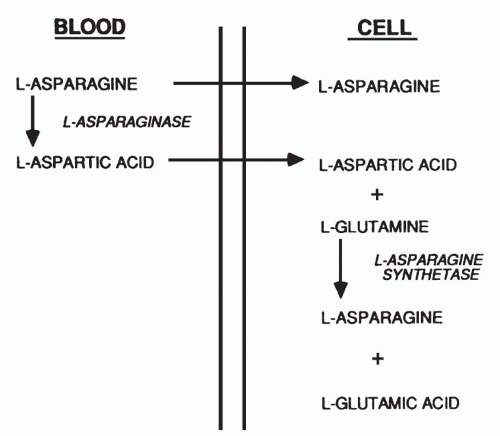
L-ASP (L-Asp) purified from
E. coli11 has been used most widely in both basic and clinical research, although L-ASP obtained from a number of other bacterial sources possesses antitumor activity. The purified bacterial enzyme has a molecular weight of 144,000 Da and is composed of four subunits, which associate as two dimers. These dimers undergo a transition to a fully active catalytic state with the binding of substrate.
12 The gene coding for the
Erwinia chrysanthemi13 enzyme has been cloned and sequenced and expressed in
E. coli.14 Preparations of enzyme from different bacterial strains and by different purification methods show slight differences in enzyme characteristics. For the bacterial enzymes, the specific activity of purified enzyme is usually 300 to 400 μmol of substrate cleaved per minute per milligram of protein; the isoelectric point lies between pH 4.6 and 5.5 for the
E. coli enzyme and is 8.6 for the
Erwinia protein; and the
Km (Michaelis-Menten constant) for asparagine is usually 1 × 10
−5 mol/L.
12,
15 The
E. coli enzyme contains 326 amino acids in each subunit
16, and the
Erwinia subunit has a molecular weight of 32,000 Da.
17 (See Ref [
9] for the amino acid sequence of the
E. coli enzyme.) The sequence and crystal structure of the
E. coli enzyme have been solved.
16 The enzyme has only a 46% homology with the
Erwinia chrysanthemi enzyme; the two enzymes lack antigenic cross-reactivity and differ in biochemical properties. For example, ammonia activates
E. coli asparaginase, whereas oxygen represses its synthesis; neither affects the
Erwinia enzyme.
17The
E. coli and
Erwinia enzymes are highly specific for L-asparagine as substrates and have less than 10% activity for the D-isomer, for
N-acylated derivatives, or for L-asparagine in peptide linkage. In contrast, the enzyme from
Saccharomyces cerevisiae has equal or greater activity with D-asparagine and with
N-substituted substrates.
18The hydrolysis of L-asparagine proceeds according to a reaction mechanism that involves an initial displacement of the amino acid NH2 group during the formation of an enzyme-aspartyl intermediate, followed by hydrolytic cleavage of the latter bond to generate free L-aspartate and active enzyme. The reaction may be summarized as E + Asn ↔ NH
3 E · Asp → E + Asp + NH
3, where E · Asp represents the enzyme-aspartyl intermediate.
18 A specific threonine in the enzyme catalyzes the nucleophilic attack and displacement of the amide group on the L-asparaginase molecule.
16 The reaction is irreversibly inhibited by the L-asparagine analog 5-diazo-4-oxo-l-norvaline, which binds covalently to the enzyme’s active site.
19