyte maturation. There is conflicting evidence about the utility of metformin in PCOS patients during IVF in the prevention of OHSS. A placebo controlled RCT of 120 PCOS patients treated with metformin 500 mg 3 times per day during IVF treatment with a long GnRH agonist protocol until menses or a positive pregnancy test revealed that the relative risk (RR) of OHSS was 0.28 (95% confidence interval [CI] 0.11 to 0.67). The metformin arm also used somewhat more gonadotropins (1350 [range, 950 to 1800] vs. 1275 [range 900 to 1750], P = .018), had fewer non-periovulatory follicles on the day of hCG administration (4.3 [range, 0 to 6] vs. 5.5 [range, 2 to 9], P = .034), and lower estradiol levels at hCG (1951 pg/mL [range, 342 to 4021], vs. 2346 [range, 709 to 4123], P = .29). Implantation rates (41 vs. 31%) and live birth rates (29 vs. 27%) per cycle were not different. In contrast, another RCT of metformin prescribed to PCOS patients during an antagonist cycle, instead of an agonist cycle, showed no reduction in the incidence of moderate to severe OHSS, and surprisingly a lower clinical pregnancy (28.6 vs. 48.7%; P = .02) and live birth rate (27.5 vs. 51.6%; P = .02) per cycle. Other interventions that may reduce the OHSS risk, including coasting, glucocorticoids, dopamine agonists, colloid infusion, GnRH-agonist-only triggers, and elective cryopreservation of all embryos with deferred transfer, are discussed later.
For women with hypothalamic amenorrhea, as with PCOS, treatment with IVF avoids a high risk of multifollicular development with gonadotropins, which is known to lead to a high risk of multiple gestation (see Chapter 30 ).
Uterine Factor
The uterus may be considered abnormal and a contributor to infertility due to many factors, including acquired defects such as fibroids, adenomyosis, polyps, or intrauterine adhesions, and also due to congenital anomalies.
Fibroids
Uterine fibroids are common, and may occur in upward of 50% of all reproductive aged women. The effect of fibroids on fertility, if any, depends on their size and location. Many investigators have reported that submucosal leiomyomas are associated with decreased pregnancy rates with IVF (see Chapter 26 ). In addition, a number of studies suggest that hysteroscopic myomectomy of submucosal myomas improves pregnancy rate with IVF.
The effect of intramural myomas on IVF outcome is less certain. Some investigators found that intramural myomas are associated with decreased pregnancy rates in IVF. In one report, 112 women with intramural myomas (the largest of which had a mean diameter of 2.3 cm) and 322 women without myomas undergoing IVF were prospectively studied. The ongoing pregnancy rate was 15.1% in the women with myomas and 28.3% in the women without myomas ( P < .003). Logistic regression demonstrated that intramural myomas were associated with a reduced OR for pregnancy (OR 0.46, 95% CI 0.24 to 0.88, P < .02) after controlling for the age of the female partner and the number of embryos available for transfer. Other investigators have reported that intramural myomas up to 7 cm in diameter that do not distort the uterine cavity have no appreciable effect on IVF outcome (myoma: n = 141 patients vs. no myoma, n = 406, OR 0.73, 95% CI 0.49 to 1.19, P = .21) after controlling for the age of the female partner. Surrey et al. examined consecutive IVF cycles in 399 women undergoing IVF with and without leiomyomas. They found that the live birth rate was not affected by the presence of intramural leiomyomas, provided that the endometrial cavity was hysteroscopically normal. They did not recommend prophylactic surgical intervention for intramural fibroids.
In contrast, a meta-analysis of 19 observational studies in 6087 IVF cycles suggests that intramural fibroids that do not distort the cavity are associated with decreased live birth rates (RR 0.79, CI 0.70 to 0.88 P < .0001). If there is, indeed, an impact of intramural fibroids on IVF live birth rates, the question remains as to whether myomectomy returns the pregnancy rate to expected levels, or whether the biology of the uterus is different among women who develop intramural fibroids. Additional large-scale studies are needed to determine if intramural myomas reduce IVF success rates and, if so, whether myomectomy is beneficial.
Adenomyosis
Adenomyosis is typically diagnosed pathologically, at the time of hysterectomy. However, diagnosis by either transvaginal ultrasound or magnetic resonance imaging is becoming more acceptable. One study of women less than 39 years undergoing their first GnRH antagonist IVF cycles with good embryo quality compared 38 women with ultrasound-diagnosed adenomyosis to 175 without, and found that the clinical pregnancy rate was significantly lower in the patients with adenomyosis (23.6% vs. 44.6%; P = .017). After adjustment for maternal age and duration of infertility, the difference remained significant (OR 0.417, CI 0.175 to 0.989 P = .047). A meta-analysis of nine studies ( n = 1865) indicated that adenomyosis was associated with lower clinical pregnancy rates following IVF (RR 0.72, 95% CI 0.55 to 0.95) and also higher miscarriage rates (RR 2.21, 95% CI 1.20 to 3.75).
Endometrial Polyps
Endometrial polyps may also decrease live birth rates with IVF. However, studies are inconsistent. If polyps do play a role in miscarriage or in lowering pregnancy rates, this appears to be associated with large polyps 2 cm or more in size. This may be due to the fact that polyps less than 1 cm have been found to regress. Indeed, in a retrospective study of 2993 IVF patients, 60 of whom were found to have a polyp less than 2 cm during their stimulation cycle, there was no difference in clinical pregnancy (43.3% vs. 44.1%, P = .45), miscarriage (10% vs. 9.8%, P = .48), or live birth rates (33.3% vs. 34.3%, P = .44).
Intrauterine Synechiae
Intrauterine adhesions are found in approximately 2% of unscreened infertile patients undergoing a diagnostic hysteroscopy for uterine cavity evaluation prior to initiating their first IVF cycle. Following hysteroscopic adhesiolysis, there are several approaches to reduce the likelihood of adhesion reformation, which in severe cases can occur in 60% of patients. Options include sequential estrogen-progestin therapy to promote reepithelialization, or placement of an intrauterine device or balloon catheter to mechanically stent open the cavity temporarily while the denuded surfaces heal. A meta-analysis of 11 RCTs demonstrated that antiadhesion therapy is effective at reducing the likelihood of recurrent adhesions at second-look hysteroscopy (OR 0.36, 95% CI 0.20 to 0.64, P = .0005; number needed to treat = 9), but no single approach is superior to any other.
Cervical Stenosis
Cervical stenosis can impair effective ET and thus lower pregnancy rates with IVF. Placing a transcervical Malecot catheter after hysteroscopic evaluation in preparation for IVF appears to improve the ease of ET in women with cervical stenosis. Other methods include cervical dilation at the initial visit, use of laminaria, and resection of cervical ridges.
Congenital Defects
Müllerian anomalies appear to be associated with a reduced pregnancy rate in IVF. Only retrospective cohort and case-control studies are available to guide patient counseling, however. In a study of 37 women with müllerian anomalies undergoing their first IVF cycle, including those with in utero diethylstilbestrol exposure, septate uterus, bicornuate uterus, or uterine didelphys, the live birth rate per initiated cycle was 8%, compared with 25% in a control group without müllerian anomalies ( P = .02). In a case-control study that matched women with and without a septate uterus undergoing two consecutive ETs in a 1 : 2 ratio, the presence of an unresected septum was associated with a significantly decreased live birth rate (3/113, 2.7% vs. 49/226, 21.7%; P = .001). Following hysteroscopic septoplasty, the difference in live birth was no longer statistically significant (43/275, 15.6% vs. 115/550, 20.9%).
Decreased Ovarian Reserve
Decreased ovarian reserve is increasingly acknowledged as a major infertility factor, and is likely second only to age as a predictor of IVF delivery rates. As a woman ages, the quantity and quality of her oocytes deteriorate and, as shown in Fig. 31.9 , the proportion of IVF pregnancies that end in miscarriage increases due to increases in oocyte aneuploidy. In addition to the chronologic age of the female partner, the biologic age of the ovary, which is an estimate of the remaining resting follicular pool as determined by ovarian reserve tests, such as cycle day 3 follicle stimulating hormone (FSH), anti-müllerian hormone (AMH), inhibin, and AFC, is also a strong predictor of IVF pregnancy rates. These markers, along with patient age, have been used to standardize the definition of expected poor responders according to the Bologna criteria, in which a patient may be classified as such if at least two of the following three criteria are present: (1) age greater than 40 years; (2) a history of prior poor response to gonadotropins using a conventional stimulation protocol (≤3 oocytes); or (3) AMH below 0.5 to 1.1 ng/mL or AFC less than 5 to 7 follicles.
Cycle Day 3 Follicle-Stimulating Hormone
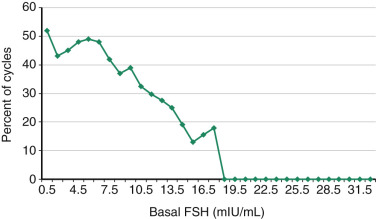
Basal FSH values, when measured in the early follicular phase and interpreted in the context of a paired estradiol value, correlate with both response to gonadotropins and likelihood of pregnancy following IVF. FSH values, in particular when above a threshold of 10 mIU/L, are inversely correlated with peak estradiol and number of oocytes retrieved. This threshold has a high specificity (>80%) for predicting poor response, but a low sensitivity (10% to 30%). A large study of 18,019 IVF cycles (mean patient age 36.2 ± 4.8 years) investigated the relationship between cycle day 3 FSH concentration and IVF delivery rates. FSH levels measured by several types of FSH assays were included, as this reflects actual clinical practice. A threshold between normal and abnormal FSH levels was then assessed. The study showed that no live births occurred in this older population with basal FSH levels of greater than 18.0 mIU/mL, and that between 1 and 7 mIU/mL live birth rates were relatively constant but underwent a decline between 8 and 12 mIU/mL, which was more precipitous beyond 13 mIU/mL ( Fig. 31.10 ). Similar trends were observed for each age group. When the interaction of age and the results of a clomiphene challenge test were examined, in women with a normal clomiphene citrate challenge test response (suggesting an adequate follicular pool), the age of the female partner remained an important prognostic variable. It should be noted that the method used to establish the threshold to separate “normal” from “abnormal” FSH levels greatly affects its predictive ability.
Anti-Müllerian Hormone
AMH is a glycoprotein growth factor synthesized by granulosa cells in preantral and antral follicles. Low AMH values correlate with a decreased response to gonadotropins. Several studies have demonstrated that AMH is inversely correlated with age and, as such, is a useful marker of ovarian reserve. An age-based nomogram of AMH is shown in Fig. 31.11 . Seifer et al. were the first to demonstrate that AMH is significantly reduced in women who responded poorly to ovarian stimulation in IVF cycles. A meta-analysis of 13 studies that evaluated the clinical utility of AMH in predicting IVF response summarized the specificity of a low value as 64% to 100%, with a sensitivity ranging from 40% to 91%, depending upon the threshold used. Importantly, AMH has not been found to be an independent predictor of IVF pregnancy. A multivariate analysis of more than 5000 autologous IVF cycles with an ultra-low AMH value (<0.17 ng/mL) indicated that while cycle cancellation occurred in 54% of all cases and there was a significantly higher rate of having either no embryos for either transfer or cryopreservation, when compared with age-matched controls with normal AMH values, the live birth rate was still 9.5% per cycle start. Accordingly, refusal of treatment based solely on a low AMH value, regardless of the cutoff used, is not encouraged.
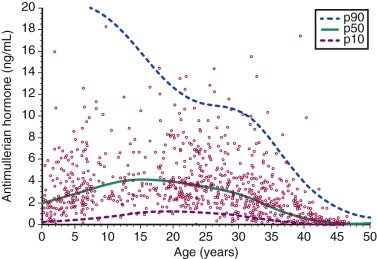
AMH also has the advantages of minimal cycle-to-cycle variation, and its levels do not change significantly across the menstrual cycle.
Inhibin
Inhibin B, a glycoprotein hormone secreted by the granulosa cells, has also been studied as a marker of ovarian reserve. Inhibin B directly inhibits pituitary secretion of FSH in a manner similar to that of estradiol, and thus was thought to be a marker of ovarian health. However, the utility of inhibin B as a marker of ovarian reserve is limited by the lack of a uniform commercial assay and poor performance characteristics of the test.
Antral Follicle Count
The AFC (the total number of follicles <10 mm visualized on transvaginal ultrasound) appears to be a better predictor of ovarian reserve than FSH, and performs similarly to a low AMH value in predicting poor response. Women with fewer than seven total antral follicles have DOR and generally have poorer outcomes in IVF. Also similar to AMH, the AFC has very limited accuracy in predicting IVF pregnancy—rather, it is helpful in choosing stimulation regimens and gonadotropin dosing, along with counseling about the risk of cycle cancellation or overresponse.
Multiple Infertility Factors
Many couples have multiple factors contributing to their low fecundability. In general, the greater the number of infertility factors, the lower the success with IVF treatment. For example, in one study that evaluated the efficacy of IVF among couples with endometriosis as the only identifiable infertility factor, the live birth rate per cycle was 31%. In women with endometriosis and a male partner with an abnormal semen analysis, the live birth rate per cycle was 16%. For women with both endometriosis and tubal disease, the live birth rate per cycle was 8%. In 2014, US data reported to the CDC revealed that 11.2% of cycles involved more than one female infertility diagnosis, and 17.9% involved both male and female infertility factors ; delivery rates per stimulated cycle were 18.7% and 24.9%, respectively. In comparison, the delivery rate for cycles with unexplained infertility, endometriosis, or male factor were, respectively, 32.3%, 29.0%, and 30.5%, whereas those for poorer prognosis categories such as DOR and uterine factor were more similar to cycles with multiple infertility diagnoses (14.6% and 20.6%, respectively).
Preimplantation Genetic Testing
Couples without infertility but who are carriers of monogenic (single gene) defects, chromosomal structural rearrangements, or who are at high risk for aneuploidy are candidates for PGT, the purpose of which is to reduce the likelihood of transferring an affected embryo(s). The testing is referred to as PGT-M for monogenic defects, PGT-SR in cases of structural rearrangements, or PGT-A for aneuploidy testing. Typically, several embryos are required for such testing because (1) the preferred stage for biopsy is the blastocyst (i.e., for trophectoderm biopsy) and not all embryos form blastocysts in vitro; (2) depending on the genetic abnormality being tested, at least 25% of those biopsiable are likely to be unsuitable for transfer (e.g., in cases of recessive conditions); and (3) in cases of concurrent comprehensive chromosomal screening at the same time as testing for single gene mutations, there may be a further reduction in the number of suitable embryos available for transfer.
Notably, PGT with trophectoderm biopsy requires a freeze-all cycle to allow for the genetic testing to be completed before transfer. Patient counseling about these considerations, along with other limitations of PGT, is critical, including the possibility of having no embryos available for biopsy, no normal embryos available for transfer, nondiagnostic results, the possibility of a false-positive or false-negative result, mosaic embryos, as well as having embryos with microduplications or microdeletions of unknown significance. PGT is discussed further later in this chapter and in Chapter 32 .
Overview of In Vitro Fertilization Statistics
- ◆
While live birth rates have increased in recent years, so has the utilization of ICSI for nonmale factor infertility, along with the use of donor oocytes and gestational carriers.
- ◆
Elective single embryo transfer (eSET) is likewise becoming more widely used, which, not unexpectedly, is resulting in a decrease in multiple gestations.
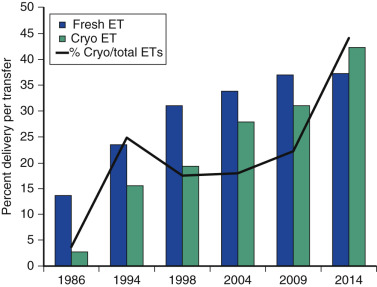
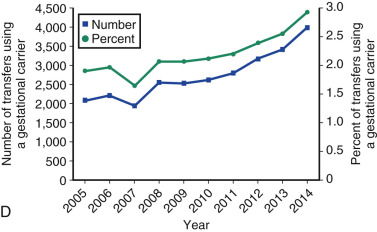
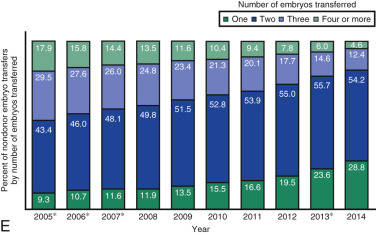
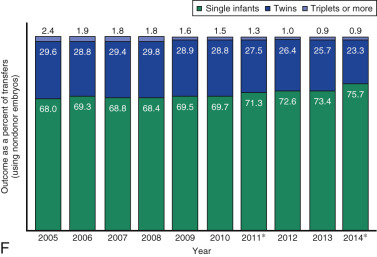
One of the most remarkable features of IVF is the continuous improvement in its efficacy. Over the last 30 years there has been a noteworthy increase in the deliveries per transfer, from less than 15% in 1986 to more than 35% in 2014 ( Fig. 31.12 ). This trend correlates with an increased number of cryopreserved cycles (see Fig. 31.12 ), and likewise an increase in the overall number of IVF cycles performed each year ( Fig. 31.13 ). Other notable trends include increases in the utilization of ICSI for nonmale factor infertility, eSET, and use of donor oocytes or gestational carriers ( Fig. 31.14A–D ). There has also been a decrease in the proportion of cycles with greater than 2 embryos transferred and, correspondingly, a reduction in the number of twin and triplet deliveries (see Fig. 31.14E and F ).
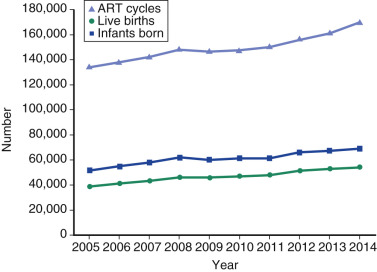
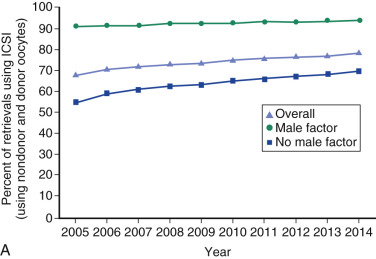
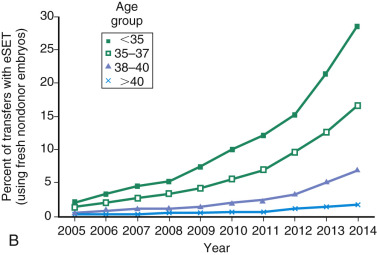
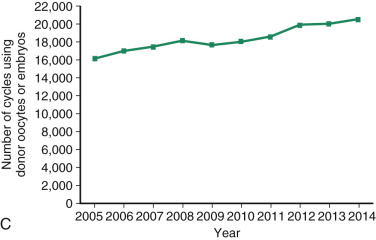
The Fertility Clinic Success Rate and Certification Act of 1992 mandates that all ART clinics in the United States report success rates to the CDC. Data from the most recent ART National Summary Report offer insight into the 458 clinics that provided outcomes to the federal government. In 2014, a total of 208,604 ART cycles were performed nationwide, of which 173,198 cycles (83%) were initiated with the intent to transfer at least one embryo. These 173,198 cycles led to 57,323 live births (an overall success rate of 33.1%), the majority of which (61.8%) were singletons. The remaining 35,406 cycles (17%) were initiated with the intent of short-term (<12 months) or long-term (≥12 months) cryopreservation of all resulting oocytes or embryos for future use (so-called banking cycles). At present, it is not possible to calculate the success rates of these cycles with the available data. Since this report was last published, there have been updated reporting requirements to SART, such that it will be possible to longitudinally track the cumulative pregnancy rates from a given stimulation.
Patient Counseling for In Vitro Fertilization
- ◆
Female age is the most critical predictor of live birth following ART, owing to an increasing burden of oocyte aneuploidy with advancing age.
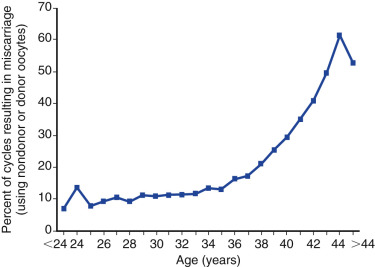
Counseling is critical for patients interested in undergoing IVF, with a particular emphasis being given to age as the most critical predictor of live birth. As shown in Fig. 31.15 from the 2014 ART National Summary Report, the clinical pregnancy and live birth rates decrease predictably with age, with a major inflection point at approximately 38 years with a corresponding increase in the rate of miscarriage (see Fig. 31.9 ). The effect of age on reproductive performance is attributed mostly to the high incidence of aneuploidy in oocytes with increasing age. The frequency of aneuploid embryos as a function of female age is shown in Fig. 31.16 .
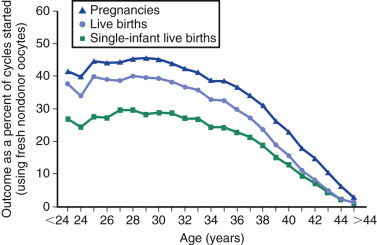
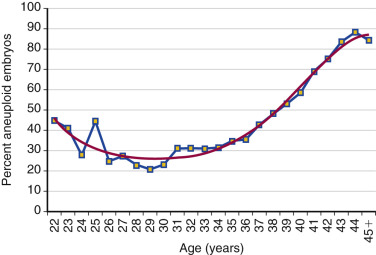
As described previously, ovarian reserve testing is most useful in selection of the stimulation protocol and prediction of responsiveness to gonadotropins, and correlates less with clinical outcomes. Thus the unusual 44-year-old woman with normal ovarian reserve testing may respond to gonadotropins but still has a delivery rate per IVF cycle start of approximately 1% (see Fig. 31.15 ). Other factors that modify the likelihood of success include primary infertility diagnosis and prior history of pregnancy and delivery.
Ovarian Stimulation
- ◆
Natural cycle and mild ovarian stimulation protocols are seldom used in the United States and, to date, have not been shown to offer any advantage over COS.
- ◆
High, normal, and poor responder protocols are available and should be chosen based on patient age, ovarian reserve testing, and prior response to stimulation.
- ◆
While hCG has conventionally been used for final oocyte maturation, in GnRH antagonist cycles, other options exist such as a GnRH-agonist-only or a GnRH-agonist/hCG dual trigger.
Natural cycle IVF and “mild stimulation” IVF are covered in detail in Chapter 30 , and so these are only briefly reviewed as follows.
Natural Cycle In Vitro Fertilization
Natural cycle IVF is possible through careful monitoring of the menstrual cycle with serum hormones and transvaginal sonography, in order to identify the time when the dominant follicle can be aspirated to yield a fertilizable oocyte. Although conceptually appealing, and the way in which the field of clinical ART was indeed born, natural cycle IVF is associated with low pregnancy rates. In one study of 74 such cycles, oocytes were only harvested in approximately 50% of cycles, and the pregnancy rate per cycle initiated was 3%. In another study of 114 natural IVF cycles, the pregnancy rate per cycle initiated was 4%. IVF is a resource-intensive treatment, and pregnancy rates in the range of 3% to 4% per IVF cycle initiated are not cost-effective.
One reason offered by advocates of natural cycle IVF is that oocytes derived from stimulated cycles may have an increased rate of aneuploidy; this concern is not supported by evidence, however. A recent prospective study demonstrated that there was no difference in the aneuploidy rate of blastocysts derived from natural ( n = 147; 44%) versus conventional stimulation cycles ( n = 6664; 42%; P = .81). The delivery rate per euploid transfer was equivalent between natural and stimulated cycles (58.7% vs. 59.0%), but natural cycles were more likely to have no oocytes retrieved, no blastocysts to biopsy or cryopreserve, and no euploid embryos to transfer.
Mild Ovarian Stimulation
“Mild stimulation” regimens, although heterogeneously defined in the literature, have been gaining in popularity (see Chapter 30 ). Such regimens may include the use of clomiphene or aromatase inhibitors with 75 to 150 IU of gonadotropins, but some publications report the total gonadotropin dose to be actually quite high. There are several RCTs to indicate that among both expected good and poor responders, a mild approach with a GnRH antagonist and low-dose gonadotropins may result in pregnancy rates comparable to those achieved using a long GnRH agonist with conventional COS. These studies are limited by the comparison of two different regimens for pituitary suppression (agonist vs. antagonist) and selection of a comparator group that would not typically be recommended for a poor responder (long agonist). Furthermore, the cumulative pregnancy rates of such an approach remain to be determined. In Europe, such treatment has been more widely adopted, perhaps in part due to the fact that many countries have legislation restricting the number of embryos that may be cultured, as well as more restrictive age limits for IVF.
Standard Controlled Ovarian Stimulation
The approach most commonly used in the United States is based on data showing that higher numbers of oocytes have been shown to optimize pregnancy rates at all ages. Consequently, success is critically dependent on generating an adequate number of mature follicles that contain developmentally competent oocytes, while avoiding OHSS. Large retrospective cohort studies suggest that obtaining enough embryos to allow for embryo selection is important in order to maximize pregnancy rates. One study of 7422 women treated with the long GnRH-agonist protocol found that pregnancy rates per initiated cycle were highest when 13 oocytes were obtained (28%). Another study of 400,135 IVF cycles performed between 1991 and 2008 also found that the pregnancy rate per cycle correlated with the number of oocytes retrieved. The median number of oocytes retrieved was nine, with a live birth rate per initiated cycle of 21%. Live birth rates correlated with the number of oocytes retrieved across all age groups, and were maximal when 15 oocytes were obtained, plateaued between 15 and 20 oocytes, and were lower if more than 40 oocytes were retrieved. Therefore the predominant approach to ovarian stimulation for IVF in the United States is to aim for the development of multiple follicles in order to allow for embryo selection, particularly in women at advanced ages (i.e., >38 years) when aneuploidy rates increase and implantation rates concomitantly decrease.
Medications used for ovarian stimulation include clomiphene, clomiphene-hMG; clomiphene-rhFSH (recombinant human FSH); hMG alone; immunopurified (highly purified) urinary human FSH (hpFSH) alone; rhFSH alone, and various combinations of these. Compared with clomiphene alone, the combination of clomiphene plus low-dose human gonadotropins (FSH or hMG) increases the number of follicles stimulated, but the number of oocytes retrieved, and the pregnancy rates are lower than expected with standard stimulation in normal responders. Though such regimens do allow for lower cost stimulations, the live birth rate per ET is low (19%). Therefore, in terms of the expected live birth rate per initiated stimulation and the cumulative live birth rate, these regimens are likely inferior to standard dosing of gonadotropins alone and may not ultimately be cost-effective.
In the following discussion, we focus on COS protocols as used for IVF in the United States. In addition, we consider decision-making in protocol selection.
Normal and High Responder Regimens
Gonadotropin-Releasing Hormone-Agonist Downregulation Protocol
In ovarian stimulation for IVF, the main purpose of a GnRH-agonist analogue or antagonist is to prevent a premature LH surge (triggered by positive feedback from high estradiol), while the follicles are still immature. Accordingly, pituitary suppression reduces premature luteinization of granulosa cells and premature ovulation.
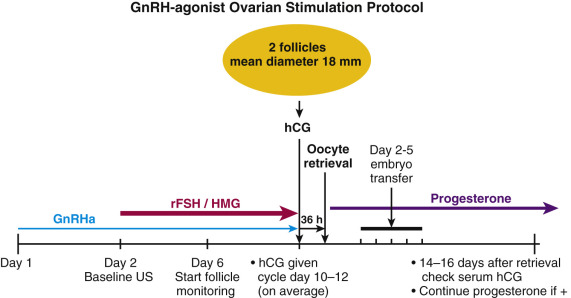
GnRH-agonist analogues differ from the native decapeptide GnRH in amino acid positions 6 and 10. They are resistant to degradation, so they have long half-lives and prolonged receptor occupancy. The initial administration of a GnRH-agonist analogue is associated with an increase in LH and FSH secretion (agonist phase). Long-term administration causes downregulation and partial desensitization of the pituitary GnRH receptor, resulting in the suppression of pituitary gonadotropin secretion. In the standard downregulation protocol, the GnRH agonist (0.5 to 1 mg daily) is begun in the midluteal phase of the prior menstrual cycle, and ovarian stimulation begins with or after the onset of the subsequent menstrual period, at which time the GnRH-agonist dose is decreased by half ( Fig. 31.17 ; Table 31.2 ). Due to the suppressive nature on the pituitary gland of prolonged GnRH-agonist exposure, hCG is required for ovulatory triggering, which has implications for expected high responders (discussed later).
| Indications | Regimen | Trigger Options | Special Considerations | |
|---|---|---|---|---|
| Normal or High Responders | ||||
| GnRH-agonist long protocol | Endometriosis, adenomyosis, poor embryo development on GnRH-antagonist regimen |
|
|
|
| GnRH antagonist | PCOS, expected high responders (e.g., oocyte donors), fertility preservation, random start |
|
|
|
| Poor Responders | ||||
| Luteal estradiol priming | Prior dysnchronous follicular cohort or poor response to another cycle type |
|
|
|
| GnRH-agonist microflare | Considered alternate to priming protocol for low responders |
|
|
|
Compared with no suppression, the addition of a GnRH-agonist analogue to regimens of ovarian stimulation for IVF-ET is associated with a lower cancellation rate, and an increase in the number of oocytes retrieved, the number of embryos available for transfer, and the clinical pregnancy rate. For example, one study demonstrated that treatment with a GnRH agonist (buserelin) plus hMG resulted in more oocytes retrieved (9.3 vs. 6.2), more embryos (4.3 vs. 2.8), and a higher clinical pregnancy rate (20% vs. 14%) than stimulation with clomiphene plus hMG. In another study, comparison of a stimulation regimen using the same medications also demonstrated that buserelin-hMG stimulation resulted in a higher pregnancy rate than that observed with clomiphene-hMG (36% vs. 18%). The type of GnRH-agonist analogue used does not appear to be crucial to obtaining the improved outcomes. Studies with D-Trp6 GnRH-agonist analogue also demonstrate improved pregnancy rates (21% vs. 12%) compared with ovarian stimulation regimens that do not use GnRH-agonists for IVF-ET.
It is important to note that women with normal ovarian reserve typically will produce optimal numbers of oocytes from downregulation regimens, but may be at an increased risk of OHSS compared with a GnRH-antagonist regimen (discussed later). In contrast, women with DOR may not respond even to high-dose gonadotropin treatment after downregulation. In some cases, employing lower doses of GnRH agonists will allow downregulation without excessive suppression of response. An RCT of a half-dose of triptorelin (1.87 mg) adequately suppressed the pituitary, but required a lower dose of FSH (42 ± 2 vs. 59 ± 3 ampules) and more mature oocytes (10.1 ± 0.54 vs. 7.4 ± 0.6), zygotes (8.2 ± 0.4 vs. 6.3 ± 0.4), and embryos (7.8 ± 0.36 vs. 5.9 ± 0.37) were obtained than when the standard 3.75 mg dose was given. There were no significant differences in pregnancy (half-dose: 38.8% vs. full-dose: 25.3%), implantation (22.6 vs. 13.8%), or miscarriage (6.1 vs. 5.0%) rates, though a trend for improved outcomes with the lower dose agonist was observed. Furthermore, the cumulative pregnancy rate was significantly higher in the lower dose agonist group (56.8% vs. 35.4%). Variations of the full-dose (1 to 0.5 mg) or low-dose (0.5 to 0.25 mg/d) long-luteal protocol include a very low dose (0.2 to 0.1 mg/d), an ultra-low dose (0.1 to 0.05 mg/d), and a pseudoluteal start overlapping with oral contraceptive pills (OCPs).
Gonadotropin-Releasing Hormone-Antagonist Protocol
A theoretical problem with the GnRH-agonist analogues is that LH secretion is stimulated at the initiation of treatment, which is not consistent with the physiology of the normal early menstrual cycle. GnRH antagonists offer the possibility of acutely suppressing LH secretion without an initial increase in LH release. Thus pituitary LH secretion can be controlled within the stimulation cycle itself; such control is not possible with the GnRH agonists, which must be begun during the previous menstrual cycle to achieve full downregulation. GnRH antagonists have extensive amino acid substitutions compared with native GnRH at positions 1 to 3, 6, occasionally 8, and 10. They act as competitive inhibitors of the GnRH receptor, such that they can off-compete endogenous GnRH, leading to an acute decrease in gonadotropin secretion within 24 hours. GnRH antagonists are typically administered as a small daily dose (cetrorelix or ganirelix, 0.25 mg daily by subcutaneous injection), usually starting on cycle day 6 to 8 or when the lead follicle reaches 14 mm in diameter or as a single large dose (cetrorelix, 3 mg subcutaneously, which has a 4-day duration of action) on approximately cycle day 8 ( Fig. 31.18 ; see Table 31.2 ). Both protocols block a spontaneous LH surge.
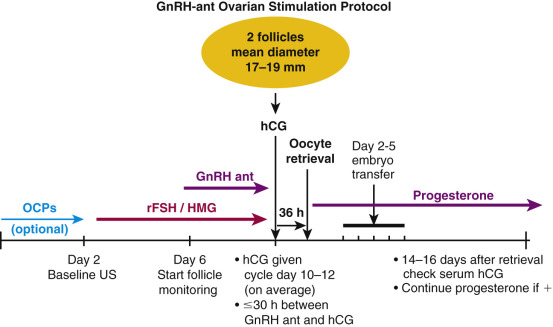
The impact of a GnRH antagonist on ovarian stimulation for IVF is dependent on the dose of GnRH antagonist administered. At small doses, the suppression of LH is minimal. With large doses, near complete suppression of LH is achieved. In one study, the impact of six doses of the GnRH-antagonist ganirelix on LH secretion and IVF outcomes was studied. A fixed daily dose of recombinant FSH was administered. Ganirelix produced a dose-dependent suppression not only of LH, but also of serum androstenedione and estradiol, with the maximal pregnancy rate per cycle with a dose of ganirelix 0.25 mg.
The use of oral contraceptives prior to GnRH-antagonist cycles is common in order to have some control over the start of cycles. Initial studies that led to FDA approval used oral contraceptives for approximately 3 weeks prior to stimulation. A three-arm RCT comparing OCP pre-treatment in GnRH antagonist ( n = 110) versus agonist ( n = 111) versus GnRH antagonist without OCP treatment ( n = 111) showed that the OCP-scheduled antagonist regimen appeared more similar to the agonist regimen rather than the no-OCP GnRH-antagonist regimen. Suppression following OCP use was more profound at the start of stimulation ( P ≤ .001), as manifested by slower follicular growth ( P ≤ .001), longer stimulation (11.7 and 10.3 vs. 9.4 days, respectively; P ≤ .001), and more recombinant (r)FSH utilized (2667 and 2222 IU vs. 1966 IU; P ≤ .001). In the three groups, both the number of oocytes (13.1, 12.9, and 11.5, respectively) and the number of good-quality embryos (5.1, 5.7, and 5.0, respectively) were similar. One meta-analysis evaluated four RCTs in 847 patients and found no difference in ongoing pregnancy rates, though duration of stimulation and gonadotropin requirements were higher after OCP pretreatment. In clinical practice, 7 to 21 days of OCPs are often prescribed to patients prior to a GnRH-antagonist cycle. Not all patients have a withdrawal bleed after oral contraceptives, so a baseline ultrasound is performed on day 2 of the menstrual period following pill cessation, or 4 days after the last pill, as the vast majority of women will have had a withdrawal bleed by that point.
One important variation is the random start GnRH-antagonist cycle, in which gonadotropins are initiated on any day of the menstrual cycle with or without a simultaneous GnRH antagonist to promote luteolysis, as needed. If the GnRH antagonist is not started at the same time as the gonadotropins, it is added at an appropriate time based on serum estradiol and lead follicle diameter to prevent the LH surge. This protocol is typically only used for fertility preservation cases in which there is a narrow time window prior to the planned gonadotoxic treatment. Preliminary data comparing the random ( n = 35) versus conventional ( n = 93) GnRH antagonist starts demonstrate an equivalent yield of total and mature oocytes and similar fertilization rates. Little is known about subsequent embryo development or pregnancy rates following a random start.
Another variation is luteal priming with oral or transdermal estradiol in an effort to promote follicular synchronization and endogenous FSH flare in the early follicular phase, as described in the section that follows on poor responders.
GnRH antagonists have several advantages compared with GnRH-agonist protocols in terms of number of days requiring injectable medication, total gonadotropin dose, risk of OHSS, trigger options for final oocyte maturation, and the effect on endometrial receptivity, as outlined in the following section. In contemporary IVF practice, GnRH-antagonist protocols are now considered the protocol of choice for many patients undergoing their first IVF cycles with an expected normal or high response.
Gonadotropin-Releasing Hormone-Agonist Versus Gonadotropin-Releasing Hormone-Antagonist Protocols
Most studies of IVF have demonstrated that both GnRH agonists in a downregulation protocol and GnRH antagonists are associated with similar rates of cycle cancellation due to a premature LH surge. A 2016 Cochrane review of 73 RCTs ( n = 12,212) comparing the long GnRH-agonist with GnRH-antagonist protocol in women with normal ovarian reserve found that with GnRH antagonists, the cancellation rate due to overresponse was lower (OR 0.47, 95% CI 0.32 to 0.69; 19 RCTs, n = 4256), but the cancellation rate due to poor response was higher (OR 1.32, 95% CI 1.06 to 1.65; 25 RCTs, n = 5230). Importantly, there was no evidence of a statistically significant difference in live birth rates (OR 1.02, 95% CI 0.85 to 1.23; 12 RCTs, n = 2303) or miscarriage (OR 1.04, 95% CI 0.82 to 1.30; 33 RCTs, n = 7022).
GnRH antagonists are often regarded as a more patient-friendly approach, as well, due to the fewer days of analogue administration (5.7 ± 2.3 vs. 25.6 ± 7.6 d; P < .001), shorter stimulation (10.6 ± 2.3 vs. 11.4 ± 1.8 d; P < .01), and less total gonadotropin usage (23.6 ± 8.5 vs. 25.6 ± 7.6 ampules hMG; P < .01).
Furthermore, in a meta-analysis of 36 RCTs ( n = 7944), there was nearly a 40% reduction in the incidence of OHSS in the GnRH-antagonist group (OR 0.61, 95% CI 0.51 to 0.72). The trigger options for final oocyte maturation are also more flexible with GnRH antagonists, including hCG only, GnRH-agonist-only, and dual hCG/GnRH agonist. As described later, these GnRH-agonist triggering options provide clinicians with the opportunity to further minimize the risk of OHSS in high responders who are planning to undergo a freeze-all cycle or a fresh transfer.
Finally, endometrial receptivity may be more physiologic in GnRH-antagonist cycles. An analysis of paired endometrial biopsies from the same patients undergoing a natural cycle versus a GnRH agonist or GnRH antagonist normalized to the LH surge indicated that the microarray signature following GnRH-antagonist exposure more closely recapitulates that of the unstimulated cycle. For example, in GnRH-agonist cycles, only 15% of genes tested showed a similar pattern of differential expression as compared with an unstimulated cycle; specifically, genes involved in cell cycle expression, such as cyclins, CDC, CDK, and members of the E2F family of transcription factors, were downregulated, while genes normally upregulated in natural cycles, including ones involved in TGF-β signaling , the complement pathway, coagulation cascade, and leukocyte migration, failed to be upregulated in GnRH-agonist cycles. In contrast, expression of 43% of these genes was shared in common between the GnRH-antagonist and unstimulated cycle.
Poor Responder Protocols
Estradiol Priming Gonadotropin-Releasing Hormone-Antagonist Protocol
A variation of GnRH-antagonist protocol utilizes a luteal estradiol patch or oral estradiol (e.g., 0.3 mg patches changed qod or 4 mg oral micronized estradiol daily), followed by GnRH-antagonist suppression ( Fig. 31.19 ; see Table 31.2 ). Following a menstrual period, gonadotropin stimulation with GnRH-antagonist suppression is utilized. The hypothetical advantage of this approach is to improve the synchrony of the follicular cohort by reducing antral follicle sizes and follicular phase heterogeneity. Once discontinued with menses or at the time of gonadotropin start, the pituitary is likewise relieved from negative inhibition, which may stimulate an increase in endogenous FSH secretion, making this protocol best suited for use in poor responders. In one report, patients using the “patch” protocol had a lower cycle cancellation rate, more oocytes retrieved, and more embryos available for transfer than in a prior cycle. A meta-analysis evaluating GnRH-antagonist cycles with and without luteal estrogen pretreatment found that more oocytes were retrieved after pretreatment.
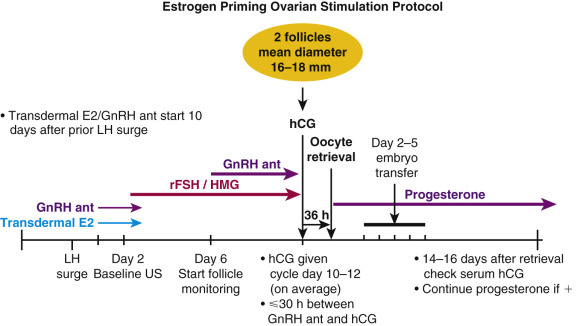
Gonadotropin-Releasing Hormone-Agonist (Micro)Flare Protocol
In the downregulation protocol, GnRH-agonist analogues are usually started in the luteal phase of the cycle preceding the IVF-ET cycle (long protocol). In the GnRH-agonist flare regimen, the GnRH agonist is begun in the early follicular phase of the IVF-ET cycle, typically between cycle days 1 and 3. The flare regimens take advantage of both the agonist and downregulation properties of GnRH-agonist analogues. In the first few days of treatment, during the early follicular phase, the GnRH agonist stimulates endogenous LH and FSH secretion from the pituitary. With continued administration of the GnRH agonist, the downregulation effects of the analogue begin, and premature LH surges are prevented.
The increased LH secretion during the follicular flare stimulates more thecal androstenedione production, which increases the substrate available for aromatization in granulosa cells. However, the increased secretion of both LH and ovarian androstenedione may not be optimal, as some studies suggest that elevated circulating LH and intrafollicular androgen levels are not associated with optimal oocyte function. In one study of women treated with a GnRH-agonist downregulation or a GnRH-agonist flare regimen, follicular fluid androstenedione concentrations were 27.0 ng/mL versus 57.3 ng/mL ( P < .05), respectively. The elevated androstenedione results in higher estradiol levels, and the increased FSH secretion may increase follicular recruitment. Rescue of a corpus luteum and premature luteinization of follicles can occur, though, which may lead to clinically significant elevations of progesterone during the cycle. This effect may be mitigated by a short OCP lead-in.
In one study, women with a previous poor response to standard long-protocol GnRH-agonist downregulation plus gonadotropin stimulation were treated with a flare regimen using microdose leuprolide acetate (20 µg every 12 hours), initiated in the early follicular phase of the treatment cycle, plus gonadotropin. Compared with a previous cycle of downregulation, microdose GnRH agonist plus gonadotropin resulted in greater ovarian stimulation, higher serum estradiol concentration, and increased numbers of follicles and oocytes retrieved. It should be noted, however, that women with normal ovarian reserve have higher cycle cancellation rates and may have lower pregnancy rates with a flare, as compared with downregulation protocol. In one large cohort study of women under 40 with normal baseline FSH levels, the GnRH-agonist flare regimen was associated with a lower delivery rate per cycle compared with the GnRH-agonist downregulation regimen (15.1% vs. 21.3%, P < .05). Other investigators have reported similar trends. The GnRH-agonist flare should not be the first choice ovarian stimulation regimen in women undergoing their first cycle of IVF or those who have a good prognosis; rather, flare protocols should be reserved for expected poor responders who are unlikely to respond well to overly suppressive downregulation protocols. Other disadvantages of a flare protocol include decreased flexibility in scheduling (unless preceded by an OCP lead-in), possible rescue of a corpus luteum or premature luteinization of granulosa cells resulting in clinically significant elevations in the serum progesterone level, and the required use of hCG for final oocyte maturation.
The “microdose” flare protocol uses diluted or low-dose GnRH agonist, typically started on day 1 or 2 of the menstrual cycle ( Fig. 31.20 ; see Table 31.2 ). The goal is to provide stimulation with enough suppression so as to avoid a spontaneous LH surge, but not to suppress endogenous LH and FSH release so that ovarian stimulation is augmented by pituitary gonadotropins. The protocol is often used with OCP pretreatment starting day 1 to 2 of menses to prevent rescue of a corpus luteum with the attendant rise in follicular progesterone levels. An RCT of 356 poor responders undergoing stimulation with a microflare protocol evaluated the optimal dose of gonadotropins to prescribe (450 vs. 600 IU of split hMG/FSH). Interestingly, there were no significant differences in the cancellation rates (26.3% vs. 19.4%), number of mature oocytes retrieved (4 [range 0 to 6] vs. 4 [range 2 to 7]), fertilization rate (62.4% vs. 57.0%), or implantation rate (29.8% vs. 30.4%). Accordingly, some programs advocate that the maximum dose of gonadotropin that should be prescribed per day is 450 IU, as higher doses have not been shown to be more effective and only result in additional medication cost.
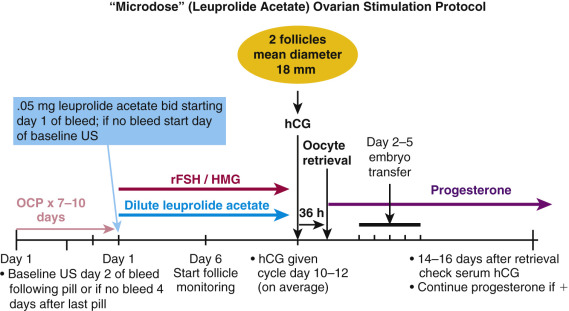
The microdose leuprolide acetate protocol has been compared with the estrogen priming protocol in RCTs of poor responders, with no difference in clinical or live birth rates reported. In one larger trial of 116 cycles, the estrogen priming protocol used higher doses of gonadotropins (3247.8 ± 634.6 vs. 2994.8 ± 611 IU), yet similar numbers of oocytes were retrieved and fertilized. There was no significant difference between the two groups in terms of the implantation rates (9.8% vs. 7.9 %) or the clinical pregnancy rates per transfer (16.3% vs. 15.6 %).
Adjunctive Medications for the Poor Responder
Several supplements, including dihydroepiandrosterone (DHEA), coenzyme Q10 (coQ10), and growth hormone (GH), have been studied as adjunctive medications to help improve follicular development in poor responders. To date, there are some encouraging preliminary findings, but most available studies are limited by small sample size and methodologic flaws. Accordingly, patients proceeding with any of the treatments outlined as follows need to be counseled appropriately.
Dihydroepiandrosterone Supplementation
DHEA, an androgen secreted by both ovaries and the adrenal glands, has been studied as an adjunct to gonadotropins to improve ovarian stimulation in poor responders. The mechanism underlying a potential beneficial effect is unclear. DHEA, via conversion from DHEAS, is responsible for 48% of follicular fluid testosterone synthesis during gonadotropin stimulation. DHEA may also increase ovarian IGF-1 expression and induce FSH receptor upregulation, amplifying gonadotropin action and the follicular microenvironment. One study supplemented women with DOR with DHEA 25 mg orally 3 times per day for 30 to 90 days, and found that serum AMH levels improved by approximately 60% during treatment, particularly in women less than 38 years; women who conceived with IVF also showed greater AMH improvements.
A 2015 Cochrane review of eight RCTs ( n = 878) comparing poor responders treated with DHEA versus placebo or no treatment demonstrated that use of DHEA was associated with higher rates of ongoing pregnancy or live birth (OR 1.88, 95% CI 1.30 to 2.71). A sensitivity analysis was conducted to remove trials at high risk of performance bias, and the effect size was decreased and no longer was statistically significant (OR 1.50, 95% CI 0.88 to 2.56; five RCTs, n = 306). Large, appropriately powered studies in well-defined patient populations are needed to confirm a benefit, and define proper dose and duration of treatment. Patients with DOR should not be counseled to delay IVF to allow the often recommended 1- to 3-month pretreatment with DHEA.
Clinically, it is relevant to note that patients on supplemental DHEA may have spuriously elevated progesterone levels, due to DHEA-S interference with the standard progesterone immunoassay. These artifactual progesterone elevations may reach thresholds shown to be detrimental in fresh IVF cycles, and as a result may be misinterpreted, leading to misguided changes in clinical management. Caution should be used in interpreting serum progesterone levels among patients on DHEA supplementation.
Coenzyme Q10
CoQ10, also known as ubiquinone, is a lipid-soluble antioxidant in the inner mitochondrial membrane that functions as an electron carrier in oxidative phosphorylation. As a critical co-factor in the generation of cellular ATP and the maintenance of proper redox homeostasis, coQ10 has been implicated in the aging oocyte. Indeed, there is a gradual and age-related decrease in tissue levels of coQ10, and in an aged mouse model, expression of key enzymes involved in coQ10 biosynthesis is decreased in cumulus cells. Treatment of these mice with coQ10 increased the oocyte yield following superovulation and likewise increased litter size. While IVF patients often ask about the role of coQ10 in human reproduction, at present there are no class I data to support coQ10 supplementation in clinical IVF.
Growth Hormone Supplementation
Off-label GH use has been studied for many years as an adjunct to gonadotropins in an effort to improve ovarian response. Evidence indicates that GH directly stimulates the development of small antral follicles to gonadotropin-dependent stages, as well as promotes maturation of oocytes. GH stimulates IGF-I. Though IGF-I has no apparent effect on primordial follicle development, both IGF-I and IGF-II stimulate growth of secondary follicles, and stimulate granulosa cell proliferation and steroidogenesis. The coadministration of GH with gonadotropins to anovulatory hypogonadotropic women reduces the gonadotropin dose required to achieve ovulation.
The literature regarding use of GH in poor responders suggests an improvement in response and live birth rates; however, the definition of poor response, the protocols employed in many of these studies, and the dose of GH vary widely. A 2010 Cochrane review included 10 RCTs of 440 subfertile couples. Meta-analysis demonstrated a statistically significant improvement in both clinical pregnancy rates (OR 3.28, 95% CI 1.74 to 6.20) and live birth rates (OR 5.39, 95% CI 1.89 to 15.35), with adjuvant use of GH among poor responders. However, the authors caution that the included studies are small and heterogeneous, so it is still unclear what optimal dose of GH to use and which subgroups of poor responders will, in fact, benefit from cotreatment.
A 2016 RCT randomized 141 patients who met Bologna criteria for poor response to a GnRH-antagonist protocol with or without GH supplementation. Users of GH required significantly less gonadotropin (3900 ± 839 vs. 4906 ± 1481 IU; P < .001), had shorter stimulations (10.8 ± 1.5 vs. 12.0 ± 1.5 days; P < .001), and a higher total and mature oocyte yield (4.5 ± 1.3 vs. 2.5 ± 1.2; P < .001). There was no difference in cancellation rate (16.1 vs. 19.2%; P = .64), but a trend toward improved fertilization (54.0 vs. 49.4%; P = .05), and a nonsignificantly higher clinical pregnancy rate per imitated cycle in the GH group (15/68, 22.1% vs. 11/73, 15.1%; P = .29).
Long-term effects of brief exposure to GH during IVF stimulation, and its cost efficacy, have not been studied.
Cycle Monitoring in Fresh In Vitro Fertilization Cycles
Specifics of routine cycle monitoring vary from program to program, but the general approach for all cycles includes the baseline exam and in-cycle and postcycle monitoring.
In GnRH-agonist cycles, documentation of pituitary downregulation is performed by assessing serum estradiol and progesterone levels after menses has begun; levels should be suppressed with, depending on the assays used, estradiol levels typically less than 50 pg/mL and progesterone less than 1.0 ng/mL. A baseline estradiol is also performed to rule out any functional ovarian cysts that could grow in response to treatment and impair the response to stimulation. Simple ovarian cysts need not necessarily prevent the start of stimulation, but persistent, complex cysts greater than 3 cm warrant surgical treatment prior to ovarian stimulation, unless the patient is known to have endometriosis and the cyst has a typical endometriotic cyst appearance.
As the pituitary is not suppressed at the start of microflare or GnRH-antagonist cycles, only a baseline ultrasound is strictly needed, but it is not uncommon to check a baseline estradiol and progesterone to ensure the patient is, indeed, in the follicular phase. For antagonist cycles with planned GnRH-only trigger, a baseline LH is helpful in predicting an adequate response to trigger; LH values less than 0.1 mIU/mL, as seen occasionally with long-term OCP use, are significantly associated with suboptimal response.
After the first 3 to 4 days of gonadotropin stimulation, serial measurements of serum estradiol and ultrasound examinations are performed to assess follicular development and to allow dose adjustments, as needed. It has also become common practice to follow serum progesterone levels during stimulation, as progesterone elevation on the day of trigger has been associated with decreased live birth rates in several cohort studies. Parameters associated with progesterone elevation include higher follicle count and the exogenous LH:FSH ratio, with a “sweet-spot” of 0.3 to 0.6 LH:FSH. In a meta-analysis of 63 eligible studies ( n = 55,199 fresh IVF cycles), late progesterone elevation was negatively associated with ongoing pregnancy or live birth; when compared with cycles with progesterone of 0.4 to 0.6 ng/mL, modest elevations to even 0.8 to 1.1 ng/mL may be detrimental (OR 0.72; 95% CI 0.56 to 0.94). The clinically relevant threshold for progesterone elevation will depend on the laboratory, but based on available data, cycles with progesterone greater than 2 ng/mL at trigger may have better pregnancy rates with a freeze-all and deferred ET.
Programs typically trigger patients when at least 1 to 2 follicles have a mean diameter of 18 mm. Mature oocytes, however, are often obtained from smaller follicles as well. In a prospective study that assessed oocyte maturity as a function of follicle size, with a flushing step between aspirated follicles of different size, the proportion of mature oocytes was inversely associated with follicle size at retrieval: greater than 18 mm ( n = 890, 89.9%), 16 to 18 mm ( n = 367, 78.7%), 13 to 15 mm ( n = 236, 72.9%), 10 to 12 mm ( n = 100, 53.0%), and less than 10 mm ( n = 34, 47.6%; P < .05). Posttrigger monitoring of urine or serum hCG, or serum LH following GnRH-agonist-only trigger, on the surge +1 day can ensure that the patient administered and responded to the trigger appropriately before proceeding to retrieval.
Following a fresh ET, monitoring requirements are minimal and typically include only a serum hCG approximately 14 to 16 days postretrieval to determine whether or not implantation occurred.
Final Follicle Maturation
There are several trigger options available for inducing final follicle maturation: urinary and recombinant hCG, recombinant LH, GnRH-agonist-only, dual hCG/GnRH agonist, and kisspeptin. Some options (recombinant LH and kisspeptin), although not available for clinical use in the United States, are included here for completeness.
In an unstimulated cycle, the midcycle LH surge induces resumption of meiosis I in the oocyte and promotes the GV-stage oocyte to progress to metaphase II. Accordingly, all trigger options use LH, exploit the structural homology between LH and hCG, or directly or indirectly stimulate endogenous LH secretion. The prescribed time from trigger to oocyte retrieval is a standard 36 hours, which is based upon the length of time from initiation of the LH surge to metaphase II arrest.
Human Chorionic Gonadotropin Trigger
Prior to the availability of recombinant hormones, the most accessible source of an LH-like analogue was urinary hCG (uhCG). The urinary preparation is extracted from pregnant women’s urine, so the source is variable and there may be batch-to-batch variation in bioactivity. It may be administered by an intramuscular or subcutaneous injection, and when used without a co-trigger like GnRH, clinically used doses range from 2000 to 10,000 U.
Approximately 15 years ago, recombinant hCG (rhCG) became commercially available. It is manufactured from transfected Chinese hamster ovary cells and purified by immunoaffinity chromatography. rhCG is administered by subcutaneous injection in either a 250 or 500 µg dose (with 250 µg comparable to 5000 U uhCG). Either can be used in any type of IVF protocol (long GnRH agonist, microflare, or GnRH antagonist).
A 2016 meta-analysis compared clinical outcomes following final follicle maturation with uhCG, rhCG, or recombinant LH (rLH). The ongoing pregnancy/live birth rates were similar between uhCG and rHCG (OR 1.15, 95% CI 0.89 to 1.49; 7 RCTs, n = 1136) and also between uhCG and rLH (OR 0.95, 95% CI 0.51 to 1.78, 2 RCTs, n = 289). There were no differences in rates of OHSS.
Gonadotropin-Releasing Hormone-Agonist-Only Trigger
When a GnRH-antagonist protocol is used, other trigger options may be prescribed, including a GnRH agonist to stimulate an endogenous surge of LH from the pituitary. Importantly, patients already on a GnRH-agonist-based protocol (long GnRH or microflare), or those with profoundly suppressed LH due to prolonged OCP use, for example, will not respond to a GnRH-agonist trigger. Given the short half-life of LH (~20 minutes) and the fact that patients given a GnRH-agonist-only trigger are never exposed to hCG following stimulation, the risk of OHSS is all but eliminated. In a 2014 meta-analysis, GnRH-agonist-only triggers resulted in an 85% reduction in the incidence of mild, moderate, or severe OHSS compared with hCG (OR 0.15, 95% CI 0.05 to 0.47; eight RCTs, 989 women). Accordingly, this has become an ideal option for oocyte donors and other high-responders, like patients with PCOS.
Physiologically, the natural LH surge resembles more closely the one induced by a GnRH-agonist trigger, due to the concurrent release of pituitary FSH. The significance of this midcycle FSH surge is not fully understood, but it has been shown to induce LH receptor expression in luteinizing granulosa cells, and promote meiotic resumption and cumulus expansion. These pleiotropic effects are supported by the findings from several RCTs that the yield of mature oocytes following a GnRH-agonist trigger is higher, as compared with using hCG as the trigger.
An important drawback to the GnRH-agonist-only trigger, though, is the resulting luteal phase deficiency, unless rescued by a bolus of hCG or intensive progesterone and estradiol support (discussed later). As a result, use of the GnRH-agonist-only trigger is restricted to oocyte donors and those undergoing a planned freeze-all cycle.
Dual Human Chorionic Gonadotropin/Gonadotropin-Releasing Hormone-Agonist Trigger
The first RCT comparing a GnRH-agonist-only trigger to an hCG trigger was terminated prematurely due to a 79% early pregnancy loss in the experimental arm, despite conventional luteal phase support. A subsequent RCT of 302 patients demonstrated that adding a low-dose bolus of hCG (1500 U) to the GnRH-agonist trigger rescued the luteal phase in these cycles, and resulted in delivery rates comparable to hCG 10,000 U. Thus the prolonged half-life of hCG (>24 hours) has a luteotropic effect and maintains progesterone secretion from the corpus luteum, allowing transfer of embryos in a stimulated cycle without sacrificing the pregnancy rates. Supplemental hCG, even in a low-dose bolus, though, can still lead to early-onset OHSS. In one retrospective study, the rate of OHSS was 8.6% with the dual-trigger ( n = 66), and 0% with GnRH agonist alone ( n = 108; P < .01).
Clinical protocols vary, with the dose of leuprolide ranging from 1 to 4 mg (given once, or in a divided dose every 12 hours × two doses) and the dose of hCG ranging from 1000 to 2500 U. The relative efficacy of these regimens has not been evaluated.
Kisspeptin Trigger
Kisspeptin is a peptide hormone that acts upstream of GnRH. Proteolytic cleavage of kisspeptin gives rise to kisspeptin-54, which binds a G-protein coupled receptor, Kiss1R, in the arcuate nucleus of the hypothalamus and stimulates pulsatile GnRH release. Limited clinical data exist, but a preliminary phase 2 study of a single subcutaneous injection of kisspeptin-54 to induce final follicle maturation in a group of high-responders demonstrated proof of concept that this also could be used as a trigger option to reduce the risk of OHSS.
Oocyte Retrieval
- ◆
Vaginal oocyte retrieval is the mainstay for oocyte extraction prior to ART, and it is typically performed 36 hours after administration of the preovulatory trigger.
- ◆
Oocyte nuclear maturity is determined by absence of the GV and presence of the first polar body. Other than the ability to support development of a viable embryo, there is currently no definitive marker for oocyte cytoplasmic maturity.
- ◆
In vitro maturation (IVM) of immature oocytes is technically possible, but the technique has not been optimized for routine use in the clinical IVF laboratory.
Oocyte retrieval is typically performed approximately 36 hours after trigger by a transvaginal ultrasound-guided technique, using a 16 to 17G needle under intravenous anesthesia. Reports of oocytes in the human fallopian tube after hCG administration suggest that a time interval between hCG administration and oocyte retrieval of about 36 hours maximizes oocyte maturation and minimizes the chance of spontaneous ovulation ( Fig. 31.21 ). Before 30 hours, oocytes in the lead follicles may not be mature. After 38 hours following hCG administration, ovulation begins and oocytes may be lost into the peritoneal cavity. The number of oocytes retrieved is largely dependent on the number of large follicles present at the time of retrieval. In general, oocytes can be harvested with a high rate of success from follicles with a mean diameter greater than 12 mm ( Fig. 31.22 ).
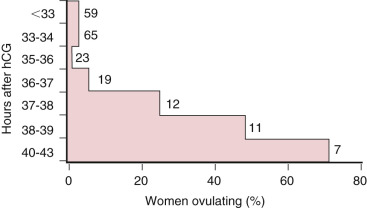
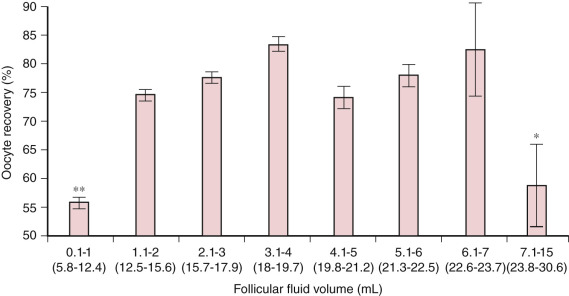
Preparation of the vagina with saline, or antibacterial agents followed by copious saline irrigation, is performed prior to retrieval. Prophylactic intravenous antibiotics are often administered, though there are no RCTs proving benefit. The ovaries of some patients may not be accessible via a transvaginal route; clinically, this may be encountered in patients with severe obesity, large uterine fibroids, müllerian anomalies, or prior oophoropexy. In these cases retrieval can be safely performed with transabdominal ultrasound guidance, or as in the early years of IVF, via a laparoscopic approach. Regardless of the route of oocyte retrieval, traps containing follicular fluid with HEPES-buffered media are passed off to the embryology laboratory for isolation of the cumulus oophorus complexes.
Assessing Oocyte Quality
Ideally, both nuclear and cytoplasmic maturation should be assessed, because both processes are required for an oocyte to fertilize successfully and support early embryonic development. Typically only 70% to 80% of retrieved oocytes have achieved nuclear maturation (i.e., have emitted the first polar body and are in metaphase II); the remaining 20% to 30% are at either prophase I or metaphase I. Following hyaluronidase stripping of the cumulus and corona radiata cells prior to ICSI, the nuclear maturity of an oocyte is readily apparent by the presence of a single polar body and the absence of the GV. For conventional IVF, nuclear maturity of the oocyte is ascertained indirectly at the fertilization check by demonstrating successful fertilization with two pronuclei and two polar bodies present. As no overt morphologic changes in the oocyte have yet been identified that are specific to cytoplasmic maturation, the proportion of retrieved oocytes that are cytoplasmically mature remains unknown. However, this proportion is likely quite small since, even after consideration of the multiple factors involved in formation of a viable healthy embryo, the overall efficiency of mature oocyte to liveborn in ART is only about 5%.
Current techniques for assessing oocyte quality fall into the two major categories: invasive and noninvasive. The invasive approach involves polar body biopsy and PGT-A, which enables, by extrapolation, determination of the oocyte chromosomal complement, as well as insight into the oocyte transcriptome. Noninvasive assessment continues to rely on visual inspection of the morphologic appearance of the surrounding cumulus oophorus with a light microscope. During the periovulatory period in response to the midcycle surge of LH or exogenous hCG, the cumulus cells secrete glycosaminoglycans, resulting in expansion of the cumulus oophorus, and a radiating appearance of the inner corona radiata cells. Although the overall disposition of the surrounding cumulus-corona cells may thus provide some insight regarding the quality of the enclosed oocyte, this association is not perfect. Nuclear maturity and emission of the first polar body is most accurately determined by visualization of the oocyte itself (see Fig. 31.2C ).
The imperfect prediction of oocyte maturity by morphologic assessment of the cumulus-corona radiata cells has led to the development of alternative noninvasive approaches to assess oocyte quality. The use of polarized light to visualize the size and shape of the meiotic spindle, and the thickness of the inner zona pellucida layer, have been correlated with outcome. However, because of the cost of the polarizing system and the sensitivity of the results to oocyte positioning, it is unlikely that this approach will ever be routinely adopted in the IVF laboratory.
Alternatively, genomic approaches are also being applied. Expression of genes specifically associated with cumulus expansion such as PTGS2 , HAS2 , and GREM1 , VCAN , and RPS6KA2 , as well as those involved in calcium signaling pathways, have all been found to have some predictive value. Other genes have been associated with oocyte meiotic stages and specific maturation conditions, and cumulus cell gene expression has been used in multivariable models to predict embryo quality and implantation. Furthermore, membrana granulosa cell genes involved in the luteinization process have been associated with oocyte quality, and a correlation has been shown between mature (as opposed to propeptide) forms of GDF9 in follicular fluid and oocyte nuclear maturation and embryo quality.
Although these molecular studies provide insight into the differential expression of biomarkers associated with developmentally competent versus incompetent oocytes, much work remains to be done to identify a gene network algorithm that can be applied clinically for identification of oocytes that have acquired both nuclear and cytoplasmic competence. Moreover, if ever established clinically, the laboratory protocols will need to be streamlined to ensure that each oocyte is individually tracked with fidelity so that its association with corresponding cumulus and/or granulosa cells is ensured.
In Vitro Oocyte Maturation
An alternate approach to the retrieval of mature oocytes following ovarian stimulation is the aspiration of immature oocytes and their subsequent maturation in vitro. In the setting of IVM, GV-stage oocytes are retrieved when the follicles reach a diameter of 6 to 12 mm, either with or without hCG priming. Compared with no priming with hCG, priming may improve maturation rates following culture ; however, such an approach should not strictly be referred to as IVM because the oocytes may have begun the maturation process in vivo under the influence of the hCG. Retrieved oocytes are typically cultured for 28 to 36 hours to allow time for progression to metaphase II, thereby simulating the timing from the LH surge in vivo. A specialized, smaller needle (19 or 20G, rather than the 16 to 17G needles generally used) is needed to remove the more adherent immature oocyte-cumulus complex from the membrana granulosa cells lining the follicle, and a lower pressure on the aspiration pump may improve oocyte yield.
The efficiency of IVM varies depending upon whether the oocytes arise from polycystic ovaries, naturally cycling ovaries, or ovaries primed with low doses of gonadotropins. There is also considerable heterogeneity in success rates among clinics, even when similar procedures are used in comparable patient populations. The majority of studies indicate that while more than 60% of IVM oocytes achieve nuclear maturation by progressing to metaphase II, a low percentage successfully acquire cytoplasmic maturation and exhibit the ability to support pronuclear formation and early embryonic development. Only 40% to 80% of fertilized IVM oocytes progress through early cleavage, and of those that do cleave and that are transferred, implantation rates of less than 15% are seen in the majority of studies. This low efficiency is further diminished for oocytes matured without their cumulus-corona cells. Therefore, attempts to salvage immature oocytes for clinical use after cumulus-corona cell removal in preparation for ICSI are not recommended with the IVM protocols currently available.
Several challenges remain before IVM can be reliably applied in ART. The IVM system requires optimization; the culture medium requires further refinement beyond the reported benefits of supplementation with activin A, or the epidermal growth factor family members amphiregulin and epiregulin, and use of a three-dimensional culture system to support the structural integrity of the oocyte-cumulus complex either in a prematuration phase or during maturation requires further investigation. Moreover, the optimal duration of IVM remains to be determined.
Collection, Assessment, and Preparation of Sperm
- ◆
Ejaculated sperm is typically used for IVF, and is prepared for either conventional insemination or ICSI via swim-up or density gradient centrifugation.
- ◆
In the setting of severe oligospermia or azoospermia, testicular and/or epididymal harvesting may yield adequate sperm for ICSI.
Sperm Collection
Semen is typically collected by masturbation or occasionally by sexual intercourse using a condom lacking spermicide. In cases of male factor infertility, retrograde ejaculation, electroejaculation, testicular biopsy, microtesticular biopsy, or epididymal aspiration may be employed (see Chapter 23 ). For men with markedly decreased sperm concentration or motility, absence of a sperm acrosome, or known fertilization abnormalities, ICSI is recommended.
Sperm Assessment
Assessment of ejaculated semen begins with determination of the time it takes for the specimen to liquefy. A normal specimen liquefies within 60 minutes at room temperature, although usually this occurs within 15 minutes. The liquefied specimen is then examined for color, volume, viscosity, and pH. Initial assessment of the raw, unprocessed specimen is then undertaken to determine the concentration, motility, agglutination of sperm, and presence of any cellular elements other than the sperm using World Health Organization 5th Edition protocols and criteria. Sperm morphology may be assessed from smears stained, for example, with Papanicolaou stain. Classification of sperm morphology typically involves assessment of the shape of the head, and defects in the neck, midpiece, and tail, and is generally performed using Kruger’s strict criteria.
Further tests may be performed on a case-by-case basis (e.g., sperm antibody assessment or viability assessment using the hypo-osmotic swelling [HOS] test). The WHO reference values of semen variables are shown in Table 31.3 . If the semen parameters are abnormal, the number of sperm isolated from the specimen may be related to both fertilization and pregnancy rate following conventional IVF. As the number of sperm in the unwashed specimen decreases below 10 to 20 × 10 6 /mL, the incidence of chromosomal abnormalities increases. Therefore nonobstructive azoospermia and severe oligospermia (<5 million/mL) are indications for karyotype evaluation (see Chapter 32 ). Interestingly, the incidence of aneuploidy in sperm appears to increase with severe morphologic defects (e.g., round-head syndrome, microcephalic, and macrocephalic sperm morphology), and with increased incidence of sperm DNA fragmentation.
| Parameter | Lower Reference Limits |
|---|---|
| Semen volume (mL) | 1.5 (1.4–1.7) |
| pH | ≥7.2 |
| Total sperm number (10 6 per ejaculate) | 39 (33–46) |
| Sperm concentration (10 6 /mL) | 15 (12–16) |
| % Total motility (progressive and non-progressive) | 40 (38–42) |
| % Progressive motility | 32 (31–34) |
| Sperm morphology (normal forms, %) | 4 (3.0–4.0) |
| Vitality (live sperm, %) | 58 (55–63) |
| Immunobead test (motile sperm with bound beads, %) | <50 |
Sperm Preparation
The liquefied semen specimen is processed for isolation of the sperm by a variety of techniques, including swim-up and centrifugation techniques using a density gradient comprising a polymer (e.g., isolate). For normal semen specimens, both techniques generate high-motility sperm for oocyte insemination. For semen with abnormal sperm characteristics (e.g., low sperm concentration), however, most studies demonstrate that sperm prepared by the gradient centrifugation technique are superior to sperm prepared by the swim-up technique. For example, in one study of sperm defined as abnormal by WHO criteria, density gradient centrifugation resulted in selection of sperm with higher scores for the acrosome reaction, the HOS test, and nuclear maturity than those selected with a swim-up procedure.
In preparation for incubation with the oocytes, sperm prepared by either the swim-up or gradient centrifugation technique are incubated in a protein-supplemented medium for up to 4 hours to initiate the process of capacitation. This preincubation period coincides with a preincubation of the oocytes after retrieval, which in turn facilitates the stabilization of microtubules and oocyte maturation.
In preparation for ICSI, the processed sperm is placed in polyvinylpyrrolidone (PVP) droplets just prior to microinjection to allow for selection and immobilization of a single spermatozoon. Further details of gamete handling prior to ICSI are described in Chapter 32 .
Incubation of Sperm With Oocytes, Intracytoplasmic Sperm Injection, and the Fertilization Check
- ◆
Normal fertilization is determined by observation of two pronuclei and two polar bodies.
- ◆
Oocytes exhibiting either fewer or more than two pronuclei are typically discarded.
For standard IVF, the number of sperm used for insemination will vary, depending on whether the oocytes are inseminated in microdrops of 100 to 200 µL medium or in a larger volume (usually 1 mL). With microdrop insemination, approximately 25,000 to 50,000 sperm are placed with each oocyte, while for insemination in a 1-mL volume, approximately 100,000 to 300,000 sperm are placed with 3 to 5 oocytes. For men with normal semen parameters, higher concentrations of sperm do not significantly improve the fertilization rate. Generally studies have indicated that co-incubation of the sperm and oocytes for 1 to 6 hours, as opposed to overnight co-incubation, results in equal or improved outcomes, possibly due to less exposure of oocytes to reactive oxygen species generated during incubation.
Approximately 16 to 18 hours later, the oocytes are examined for fertilization. The cumulus-corona radiata cells are gently removed from the oocyte, which is then examined under an inverted microscope with Hoffman illumination at around 100× magnification. A zygote with two pronuclei (PNs) and two polar bodies is morphologic evidence of normal fertilization. All oocytes with either less than or more than 2PNs are discarded. The 2PN zygotes are then set up in culture either separately or in groups.
For ICSI, cumulus masses and residual corona radiata cells are removed 3 to 5 hours postretrieval by mechanical and enzymatic denuding via brief pipetting in the presence of hyaluronidase. Nuclear maturity status is assessed, and each mature metaphase II oocyte is microinjected with a single, immobilized spermatozoon. Further details of gamete handling and the microinjection procedure are discussed in Chapter 32 .
ICSI is primarily indicated for treatment of severe male factor infertility; however, other indications include prior total fertilization failure (TFF) with conventional IVF, a high titer of antisperm antibodies, limited sperm supply from a banked specimen (e.g., in a patient who subsequently was rendered sterile by gonadotoxic treatment), human immunodeficiency virus serodiscordance, fertilization of cryopreserved oocytes or oocytes following IVM, and PGT. Use of ICSI in general and also for nonmale factor infertility has increased over the last 10 years, such that 78% of all cases reported to the CDC in 2014 were ICSI (see Fig. 31.14A ). In a large retrospective study of the 317,996 cases of nonmale factor infertility treated with ICSI from 1996 to 2012 in the United Sates, ICSI was associated with lower rates of implantation (23.0% vs. 25.2%; adjusted RR, 0.93; 95% CI, 0.91 to 0.95) and live birth (36.5% vs. 39.2%; adjusted RR, 0.95; 95% CI, 0.93 to 0.97). These lower rates are more likely associated with the genomic background of the males than to the ICSI procedure per se.
Embryo Culture Systems
- ◆
Multiple culture dish platforms and commercial media are available, but none have been shown to be superior to others.
- ◆
Compared with atmospheric oxygen (20%), low oxygen tension (5%) may modestly improve blastulation rates and clinical outcomes.
Dish Platforms
Standard systems used for culturing human embryos are not physiologic. Typical systems comprise a culture dish containing: (1) 25 to 50 µL volumes of medium placed either onto the surface of the dish in microdrops or into wells sunk into the dish surface; or (2) a larger volume of medium that covers the entire surface area of the dish. Regardless of which setup is used, all provide two-dimensional, relatively static fluid environments on an inert substrate. In contrast, the embryo in vivo not only is exposed to a four-dimensional microenvironment that is chemically dynamic and changes with time (in terms of chemokines, nutrients, energy substrates, oxygen tension, etc.), but also is in contact with epithelial surfaces rich in glycoprotein.
Due to the nonphysiologic nature of current IVF systems, new systems and culture platforms have been designed specifically to address the physical and chemical requirements of the embryo. These include the Well of the Well, or WOW, system, and a microfluidic, microchannel approach, which aims to perform almost all steps of the IVF process with minimal embryologist intervention. However, none of these innovations have yet to be integrated into routine IVF, and it is unclear whether there is any increase in embryo viability and thus implantation rates over the traditional systems currently in use.
Embryo Culture Media
Substantial improvements in culture media for clinical IVF have occurred since the culture of the first viable human embryo in the 1970s. In contrast to the use of Ham’s F10 medium or Earle’s balanced salt solution supplemented with maternal serum for use in these early days of clinical IVF, many commercial culture media are now available for growing human embryos in culture.
Prior to the development of these media, early attempts to culture human embryos to the blastocyst stage required the use of feeder cells in order to overcome cleavage stage block. However, significant improvements in human IVF culture media were made in the 1990s using two different approaches: the “back to nature” approach and the “let the embryo choose” approach. These approaches resulted in the successful culture of human embryos to the blastocyst stage without feeder cell support.
The “back to nature” approach drew upon assessment of the energy substrate profiles in fallopian tube and uterine fluids. The results indicated that, in contrast to the fallopian tube, the glucose concentration in the uterine fluid was relatively high, but the lactate and pyruvate concentrations were relatively low. These observations led to the development of a pair of media, reflecting these differences in energy substrate concentration, that are used in sequence for support of human blastocyst formation: the first medium, relatively high in lactate and pyruvate, is used from days 1 to 3 of culture, and the second medium, relatively high in glucose, is used from day 3 onward. The resultant paired media comprise the sequential media system.
The “let the embryo choose” approach is based on the determination of blastocyst formation rates following systematic adjustments in medium composition in numerous experiments using the simplex optimization approach. This approach has resulted in the development of a single medium for the culture human embryos from day 1 to day 5/6. This system is referred to as the single-step system.
Numerous studies support the efficacy of both sequential and single-step systems for growing human embryos to the blastocyst stage. The weight of the evidence indicates that neither system is superior to the other; specifically, in a 2016 meta-analysis, there was no difference in the ongoing pregnancy rate per randomized woman (RR 0.9, 95% CI 0.7 to 1.3, two RCTs, n = 246). Single-step media use was associated with an increased overall blastocyst formation rate per randomized oocyte/zygote, but no difference in high-quality blastocysts. Cumulative pregnancy rates in the two culture systems have not been evaluated.
Optimal Temperature, pH, and Oxygen Tension for In Vitro Culture
Human embryo culture, like other mammalian cell culture, is typically performed at 37°C. Limited data indicate that both the ovary and the preovulatory follicle may be 1°C to 2°C cooler than the remainder of the body ; sparse retrospective data suggest that fertilization rates and clinical pregnancy rates may be improved in a cooler environment. In contrast, the only RCT performed to date comparing sibling embryos cultured in 37°C versus a perhaps more physiologic 36°C demonstrated that the rate of usable blastocyst formation was higher in the 37°C group. Therefore, at least today, this is the preferred and standard temperature in most laboratories.
The optimal pH for clinical IVF has not been defined. Most commercial media manufacturers recommend culture within the range of 7.2 to 7.4, and these recommendations may vary according to the stage at which the medium is used (fertilization vs. cleavage stage vs. extended culture). Importantly, the external pH in the media drop does not reflect the internal pH within the embryo, and varying amounts of lactate, pyruvate, and certain amino acids in culture media can lead to differences in intracellular buffering. Accordingly, the ideal pH may be specific to each medium, and practically can be determined within a given lab with routine quality control testing. The pH can be adjusted by altering incubator CO 2 tension or protein concentration; in terms of incubator management, it is important to recognize that the pH scale is logarithmic, such that a 0.2 unit change in pH translates into a greater than 60% change in H + concentration.
More data are available to guide recommendations for the optimal oxygen tension for human embryo culture. Traditionally embryos have been cultured in atmospheric (20%) oxygen, but over the last 10 years there has been a paradigm shift in many laboratories to culture in “low” oxygen tension (5%), due to potential improvement in blastocyst development and pregnancy rates. A detrimental effect of 20% oxygen on in vitro development of bovine embryos was shown 20 years ago, and experiments in the mouse have revealed that this effect is irreversible. Consistent with these observations, a prospective randomized trial showed that culture of embryos in low versus atmospheric oxygen tension resulted in a significant increase in both the conversion rate of human zygotes to blastocysts (47.8% vs. 42.1%; P = .02), as well as the live birth rate (42.1% vs. 32.2%; P = .04). A 2016 meta-analysis demonstrated a modest improvement in both clinical pregnancy (OR 1.11, 95% CI 1.04 to 1.18; 9 RCTs, n = 5501) and live birth (OR 1.14, 95% CI 1.04 to 1.25; 8 RCTs, n = 5401) following culture in low oxygen tension. The effect of low oxygen on clinical pregnancy rate remained in subanalysis of cleavage versus blastocyst transfer; however, for live birth it was only apparent following blastocyst transfer (OR 1.3, 95% CI 1.02 to 1.66; 1 RCT, n = 160). Therefore the beneficial effect of low oxygen tension may manifest only after day 3 (corresponding to the time of embryonic genome activation, and also when the embryo enters the uterine cavity in vivo), although further studies are required to investigate this possibility. Notably, as the uterine oxygen tension in the human is approximately 2%, it remains to be determined whether a further reduction in the incubator oxygen tension would prove beneficial for extended culture.
Single Versus Group Culture
Animal and preliminary human data indicate that the blastocyst formation rate may be improved when embryos are cultured together within a shared drop of media. For example, one RCT randomized sibling human zygotes to individual (1 embryo/drop) or group (3 to 5 embryos/drop) culture; embryos in group culture were significantly more likely to blastulate (212/380, 55.8% vs. 124/304, 40.8%; P < .001) and to form high-quality blastocysts (122/154, 79.4% vs. 66/96, 68.8%; P < .05). Embryo density in group culture was not associated with blastulation; that is, group culture of 3, 4, and 5 embryos together led to similar blastocyst rates: 104/178 (58.4%), 74/141 (52.5%), and 34/60 (56.7%). There was no difference in clinical pregnancy or live birth rates, but the study was underpowered for these endpoints. Further studies are needed to clarify the role of group culture. It should be noted that in most IVF laboratories, embryos are cultured in individual microdrops to allow tracking of development from the 2PN stage onward.
Undisturbed Culture and the Role of Media Renewal on Day 3
With the advent of time-lapse imaging (TLI) systems, it has become possible to culture an embryo from the time of ICSI to the time of transfer, cryopreservation, or discard without ever removing the culture dish from the incubator. Given that environmental factors such as light exposure, variations in temperature, and atmosphere may be embryotoxic, an undisturbed culture system would seem preferable to the multiple incubator excursions currently performed for morphologic evaluations and media renewal. Furthermore, limited data exist evaluating whether or not media need to be changed on day 3 for the duration of extended culture; by doing so, one might limit accumulation of toxic metabolites like ammonium, but similarly may negate any beneficial effects of autocrine, embryotrophic substances.
The effect of undisturbed culture versus standard day 3 morphology and media renewal in the same medium has only been evaluated in one prospective study to date. A 2016 RCT randomized donor oocytes immediately following ICSI to undisturbed culture in a single-step medium until day 5, or to the same single-step medium with a renewal step on day 3. All embryos from the same cohort were cultured in the same TLI system. Blastocyst rates were equivalent in the two groups (no renewal: 66.8% vs. renewal: 68.3%). Likewise, there were no differences in the proportion of high-quality blastocysts or implantation rates. Thus, from the limited data available, culture in a single-step, undisturbed environment appears equivalent to culture in a system with a day 3 renewal step.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree