Nausea and vomiting can be symptoms associated with cancer, but it was the introduction of cytotoxic chemotherapy of high emetic potential to treat cancer that stimulated research on the mechanisms of emesis. This has resulted in the introduction of two new classes of antiemetics, namely, the 5 hydroxytryptamine 3 receptor antagonists (5HT3 RA) and the neurokinin 1 receptor antagonists (NK1 RA), which have made a major impact on the control of emesis (
Table 39-1).
Three distinct types of emesis are associated with chemotherapy. The most common is postchemotherapy emesis occurring in the 24 hours after chemotherapy.
1 Delayed emesis is thought to commence around 18 hours after treatment and can last for at least 5 days.
2 For patients who experience emesis with chemotherapy, anticipatory emesis can occur with subsequent cycles as a conditioned response.
3The most important predictor of acute postchemotherapy emesis is the type, dose, and schedule of the chemotherapy (
Table 39-2). Drugs of high emetic potential have a 90% or greater chance of causing emesis if no antiemetic prophylaxis is given.
4 The paradigm example is cisplatin, which when given over an hour at greater than 60 mg/m
2 will cause almost all patients to vomit, and is associated with both acute and delayed emesis. Trials to test new antiemetic drugs frequently focus on cisplatin-induced emesis. Drugs such as anthracyclines or cyclophosphamide have moderate emetic potential (between 30% and 90% chance of emesis). The combination of these drugs (AC) is of high emetic potential, however.
A number of patient characteristics predict emesis after chemotherapy.
5 Younger patients are more prone to vomiting than older, as are women compared to men. Patients who have previous vomiting with chemotherapy, or motion sickness, or vomiting with pregnancy are more likely to vomit postchemotherapy. Those patients with a prolonged history of heavy alcohol consumption vomit less after chemotherapy.
Nausea and vomiting are among the most distressing side effects of chemotherapy.
6,
7 These side effects are also associated with fatigue, anorexia, and insomnia.
8 Doctors and nurses tend to underestimate nausea and vomiting, particularly in the delayed phase, which can lead to undertreatment.
9
5HT3 Receptor Antagonists
The initial revolution in the control of chemotherapy-induced emesis came with the introduction of a new class of antiemetics, the 5HT3 RAs. The antiemetics previously available, such as the substituted benzamides, the phenothiazines, and butyrophenones, had very limited efficacy against chemotherapy-induced emesis and had unpleasant side effects, including somnolence, depression, dysphoria, and tardive dyskinesia.
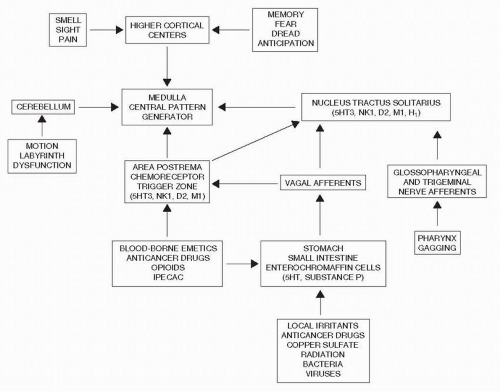
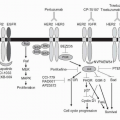
Mechanistic studies revealed that chemotherapy causes the release of 5-hydroxtryptamine from the enterochromaffin cells in the small intestine. This 5HT stimulates the vagal afferents that connect to the dorsal brainstem, the nucleus tractus solitarius, and the area postrema (
Fig. 39-1). Efferent fibers then go to an area known as the central pattern generator more ventrally in the brain stem, and the vomiting reflex is initiated.
10,
11 The area postrema also has direct contact with the blood and cerebrospinal fluid, sitting as it does in the floor of the 4th ventricle, and can also be stimulated via that route.
The first of the 5HT3 RAs, ondansetron, when given in combination with dexamethasone before chemotherapy of high emetic potential achieved control of acute postchemotherapy emesis in over 80% of patients with a rate of around 65% if used as a single agent (
Fig. 39-2).
12 The side-effect profile was favorable, with the main toxicities being mild headache, constipation, and transient elevation in liver transaminases. Randomized studies and meta-analyses did not demonstrate differences in the therapeutic effect of ondansetron, granisetron, tropisetron, or dolasetron, although in
Jordan’s meta-analysis, there were some differences in subset analyses of the granisetron and tropisetron comparison.
13,
14The 5HT3 RAs are metabolized by the liver through hydroxylation followed by glucuronide or sulfate conjugation. The metabolites do not seem to contribute to antiemetic activity. Currently, the 5HT3 RAs are given by single daily dosing rather than the initial multiple day schedules in the original ondansetron studies.
15 Oral formulations have also been shown to be as effective as the intravenous dosing (
Table 39-3).
16The clinical trials of the initial 5HT3 RAs such as ondansetron showed that they demonstrated low efficacy for cisplatin-induced delayed emesis.
17 These first-generation 5HT3 RAs only had modest activity for the delayed emesis associated with chemotherapy of moderate emetic potential.
18
Palonosetron
The initial second-generation 5HT3 RA, palonosetron, has different properties and efficacies than the first-generation 5HT3 RAs (
Fig. 39-2). Palonosetron has a mean elimination half-life of approximately 40 hours as compared to 4 to 9 hours with the first-generation 5HT3 RAs, but the same favorable safety profile.
19 It also exhibits a 30-fold higher binding affinity for the receptor than the first-generation drugs. The binding differs in that whereas ondansetron and granisetron show simple bimolecular binding, palonosetron demonstrates allosteric binding with positive cooperativity to inhibit receptor function over a longer time and triggers either inactivation or internalization of the 5HT3 receptor.
20In the early phase studies of palonosetron, the side effects of headache, constipation, and dizziness were similar to other 5HT3 RAs and no cardiac safety issues were noted. Of the first three large phase III trials, two were for chemotherapy of moderate emetic potential, comparing single intravenous doses of palonosetron with ondansetron or dolasetron.
21,
22 Gralla et al.
21 found that single-dose palonosetron (0.25 and 0.75 mg) was not inferior to ondansetron (32 mg) for the response of no vomiting, no rescue for acute emesis, and was considered superior in the control of delayed emesis. Also there was a suggestion that nausea was better controlled than with the first-generation 5HT3 RAs.
21 Similarly, Eisenberg et al.
22 demonstrated a similar outcome in the comparison of palonosetron with 100 mg of dolasetron. The third trial compared palonosetron with ondansetron in the prophylaxis of chemotherapy of high
emetic potential and showed noninferiority for acute, delayed, and overall emesis.
23The next step in the assessment of palonosetron was to evaluate it in combination with both dexamethasone and as part of triple prophylactic antiemetic therapy with dexamethasone and an NK1 RA to ascertain whether palonosetron should replace the older 5HT3 RAs in antiemetic regimens with chemotherapy of high or moderate emetic potential.
A pooled analysis of the two studies with chemotherapy of moderate emetic potential had suggested that although the occurrence of acute chemotherapy-induced nausea and vomiting is the strongest predictor of delayed emesis, the efficacy of palonosetron in the
delayed phase was not just a carryover effect but directly due to the activity of palonosetron.
24Saito et al.
25 compared palonosetron and dexamethasone with granisetron and dexamethasone in 1,114 patients receiving chemotherapy of high emetic potential (including the AC combination). The complete response rate for acute emesis was 75.3% in the palonosetron arm versus 73.3% in the granisetron arm of the study.