Richard J. Wallace Jr., Julie V. Philley, David E. Griffith
Antimycobacterial Agents
Drugs for mycobacterial infections are discussed in three groups: those primarily for the treatment of infections caused by Mycobacterium tuberculosis, drugs for infections caused by nontuberculous (atypical) mycobacteria (NTM), and agents principally for the treatment of leprosy. Approaches to antituberculous chemotherapy have been affected by the increasing prevalence of multidrug-resistant M. tuberculosis (MDR-TB), defined as resistance to at least isoniazid (INH) and rifampin,1–5 and extensively drug-resistant M. tuberculosis (XDR-TB), defined as resistance to at least INH and rifampin, resistance to any fluoroquinolone, and resistance to at least one second-line injectable drug,5–10 and by the special impact on M. tuberculosis of those infected with human immunodeficiency virus (HIV).5,11,12
MDR-TB and XDR-TB strains have necessitated the use of drugs that are considered second-line agents, as well as drugs that must be administered by injection.13 The list of agents active against MDR-TB and XDR-TB strains remains limited; however, recent development and introduction of new antituberculous drugs has significantly improved the outlook for successful treatment of M. tuberculosis strains resistant to “standard” first-line and second-line agents. The current pace of new tuberculosis drug development is unprecedented and cause for optimism even in the presence of increasing MDR-TB and XDR-TB disease prevalence. It still must be emphasized that to prevent the emergence of acquired drug resistance and to treat optimally both drug-susceptible and drug-resistant M. tuberculosis infection universal supervised therapy is essential.14,15
MDR-TB and XDR-TB infections are of increased concern in persons immunologically disabled by HIV with or without acquired immunodeficiency syndrome (AIDS) because the host contribution to controlling the infection is severely diminished. HIV-infected individuals have a number of other special problems. They are especially prone to adverse drug reactions.16,17 The susceptibility of protease inhibitors (PIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) to hepatic metabolism induced by rifamycins, especially rifampin and rifapentine, necessitates the ongoing need for regimens that exclude these agents.16,17 In addition, malabsorption of antituberculous drugs can occur in AIDS patients, resulting in suboptimal antituberculous serum drug levels, which predisposes to acquired drug resistance.18
The early years of HIV disease saw a dramatic increase in the prevalence and severity of infections caused by M. avium and other NTM, especially in their disseminated forms. Fortunately, the combination of the macrolide-azalide group with ethambutol was active against M. avium and other NTM, even in patients with markedly impaired cell-mediated immune defenses. In recent years, with great advancement in antiretroviral therapy and the use of disseminated M. avium prophylaxis, the incidence of disseminated disease in advanced HIV infection has markedly declined. The effects of HIV infection and AIDS on leprosy and its chemotherapy do not appear to be as great as expected.
Traditionally, antimicrobial agents for tuberculosis have been classified as first-line drugs, having superior efficacy with acceptable toxicity, and second-line drugs, having less efficacy and greater toxicity. Several excellent reviews of antimycobacterial agents and therapy are available,13,19–24 including guidelines for therapy for MDR-TB infection.13,19,21 Effective treatment of both MDR-TB and XDR-TB strains requires detailed information about extended drug susceptibilities to guide individualized treatment regimens.8,13,25
Antituberculous drugs differ in their mechanism of bactericidal action and in their delivery to tuberculous lesions. Three of the first-line agents—INH, rifampin, and ethambutol—are active against the large populations of tubercle bacilli in cavities. Streptomycin (now considered a second-line agent), other aminoglycosides, and capreomycin penetrate cells poorly and are inactive at acidic pH. Pyrazinamide (PZA; the fourth first-line agent), which is inactive at the neutral or slightly alkaline pH that may occur extracellularly, is active only in acidic environments such as within macrophages. Slowly replicating organisms in necrotic foci are killed by rifampin and somewhat less readily by INH.
First-line antituberculous agents, except ethambutol, are bactericidal. The bactericidal activities of both INH and rifampin against tubercle bacilli in cavitary, intracellular, or necrotic foci provide the basis for the efficacy of short-course INH-rifampin regimens. A combination of three bactericidal agents that are active against intracellular organisms—INH, rifampin, and PZA—is essential for the 2-month initiation phase of the standard 6-month regimen currently recommended for drug-susceptible disease in the United States. A residual population consisting of virtually nonreplicating dormant tubercle bacilli within necrotic foci is especially difficult to eradicate, perhaps explaining the minimum of 4 months of continuation phase therapy needed even in persons with competent immune defenses.
Much of the following discussion of individual antimycobacterial agents is taken from existing guidelines. In collaboration with other organizations, the Centers for Disease Control and Prevention (CDC) will be updating treatment guidelines for latent M. tuberculosis infection (LTBI) and established tuberculosis probably close to the publication of this volume. Additionally, new technologies such as the widely available TB GeneXpert and gene sequencing (available through the CDC) offer the clinician the ability to rapidly (within days) detect drug-resistant M. tuberculosis strains to avoid ineffective and toxic empirical second-line medication regimens for tuberculosis. The reader is strongly encouraged to monitor the CDC website for timely review of the anticipated new guidelines and details about the indications and procedures for utilizing the tubercular drug resistance rapid identification technologies.
Chemical structures of the major drugs discussed in this chapter are shown in Figure 38-1.
First-Line Antituberculous Drugs
Isoniazid
Derivation and Structure.
Isoniazid, isonicotinic acid hydrazide (INH), a synthetic agent, was introduced in 1952.
Mechanism of Action.
Isoniazid is bactericidal against actively growing M. tuberculosis and bacteriostatic against nonreplicating organisms. It acts by inhibition of synthetic pathways of mycolic acid, an important constituent of mycobacterial cell walls. It also likely inhibits the catalase-peroxidase enzyme, coded for by the gene katG.
Antimicrobial Activity and Resistance.
Against M. tuberculosis, 0.025 to 0.05 µg/mL of INH is inhibitory, and higher concentrations are bactericidal against replicating organisms. When INH is administered alone as monotherapy in the presence of active tubercular disease, resistance to INH will emerge. Initially susceptible isolates become resistant in over 70% of cases treated with INH monotherapy for 3 months. Resistance results from selection under antimicrobic pressure of resistant mutants of M. tuberculosis that number 1 in 106 among untreated bacillary populations. Large populations like the 109 to 1010 bacilli in pulmonary cavities are especially likely to contain significant numbers of inherently resistant tubercle bacilli. Low-level INH resistance, defined as an INH minimal inhibitory concentration (MIC) for M. tuberculosis of greater than 0.1 µg/mL but less than 1.0 µg/mL is most commonly associated with point mutations or short deletions within the catalase-peroxidase gene (katG), which still produces some enzymatic activity, whereas high-level resistance, defined as an INH MIC for M. tuberculosis of greater than 1.0 µg/mL, is associated with major deletions within the gene with loss of all enzymatic activity.26,27 Resistance in the regulatory region of a second gene involved in mycolic acid synthesis (inhA) also confers INH resistance.26,27 The incidence of INH resistance among new cases of tuberculosis in 2011 was 7% in persons born in the United States and 11% in persons born outside the United States, including immigrants from Africa, Southeast Asia, Eastern Europe, and Central America, where INH resistance is more common.11,12,28
Pharmacology.
INH is well absorbed orally or intramuscularly and is distributed throughout the body. Cerebrospinal fluid (CSF) levels are generally about 20% of plasma concentrations but may approach plasma levels in the presence of meningeal inflammation. Co-administration with vitamin C appears to inactivate INH suspensions markedly.29
Metabolism of INH occurs initially by hepatic N-acetyltransferase. Diminished acetylation capacity is inherited as an autosomal recessive trait that varies from a 5% prevalence rate in Canadian Eskimos to 83% in Egyptians. Ten percent to 15% of Asians are “slow” acetylators, as are 58% of American whites. Six hours after a 4-mg/kg oral dose, slow acetylators exhibit plasma INH levels of more than 0.8 µg/mL and rapid acetylators have levels of less than 0.2 µg/mL.20 The striking bimodal distribution of plasma half-lives of INH depending on acetylator status generally does not affect the outcome with daily therapy, because plasma levels are maintained well above inhibitory concentrations. Metabolically altered INH is principally excreted in urine along with lesser amounts of unaltered drug. Dosage modification in renal insufficiency is not necessary. The benefit of dosage adjustment with significant hepatic disease is not established. Table 38-1 summarizes dosage modifications for INH and other antituberculous drugs in hepatic or renal failure.
TABLE 38-1
Need for Dosage Modification for Antituberculous Drugs in Hepatic or Renal Failure
| ANTIMICROBIAL DRUG | MODIFY IN HEPATIC FAILURE | MODIFY IN RENAL FAILURE |
| Isoniazid | No | No |
| Pyrazinamide | Yes | Yes |
| Ethambutol | No | Yes |
| Rifampin | ?Yes | No |
| Rifabutin | ?Yes | No |
| Amikacin | No | Yes |
| Capreomycin | No | Yes |
| Kanamycin | No | Yes |
| Streptomycin | No | Yes |
| Quinolones | No | Yes (levofloxacin, not moxifloxacin) |
| Para-aminosalicylic acid | No | Yes |
| Ethionamide | Yes | No |
| Cycloserine | No | Yes |
Adverse Reactions
INH has infrequent major toxicities, most notably hepatitis. Ten to 20 percent of INH recipients have asymptomatic minor elevations in serum aspartate aminotransferase levels that usually resolve even with continued therapy.30 A meta-analysis of six studies estimated the rate of clinical (symptomatic) hepatitis in patients given INH alone to be approximately 0.6%.31 Recent data indicate that the incidence of clinical hepatitis is even lower. Hepatitis occurred in only 0.1% to 0.15% of 11,141 persons receiving INH alone as treatment for LTBI in an urban tuberculosis control program.32 Early estimates of the incidence of severe or major INH hepatotoxicity were provided from the results of a large multicenter U.S. National Institutes of Health (NIH)–sponsored trial. Fatal hepatitis occurred in 8 of nearly 14,000 patients receiving INH.33 All but one of the deaths occurred in one study center, and patients did not receive routine monitoring for toxicity during the trial.33 More recent studies, however, suggest that the rate of fatal INH-related hepatitis is substantially lower.32,34,35 The likely explanation is the adoption in the early 1980s of uniform clinical toxicity monitoring for patients receiving INH for treatment of LTBI.34,35 Hepatotoxicity can occur at any time but generally occurs after weeks to months of therapy rather than days to weeks after treatment is begun. INH hepatotoxicity is correlated with age, presumably owing to a diminished capacity for repair of INH-induced hepatocellular damage in the elderly. Undernutrition may also play a role in the expression of INH hepatotoxicity.36 Hepatotoxicity is increased in several groups: alcoholic patients; patients with preexisting liver damage33; pregnant women and women up to 3 months postpartum37; patients also taking INH in combination with acetaminophen38; patients receiving other potentially hepatotoxic agents such as rifampin35; patients with active hepatitis B; and HIV-seropositive patients on highly active antiretroviral therapy.39 Histologically, hepatocellular damage can progress to submassive necrosis. Although active hepatitis B is considered a contributing factor to INH hepatotoxicity,40,41 INH has been safely administered to some with acute hepatitis42 and for LTBI to persons chronically infected with hepatitis B and C.39,43,44 Educating patients about the recognition of symptoms of INH-induced liver disease is key in preventing its progression. As noted, routine clinical monitoring of patients receiving INH is mandatory.35 Routine monitoring of serum hepatic enzyme concentrations is not indicated for all patients at the start of treatment of LTBI. Baseline testing is recommended for patients whose initial evaluation suggests a liver disorder, patients infected with HIV who are receiving highly active antiretroviral therapy, pregnant women and those in the immediate postpartum period (i.e., within 3 months of delivery), persons with a history of chronic liver disease (e.g., hepatitis B or C, alcoholic hepatitis, or cirrhosis), persons who use alcohol regularly, and others who are at risk for chronic liver disease.35,39 Baseline testing is no longer routinely indicated in persons older than 35 years of age.35,39 Laboratory monitoring during treatment of LTBI is indicated for patients whose baseline liver function test results are abnormal and for other persons at risk for hepatic disease.35,39,45 Although biochemical monitoring may contribute to patient confidence and adherence with the treatment regimen, the contribution of routine biochemical monitoring to the safety of INH administration is not proven. The importance of routine clinical monitoring is clear, however, and must be applied rigorously with or without biochemical monitoring.
The most feared INH-related toxicity is fulminant hepatic failure necessitating liver transplantation or resulting in death. The CDC conducted a detailed analysis of 17 patients with severe INH-related hepatoxicity over a 4-year period.46 The estimated incidence of death and liver transplantation was 1/150,000 to 1/220,000 patients receiving INH therapy for LTBI. Although continuation of INH after the onset of hepatitis-related symptoms was associated with severe hepatotoxicity, some patients still developed severe hepatotoxicity after stopping INH within days to a week of symptom onset. Although it is not universally protective, patients should be strongly advised to discontinue INH therapy at the onset of symptoms consistent with incipient hepatitis, such as nausea, loss of appetite, and dull midabdominal pain. Perhaps most disconcerting, there were two children younger than 15 years of age in this series of patients. The overall conclusions of this analysis were that severe hepatotoxicity was idiosyncratic, occurred at any time during INH treatment, occurred even with careful clinical and biochemical monitoring and with appropriate (recommended) doses of INH, and could occur in children.46
Most hepatotoxicity subsides after INH discontinuation. Cautious readministration of INH after a resolution of hepatitis for selected patients has been reported to be well tolerated and safe,47 although consideration should be given in these patients to alternative therapies for LTBI, such as rifampin.35,39 Recognition of the frequency and severity48 of INH hepatotoxicity has not curtailed therapeutic usage but has led to a revision of indications for treatment of LTBI, with special caution indicated for groups identified at high risk for INH hepatotoxicity (see earlier discussion).35,39,48
Neurotoxicity.
Peripheral neuropathy has been described in 17% of recipients of 6 mg/kg/day of INH but is less frequent when adults receive the standard dose of 300 mg/day. Poor nutrition or underlying alcoholism, diabetes mellitus, or uremia predisposes to neuropathy, which is more frequent in slow acetylators who have higher plasma levels of unaltered drug. Increased pyridoxine excretion is promoted by INH. Pyridoxine, 10 to 50 mg daily, can ameliorate the neuropathy without interfering with the antimycobacterial effect. Current guidelines suggest that pyridoxine use is necessary only for patients with underlying predispositions for neuropathy, as outlined earlier, but pyridoxine use is essentially universal for patients receiving INH. It is noteworthy that neuropathy is a manifestation of excessive pyridoxine dosing.
INH-induced central nervous system (CNS) toxicity can produce aberrations ranging from memory loss to psychosis or seizures. Optic neuropathy has been reported and can be confused with ethambutol-related optic neuritis in patients receiving both drugs. Toxic CNS reactions are not necessarily related to pyridoxine deficiency but have responded to its administration.20
Hypersensitivity Reactions.
Fever, which may be sustained or “spiking,” skin eruptions, or hematologic abnormalities can occur. INH recipients can develop positive antinuclear antibody reactions and rarely manifest a lupus-like syndrome that is reversible on discontinuation of the INH.
Overdose.
Accidental ingestion of INH by children or ingestion during a suicide attempt may result in metabolic acidosis, hyperglycemia, seizures, and coma. High-dose pyridoxine usually reverses these toxicities.
Significant Drug Interactions.
Particular caution is indicated when administering INH to those with a seizure disorder. INH may alter metabolism of antiseizure medications, so it is important to monitor the blood levels of seizure medications for patients taking INH. Phenytoin (Dilantin) toxicity is potentiated by INH. Mental changes, nystagmus, and ataxia can result, especially in slow acetylators whose high INH levels inhibit phenytoin metabolism. Theophylline toxicity has been reported with co-administration of INH. INH decreases the efficacy of clopidogrel by decreasing the metabolism of the parent compound to the more biologically active metabolite. Combined INH and rifampin therapy predisposes to elevation of plasma hepatic enzymes. Plasma INH concentrations are increased by para-aminosalicylic acid (PAS) through interference with acetylation. Although significant drug interactions are less frequent with INH than with rifampin, it is important to check for all potential drug-drug interactions for any patient taking INH.
Usage.
INH is indicated for all clinical forms of tuberculosis. It is used alone for therapy for LTBI for selected purified protein derivative (PPD) skin test or interferon-γ release assay (IGRA) reactors at high risk for developing active tuberculosis disease.35 The most recent CDC/American Thoracic Society (ATS) guidelines state that INH is considered safe in pregnancy, but the risk for hepatitis may be increased both before and after delivery. Supplementation with pyridoxine is recommended if INH is administered during pregnancy.19,35 It is approved for treating active tuberculosis in pregnant patients, but it should be given with caution, and then only for selected high-risk patients with LTBI (e.g., HIV-seropositive patients or recent contacts to individuals with active tuberculosis).35
Availability and Dosage.
INH is available generically (tablets, syrup, injectable solutions) and under brand names—INH tablets or Nydrazid injectable solution. Dosage forms include 100- and 300-mg tablets; syrup containing 10 mg/mL; 100 mg/mL solution for parenteral injection; and combination capsules combining 150 mg of INH with 300 mg of rifampin (Rifamate) or tablets of 50 mg with 120 mg of rifampin and 300 mg of PZA (Rifater). The usual adult dosage is 5 mg/kg/day (preferably 300 mg once daily). A higher dosage (10 to 15 mg/kg; maximum, 300 mg/day) has been recommended for infants and children.
With the routine use of directly observed therapy for all patients, twice- or three-times-weekly high-dose INH, 15 mg/kg orally (maximum 900 mg), is combined with rifampin, 10 mg/kg (maximum 600 mg orally), and PZA, 50 mg/kg (maximum 4 g), after an initial period of daily drug therapy for drug-susceptible tuberculosis. For most patients in the United States with tuberculosis, these drugs are combined with ethambutol, 50 mg/kg orally (maximum 4 g) twice weekly, or streptomycin, 20 mg/kg intramuscularly, until susceptibilities are available. A reliable urine test is available to confirm INH ingestion.51 Although the preferred parenteral route is intramuscular injection, INH for injection can be administered safely intravenously.52
Rifampin (see also Chapter 27)
Derivation and Structure.
Rifampin (termed rifampicin in the United Kingdom) is a semisynthetic derivative of a complex macrocyclic antibiotic, rifamycin B, produced by Streptomyces mediterranei. It was introduced for clinical trials in tuberculosis in 1967.
Mechanism of Action.
Rifampin inhibits DNA-dependent RNA polymerase; human RNA polymerase is insensitive.
Antimicrobial Activity and Resistance.
Rifampin is bactericidal against actively replicating M. tuberculosis to a degree comparable to INH with MICs of 0.005 to 0.2 µg/mL. It is also active against intracellular, slowly replicating bacilli and somewhat against nearly dormant organisms in necrotic foci. Rifampin’s efficacy is indicated in susceptible pulmonary tuberculosis by sputum conversion 2 weeks earlier with rifampin-containing regimens than with regimens without the drug. Resistance emerges rapidly if the drug is given as monotherapy. Approximately 95% of resistance to rifampin results from a point mutation or deletion within an 81-base pair region of the gene encoding the β-subunit of RNA polymerase (rpoB).27,53 Mutations in the rpoB gene can be rapidly detected with the tuberculosis GeneXpert technology that is widely available in the United States. The prevalence of rifampin resistance among new cases of tuberculosis in the United States is currently less than 1%.4,11,12 Isolated resistance to rifampin in the United States has been strongly associated with HIV infection.54 Resistance to rifampin is associated in all instances with cross-resistance to rifapentine and in most instances with rifabutin, especially in the presence of high-level resistance. Rifampin resistance coupled with resistance to INH and other antituberculous agents defines either MDR-TB or XDR-TB isolates, depending on the number and type of antituberculous agents to which the M. tuberculosis isolate is resistant.5
Pharmacology.
Rifampin is well absorbed orally, yielding peak plasma concentrations of 7 to 8 µg/mL after a dose of 600 mg. It is widely distributed throughout the body. CSF concentrations range from undetectable to 0.5 µg/mL in healthy persons and reach 50% of plasma concentrations with meningeal inflammation. Rifampin’s high lipid solubility enhances phagosomal penetration. Rifampin is deacetylated to an active form that undergoes biliary excretion and enterohepatic recirculation. Because of autoinduction of rifampin metabolism (cytochrome P-450–coupled),55 biliary excretion increases with continued therapy. Induction of rifampin’s metabolism with consequent reduction in its half-life and plasma concentrations becomes maximal after approximately six doses.56 Excretion is primarily into the gastrointestinal tract, with lesser amounts in the urine. The plasma concentration and urinary excretion increase in hepatic failure. Probenecid blocks hepatic uptake, causing decreased biliary excretion. There are no recommendations for rifampin dosage modification (i.e., dose reduction) with either hepatic or renal insufficiency. Rifampin is removed by hemodialysis or peritoneal dialysis.57
Adverse Reactions.
Minor adverse reactions are rather frequent with rifampin, but cessation of therapy because of adverse effects was necessary in only 6 of 372 patients taking the drug for 20 weeks.58
Hepatitis.
Rifampin’s major adverse effect is hepatitis. Minimal abnormalities in liver function tests are common in those taking rifampin and usually resolve, possibly because of autoinduction of its metabolism even with continuation of the drug. Characteristically, elevations of bilirubin and alkaline phosphatase levels result, whereas elevation of hepatocellular enzyme concentrations can be caused by rifampin, INH, or both. Alcoholic patients with preexisting liver damage appear to be especially prone to rifampin-induced liver reactions. There is no clear evidence that rifampin monotherapy for LTBI is associated with fulminant hepatic failure as reported with INH therapy for LTBI.
Effects on Immune Parameters.
Rifampin has widespread effects on humoral and cell-mediated immunity, but they appear to be of no clinical significance.
Hypersensitivity Reactions.
Flushing, fever, pruritus without rash, urticaria, cutaneous vasculitis, eosinophilia, thrombocytopenia, hemolysis, or renal failure due to interstitial nephritis can occur because of rifampin. A systemic flulike syndrome, at times associated with thrombocytopenia, has been described almost exclusively with intermittent, high-dose therapy.
Miscellaneous Adverse Reactions.
Widespread distribution of rifampin is reflected in an orange color appearing in urine, feces, saliva, sputum, pleural effusions, tears, soft contact lenses, sweat, semen, and CSF. With overdosage, a red man syndrome of skin discoloration has been described. Gastrointestinal upset is frequent but is usually ameliorated by a temporary reduction in dosage.
Significant Drug Interactions.
By induction of microsomal cytochrome P-450–mediated enzymatic activities, rifampin causes increased hepatic metabolism of many substances. Rifampin interaction with more than 100 drugs has been described.16,17,59–61 A compilation of this expanding list of compounds is given in Table 38-2. The induction period with rifampin may last for weeks after the drug is discontinued. The recent introduction of PIs and NNRTIs for the treatment of HIV infection has complicated the treatment of tuberculosis in this setting (see Table 38-2). Because rifampin induces metabolism of PIs and NNRTIs, rifampin should not be co-administered with these agents.16,17,62 The most recent guidelines for co-administration of rifamycins with antiretroviral agents are summarized in Table 38-3 and can be found on the NIH website (http://aidsinfo.nih.gov/guidelines), which is a “living document” with ongoing updates.17 Assistance can also be found at the CDC website (www.cdc.gov/tb/publications/guidelines/TB_HIV_Drugs/default.htm).17 As new antiretroviral agents and more pharmacokinetic data become available, treatment recommendations are likely to be modified, so the reader is encouraged to check these websites periodically for updates.
TABLE 38-2
Mycobacterium tuberculosis Disease in Human Immunodeficiency Virus (HIV) Coinfection

Rating of Recommendations: A = Strong; B = Moderate; C = Optional.
Rating of Evidence: I = data from randomized controlled trials; II = data from well-designed nonrandomized trials or observational cohort studies with long-term clinical outcomes; III = expert opinion
Modified from Department of Health and Human Services: Guidelines for the Use of Antiretroviral Agents in HIV-1 Adolescents and Adults. Last updated 3/27/2012. For the most up-to-date guidelines, see http://aidsinfo.nih.gov/guidelines.
TABLE 38-3
Recommendations for Co-administering Antiretroviral Drugs with Rifampin
| RECOMMENDED CHANGE IN DOSE OF ANTIRETROVIRAL DRUG | RECOMMENDED CHANGE IN DOSE OF RIFAMPIN | COMMENTS | |
| Non-nucleoside Reverse Transcriptase Inhibitors | |||
| Efavirenz | None (some experts recommend 800 mg for patients >60 kg) | No change (600 mg/day) | Efavirenz AUC ↓ by 22%; no change in rifampin concentration. Efavirenz should not be used during the first trimester of pregnancy. |
| Nevirapine | Nevirapine and rifampin should not be used together | Nevirapine AUC ↓ 37%-58% and Cmin ↓ 68% with 200 mg twice daily | |
| Rilpivirine | Rifampin and Rilpivirine should not be used together | Rilpivirine AUC ↓ by 80% | |
| Etravirine | Etravirine and rifampin should not be used together | Marked decrease in etravirine predicted, based on data on the interaction with rifabutin | |
| Single Protease Inhibitors | |||
| Ritonavir | Rifampin and ritonavir should not be used together | Significantly ↓ PI exposure (>75%) despite ritonavir boosting | |
| Fosamprenavir | Rifampin and fosamprenavir should not be used together | ||
| Atazanavir | Rifampin and atazanavir should not be used together | Atazanavir AUC ↓ by >95% | |
| Indinavir | Rifampin and indinavir should not be used together | Indinavir AUC ↓ by 89% | |
| Nelfinavir | Rifampin and nelfinavir should not be used together | Nelfinavir AUC ↓ 82% | |
| Saquinavir | Rifampin and saquinavir should not be used together | Saquinavir AUC ↓ by 84% | |
| Dual Protease-Inhibitor Combinations | |||
| Saquinavir/ritonavir | Saquinavir 400 mg + ritonavir 400 mg twice daily | No change (600 mg/day) | Use with caution; the combination of saquinavir (1000 mg twice-daily), ritonavir (100 mg twice-daily), and rifampin caused unacceptable rates of hepatitis among healthy volunteers |
| Lopinavir/ritonavir (Kaletra) | Increase the dose of lopinavir/ritonavir (Kaletra) to 4 tablets (200 mg of lopinavir with 50 mg of ritonavir) twice daily | No change (600 mg/day) | Use with caution; this combination resulted in hepatitis in all adult healthy volunteers in an initial study |
| “Super-boosted” lopinavir/ritonavir (Kaletra) | Lopinavir/ritonavir (Kaletra)—2 tablets (200 mg of lopinavir with 50 mg of ritonavir) + 300 mg of ritonavir twice daily | No change (600 mg/day) | Use with caution; this combination resulted in hepatitis among adult healthy volunteers. However, there are favorable pharmacokinetic and clinical data among young children. |
| Atazanavir/ritonavir | The standard dose of ritonavir-boosted atazanavir (300 mg once daily with 100 mg of ritonavir) should not be used with rifampin | Atazanavir trough concentration ↓ by >90% | |
| Tipranavir/ritonavir | Rifampin and tipranavir/ritonavir should not be used together | ||
| Darunavir/ritonavir | Rifampin and darunavir/ritonavir should not be used together | ||
| CCR-5 Receptor Antagonists | |||
| Maraviroc | Increase maraviroc to 600 mg twice daily | No change (600 mg/day) | Maraviroc Cmin ↓ by 78%. No reported clinical experience with ↑ dose of maraviroc with rifampin. |
| Integrase Inhibitors | |||
| Raltegravir | No change | No change (600 mg/day) | Increase raltegravir dose to 800 mg orally twice daily; monitor for antiretroviral efficacy or switch to rifabutin |
| Elvitegravir/cobicistat/tenofovir/emtricitabine (Stribild) | Co-administration should be avoided | Cobicistat and elvitegravir concentrations may be significantly ↓. Consider an alternative antimycobacterial or alternative antiviral regimen. | |
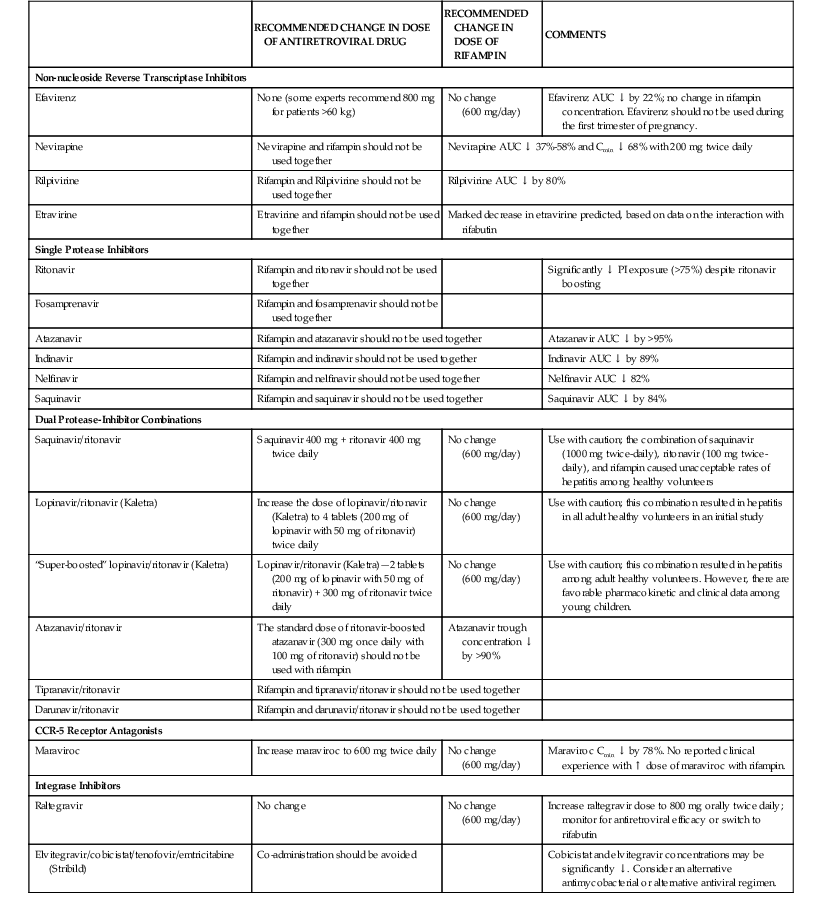
AUC, area under the curve; PI, protease inhibitor.
Modified from UpToDate, 2013. For the most up-to-date information, see http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf
In general, the co-administration of antituberculosis drugs and antiretroviral drugs should be initiated and guided by clinicians experienced in the treatment of these patients and familiar with the potential drug-drug interactions.
Competition for excretion with contrast agents used for biliary tract imaging may cause inability to visualize the gallbladder. Probenecid interferes with renal excretion, whereas PAS may interfere with gastrointestinal absorption.
Usage.
Rifampin is indicated for treatment of all forms of pulmonary and extrapulmonary tuberculosis. It is recommended for treatment of LTBI as an alternative choice to INH.35 The available data suggest that the efficacy of rifampin for treating LTBI appears comparable to that of INH, and, as noted, it appears to be less frequently associated with severe hepatotoxicity. Rifampin in combination with PZA was shown to be effective in HIV-positive patients for prophylaxis when given for only 2 months,63 but for reasons that have yet to be elucidated the combination of rifampin and PZA for treatment of LTBI was associated with an unanticipated high rate (almost 6%) of severe or fatal hepatotoxicity, especially in HIV-seronegative patients.64,65 The rifampin/PZA combination is, therefore, not currently recommended for treatment of LTBI, although the use of this regimen might rarely be indicated for carefully selected patients under very unusual and stringent circumstances with close clinical and biochemical monitoring and supervision by experienced clinicians. Rifampin is a category C drug, but it is approved for use in pregnant patients with active tuberculosis. It should only be used in pregnancy (as with INH) for high-risk patients with LTBI, and then only if INH is not appropriate.
Availability and Dosage.
Rifampin is supplied in the United States as Rifadin, available in 150- or 300-mg capsules and in combination 300-mg capsules with 150-mg INH (Rifamate) or in 120-mg tablets with 50-mg INH and 300 mg of PZA (Rifater). Rifampin for intravenous infusion (600 mg/vial, Rifadin) should not be used intramuscularly. The usual oral dosage is 10 mg/kg/day (maximum, 600 mg) for adults and 10 to 20 mg/kg/day for children (not to exceed 600 mg/day). A 600-mg twice-weekly schedule generally has been well tolerated. The current rifampin dosing recommendations have raised concerns that some patients may be receiving suboptimal rifampin doses.66 For instance, with current recommendations for 10-mg/kg, maximal 600 mg, dosing, a 60-kg individual would receive the same rifampin dose as a 100-kg person. Studies are ongoing to investigate the safety and efficacy of weight-adjusted rifampin doses higher than the 600-mg dose. Rifampin from opened capsules can be suspended (usually 10 mg/mL) in simple or flavored sugar syrups that should not include ascorbic acid, which can inactivate rifampin.29 Suspensions can be refrigerated up to 2 weeks.
Pyrazinamide
Derivation and Structure.
PZA is a synthetic pyrazine analogue of nicotinamide.
Mechanism of Action.
The mechanism of action of PZA remains unknown.
Antimicrobial Activity and Resistance.
PZA is bactericidal for tubercle bacilli at 12.5 µg/mL. Its optimal activity appears to be against semi-dormant organisms in an acid pH environment, like that existing intracellularly in phagolysosomes. Despite good activity at acid pH in vitro and inhibitory concentrations within monocytes,67 PZA exhibits low activity alone in pretreated macrophages.68 Resistance rapidly evolves if PZA is used alone. Primary resistance is seen in less than 1% of isolates, but nearly 50% of INH-rifampin–resistant MDR-TB isolates are PZA resistant.2 Most isolates resistant to PZA have mutations in the gene encoding pyrazinamidase (pncA) (Table 38-4).69,70 This results in the loss of pyrazinamidase activity, an enzyme that converts PZA to the active form of pyrazinoic acid.
TABLE 38-4
Mechanism of Action and Recognized Mutational Resistance in Commonly Used Antituberculous Agents
| DRUG | MECHANISM OF ACTION | SITE OF MUTATIONAL RESISTANCE (GENE) |
| Isoniazid | Inhibits mycolic acid synthesis | inhA (regulatory region) (mycolic acid gene) |
| Catalase/peroxidase enzyme | katG (catalase/peroxidase gene) | |
| Rifampin | Inhibits RNA polymerization | β subunit rpoB (RNA polymerase gene) |
| Pyrazinamide | Unknown | pncA (pyrazinamidase gene) |
| Ethambutol | Inhibits cell wall synthesis (blocks arabinosyl transferase) | embB (gene for arabinosyl transferase enzyme) |
| Streptomycin | Inhibits protein synthesis | rpsL (gene for ribosomal S12 protein); 16-S ribosomal RNA gene |
| Amikacin | Inhibits protein synthesis | 16-S ribosomal RNA gene (? amikacin-binding site) |
| Capreomycin | Inhibits cell wall synthesis | Unknown |
| Quinolones | Inhibits DNA structure | gyrA (gyrase A gene) |
Pharmacology.
Well-absorbed orally, PZA is widely distributed throughout the body, attaining concentrations above that needed to inhibit tubercle bacilli. Peak plasma concentrations are approximately 50 µg/mL, with a half-life of 12 hours, making once-daily or less frequent dosing practical. PZA crosses inflamed meninges and has been recommended in combination regimens for tuberculous meningitis.71 It is metabolized by the liver, and metabolic products, including principally pyrazinoic acid, are excreted mainly by the kidneys, requiring dosage modification in renal failure. PZA is dialyzable, so dosing is recommended after dialysis sessions consistent with dosing recommendations for INH, rifampin, and ethambutol.57
Adverse Reactions.
The most common side effects are nausea and vomiting. Hepatotoxicity occurred in nearly 15% of PZA recipients in early trials that employed dosages of 40 to 50 mg/kg/day for prolonged periods. Current regimens of 20 to 35 mg/kg/day are safer,72 although recent data suggest that PZA is the most common cause of hepatotoxicity in multidrug regimens also containing INH and rifampin.73,74 Patients with preexisting liver disease should have symptoms and hepatic function tests monitored closely. PZA is also a frequent cause of hypersensitivity reactions and nongouty polyarthralgia in these multidrug regimens.73 Other adverse reactions (1% of patients or less) include interstitial nephritis,75 rhabdomyolysis with myoglobinuric renal failure,76 and photosensitivity. Asymptomatic urate retention occurs in 50% of PZA recipients, with symptomatic gout usually occurring in patients with preexisting gout.72
Usage.
PZA is included as an essential component of multidrug 6-month short-course chemotherapy.19,73 Without PZA for the first 2 months initiation phase of therapy, relapse rates after 6 months of therapy are unacceptable.19 Efficacy with intermittent administration makes PZA suitable for directly observed therapy regimens. PZA is a class C drug and should be used with caution in pregnancy. Although PZA is recommended for routine use in pregnant women by the World Health Organization (WHO), the drug has not been recommended for general use in pregnant women in the United States by the Food and Drug Administration (FDA) and the CDC because of insufficient data to determine safety.19 The practical consequence of this policy is that pregnant women in the United States with active tuberculosis require 9 months of therapy, because PZA is not included in the first 2 months of therapy.
Availability and Dosage.
PZA is available in 500-mg tablets or as 300-mg tablets in combination with INH (50 mg) and rifampin (120 mg) (Rifater). Dosage is 20 to 25 mg/kg/day (maximum, 2.0 g) orally once or in two divided doses. PZA has been well tolerated in a twice-weekly dosage of 50 mg/kg (not to exceed 4 g/day) for short-course regimens.
Ethambutol
Derivation and Structure.
Ethambutol (ethylenediiminobutanol) was discovered in 1961 among synthetic compounds screened for antituberculous activity.
Mechanism of Action.
Ethambutol inhibits arabinosyl transferase enzymes that are involved in arabinogalactan and lipoarabinomannan biosynthesis within the cell wall.77
Antimicrobial Activity and Resistance.
Ethambutol is bacteriostatic in vitro or within macrophages67 at concentrations of 1 µg/mL against susceptible strains of M. tuberculosis. Primary ethambutol resistance in the United States is only approximately 2%.11,12 Ethambutol’s principal role has been as a “companion” drug to curtail resistance. However, resistance rates as high as 80% for ethambutol in INH-rifampin–resistant isolates from New York City apparently indicate limited utility against MDR-TB.2 Ethambutol resistance relates to point mutations in the arabinosyl transferase enzyme EmbB, which is coded for by the embB gene.78
Pharmacology.
Ethambutol administered orally is 75% to 80% absorbed, yielding peak plasma concentrations of 5 µg/mL after a dose of 25 mg/kg. It is distributed throughout the body, including the CSF. Although little ethambutol crosses normal meninges, levels 10% to 50% of those in plasma occur in CSF with meningeal inflammation. After conversion of approximately 25% of absorbed ethambutol to inactive metabolites, 80% of the parent drug, together with metabolites, is excreted in urine. Consequently, it becomes necessary to modify the dosage in significant renal failure.
Adverse Reactions.
The major toxicity of ethambutol is neuropathy, including peripheral neuropathy and retrobulbar optic neuritis. Characteristically, patients complain of bilateral blurry vision and are found to have impairment of visual acuity and red-green color vision. Common in association with high-dose (50 mg/kg/day) therapy with prolonged administration, and more common with 25 mg/kg/day than with 15 mg/kg/day dosing, retrobulbar neuritis is usually slowly reversible. Visual loss has rarely occurred in elderly persons receiving as little as 15 mg/kg/day.79 The administration of ethambutol at 25 mg/kg on a three times weekly basis appears to be associated with a reduced risk for visual toxicity in this patient population compared with daily dosing at 15 mg/kg.80 This observation is important because baseline visual acuity is often impaired in the elderly and associated with other diseases such as cataracts that are associated with blurred vision. Recipients of ethambutol should be instructed to report symptoms of blurry vision promptly and to discontinue the drug until confirmatory visual testing can be done. Visual acuity and red-green color perception testing is recommended at baseline, whenever a change in visual symptoms occurs, and monthly for patients taking ethambutol for more than 2 months or at higher than usual doses.19 Monthly testing in patients on 15 mg/kg can be useful in establishing the range of visual abnormalities in those already visually impaired.
Gastrointestinal intolerance is infrequent. Hyperuricemia occurs because of decreased renal uric acid excretion. Hypersensitivity reactions are rare and include dermatitis, arthralgias, and fever.
Significant Drug Interactions.
There are no significant drug interactions with ethambutol.
Usage.
Ethambutol is routinely included as the fourth drug along with INH, rifampin, and PZA in initial therapy for tuberculosis before the availability of drug susceptibility information. Ethambutol is included to protect against the emergence of rifampin resistance in patients with occult INH resistance who, if receiving only INH, rifampin, and PZA, would be functionally on only INH and PZA. It is also routinely used in treatment regimens for patients with isolates resistant to INH or rifampin, or both. Ethambutol has no detectable effects on the fetus and is approved for treatment of tuberculosis in the United States.
Availability and Dosage.
Ethambutol is available as ethambutol hydrochloride (Myambutol) supplied in 100- or 400-mg tablets. The usual dosage is 15 to 25 mg/kg/day initially, followed after 60 days by 15 mg/kg/day (maximum, 1600 mg) as a single daily dose.
Streptomycin
Derivation, Structure, and Pharmacology.
Streptomycin, an aminoglycoside antibiotic introduced in the 1940s, was the first drug to reduce tuberculosis mortality. Its structure, mechanism of action, and pharmacology are covered in other chapters. Briefly, intramuscular injection of 1 g yields peak plasma concentrations of 25 to 45 µg/mL. It is virtually excluded from the CNS.
Antimicrobial Activity and Resistance.
Streptomycin is bactericidal against M. tuberculosis in vitro but is inactive against intracellular tubercle bacilli. Concentrations of 4 to 10 µg/mL of plasma are inhibitory. The rapid emergence of resistance to streptomycin was quickly recognized as a consequence of single-drug therapy. Approximately 1 in 106 tubercle bacilli is spontaneously resistant to streptomycin. Primary resistance to streptomycin is seen most often in patient populations having a high incidence of INH resistance. In MDR-TB disease outbreaks, approximately 80% of INH-rifampin–resistant isolates are also streptomycin resistant.2 Streptomycin resistance relates to mutational changes involving ribosomal binding protein or the ribosomal binding site.27,81,82 Isolates resistant to streptomycin are not cross-resistant to amikacin, kanamycin, or capreomycin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree